
Exhibit 99.03

© 2018 Tonix Pharmaceuticals Holding Corp. November 2018 Version P0141 10 - 31 - 18 (Doc 0404) Investor Presentation
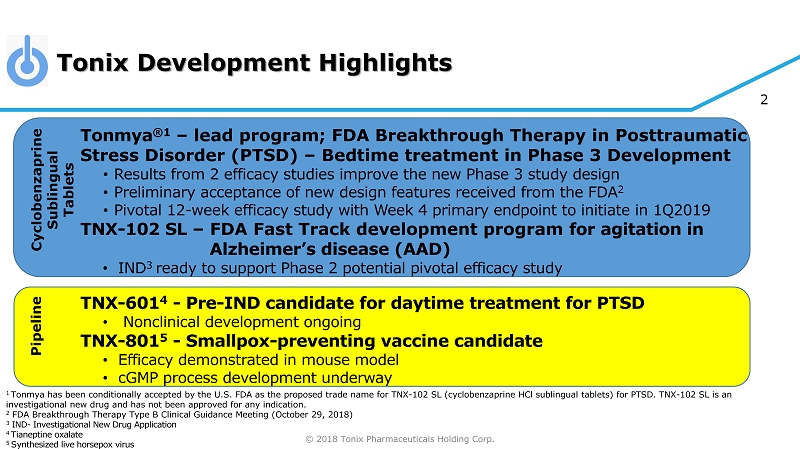
© 2018 Tonix Pharmaceuticals Holding Corp. 2 Tonmya ®1 – lead program; FDA Breakthrough Therapy in Posttraumatic Stress Disorder (PTSD) – Bedtime treatment in Phase 3 Development • Results from 2 efficacy studies improve the new Phase 3 study design • Preliminary acceptance of new design features received from the FDA 2 • Pivotal 12 - week efficacy study with Week 4 primary endpoint to initiate in 1Q2019 TNX - 102 SL – FDA Fast Track development program for agitation in Alzheimer’s disease (AAD) • IND 3 ready to support Phase 2 potential pivotal efficacy study TNX - 601 4 - Pre - IND candidate for daytime treatment for PTSD • Nonclinical development ongoing TNX - 801 5 - Smallpox - preventing vaccine candidate • Efficacy demonstrated in mouse model • cGMP process development underway 1 Tonmya has been conditionally accepted by the U.S. FDA as the proposed trade name for TNX - 102 SL (cyclobenzaprine HCl sublingual tablets) for PTSD. TNX - 102 SL is an investigational new drug and has not been approved for any indication. 2 FDA Breakthrough Therapy Type B Clinical Guidance Meeting (October 29, 2018) 3 IND - Investigational New Drug Application 4 T ianeptine oxalate 5 S ynthesized live horsepox virus Tonix Development Highlights Cyclobenzaprine Sublingual Tablets Pipeline
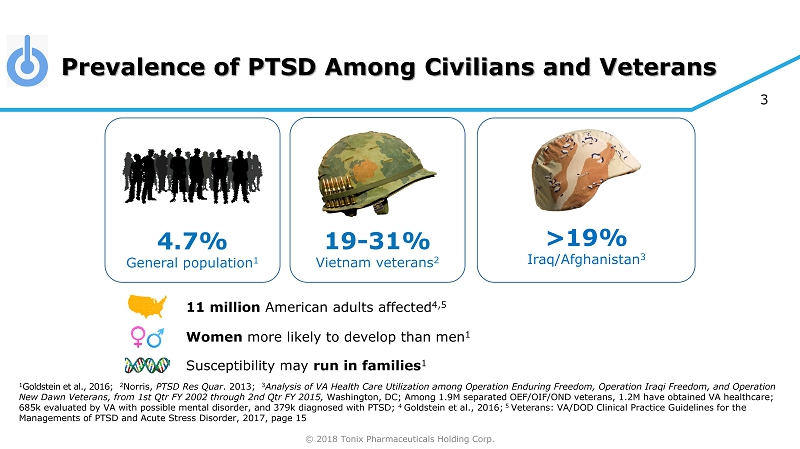
© 2018 Tonix Pharmaceuticals Holding Corp. 3 Prevalence of PTSD Among Civilians and Veterans 1 Goldstein et al., 2016 ; 2 Norris, PTSD Res Quar . 2013; 3 Analysis of VA Health Care Utilization among Operation Enduring Freedom, Operation Iraqi Freedom, and Operation New Dawn Veterans, from 1st Qtr FY 2002 through 2nd Qtr FY 2015, Washington, DC ; Among 1.9M separated OEF/OIF/OND veterans, 1.2M have obtained VA healthcare; 685k evaluated by VA with possible mental disorder, and 379k diagnosed with PTSD; 4 Goldstein et al., 2016; 5 Veterans: VA/DOD Clinical Practice Guidelines for the Managements of PTSD and Acute Stress Disorder, 2017, page 15 >19% Iraq/Afghanistan 3 4.7% General population 1 19 - 31% Vietnam veterans 2 11 million American adults affected 4,5 Women more likely to develop than men 1 Susceptibility may run in families 1
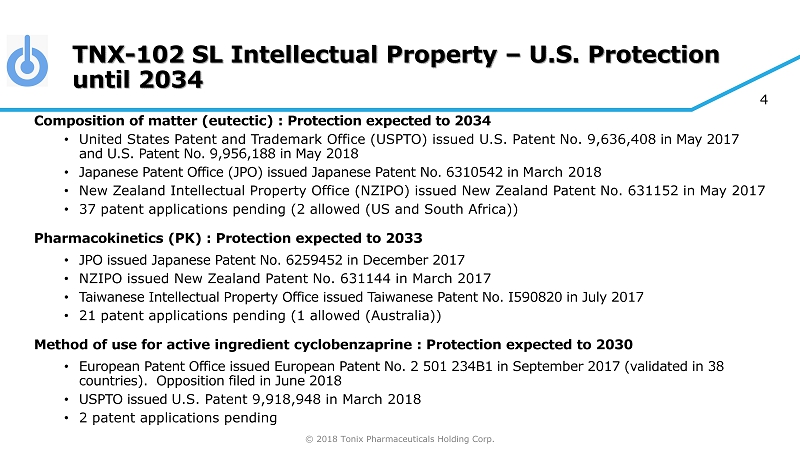
© 2018 Tonix Pharmaceuticals Holding Corp. 4 TNX - 102 SL Intellectual Property – U.S. Protection until 2034 Composition of matter (eutectic) : Protection expected to 2034 • United States Patent and Trademark Office (USPTO) issued U.S. Patent No. 9,636,408 in May 2017 and U.S. Patent No. 9,956,188 in May 2018 • Japanese Patent Office (JPO) issued Japanese Patent No. 6310542 in March 2018 • New Zealand Intellectual Property Office (NZIPO) issued New Zealand Patent No. 631152 in May 2017 • 37 patent applications pending (2 allowed (US and South Africa)) Pharmacokinetics (PK) : Protection expected to 2033 • JPO issued Japanese Patent No. 6259452 in December 2017 • NZIPO issued New Zealand Patent No. 631144 in March 2017 • Taiwanese Intellectual Property Office issued Taiwanese Patent No. I590820 in July 2017 • 21 patent applications pending (1 allowed (Australia)) Method of use for active ingredient cyclobenzaprine : Protection expected to 2030 • European Patent Office issued European Patent No. 2 501 234B1 in September 2017 (validated in 38 countries). Opposition filed in June 2018 • USPTO issued U.S. Patent 9,918,948 in March 2018 • 2 patent applications pending

© 2018 Tonix Pharmaceuticals Holding Corp. 5 Tonmya® 1 - Breakthrough Therapy for Treatment of PTSD Targets Sleep Disturbance Tonmya - first investigational new drug to show treatment effect in military - related PTSD in two large, 12 - week, multi - center studies Retrospective analyses showed Tonmya reduced PTSD severity in participants who experienced index traumas during military service in 2001 or later • The severity of PTSD symptoms were measured by the CAPS - 5 2 assessment scale, the endpoint required by FDA for marketing approval Breakthrough Therapy Designation from FDA in December 2016 Collaboration with Army: Tonix - USAMMDA CRADA signed 2015 1 Tonmya has been conditionally accepted by the U.S. FDA as the proposed trade name for TNX - 102 SL (cyclobenzaprine HCl sublingual tablets) for PTSD. TNX - 102 SL is an investigational new drug and has not been approved for any indication. 2 Clinician Administered PTSD Scale for DSM - 5

© 2018 Tonix Pharmaceuticals Holding Corp. 6 Need for Effective and Safe Therapies for Treatment of Military PTSD PTSD is signature wound of last 25 years of war • Affects servicemember health and performance, force readiness, retention • Believed to be the underlying cause of suicide in many cases No products FDA approved for PTSD since Pfizer’s Zoloft® (sertraline) and GSK’s Paxil® (paroxetine) circa 2000 • Neither has shown efficacy in military - related PTSD • Male PTSD patients often unresponsive or intolerant of current treatments • Side effects relating to sexual dysfunction (particularly in males), sleep and weight gain are commonly reported DoD is Working to Understand and Treat PTSD • Increased scrutiny of PTSD discharges for behavioral problems • Wider recognition that PTSD is a service - related disability

© 2018 Tonix Pharmaceuticals Holding Corp. 7 Key Features Tonmya is believed to treat PTSD by improving sleep quality • The brain naturally processes memories during sleep • PTSD sufferers’ emotionally charged memories disturb sleep and disrupt the natural processing of memories during sleep • Tonmya is believed to normalize memory processing and facilitate extinction consolidation (breaking the link between “triggers” and PTSD symptoms) Tonmya is NEITHER a benzodiazepine nor a narcotic • The active ingredient, does NOT interact with the same receptors as traditional hypnotic sleep drugs associated with retrograde amnesia; is NOT an opiate Tonmya is non - addictive • The active ingredient of Tonmya, cyclobenzaprine, is the active ingredient of an orally ingested immediate release tablets ( Flexeril ® ), approved 40 years ago • Flexeril’s current labeling indicates no abuse and dependence concern at higher doses than Tonmya ( 15 - 30 mg/day v. 5.6 mg/day ); NDA can be filed without drug abuse and dependency assessment studies Once - daily sublingual dose taken at bedtime enhances patient adherence
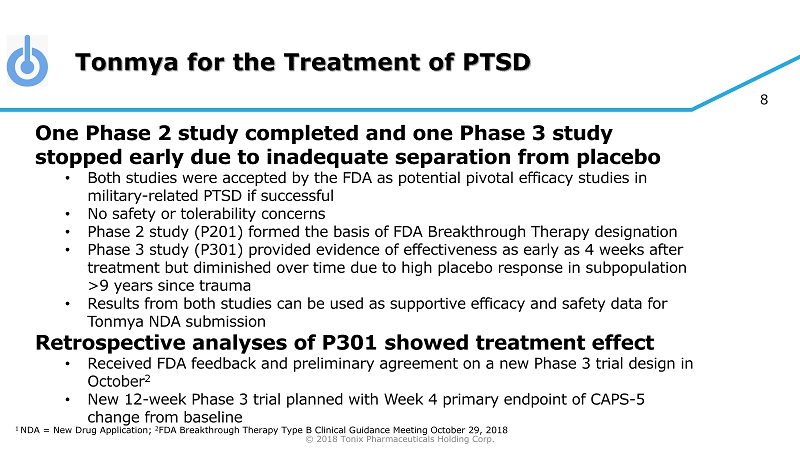
© 2018 Tonix Pharmaceuticals Holding Corp. 8 Tonmya for the Treatment of PTSD One Phase 2 study completed and one Phase 3 study stopped early due to inadequate separation from placebo • Both studies were accepted by the FDA as potential pivotal efficacy studies in military - related PTSD if successful • No safety or tolerability concerns • Phase 2 study (P201) formed the basis of FDA Breakthrough Therapy designation • Phase 3 study (P301) provided evidence of effectiveness as early as 4 weeks after treatment but diminished over time due to high placebo response in subpopulation >9 years since trauma • Results from both studies can be used as supportive efficacy and safety data for Tonmya NDA submission Retrospective analyses of P301 showed treatment effect • Received FDA feedback and preliminary agreement on a new Phase 3 trial design in October 2 • New 12 - week Phase 3 trial planned with Week 4 primary endpoint of CAPS - 5 change from baseline 1 NDA = New Drug Application; 2 FDA Breakthrough Therapy Type B Clinical Guidance Meeting October 29, 2018
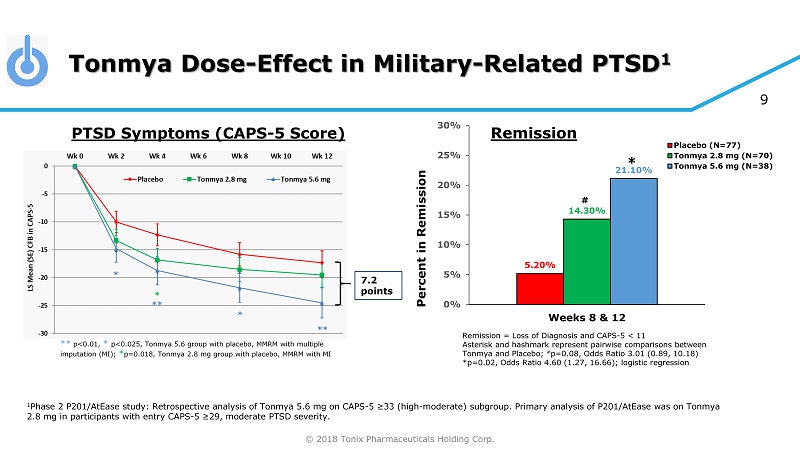
© 2018 Tonix Pharmaceuticals Holding Corp. 9 Tonmya Dose - Effect in Military - Related PTSD 1 1 Phase 2 P201/AtEase study: Retrospective analysis of Tonmya 5.6 mg on CAPS - 5 ≥33 (high - moderate) subgroup. Primary analysis of P 201/AtEase was on Tonmya 2.8 mg in participants with entry CAPS - 5 ≥29 , moderate PTSD severity. 7.2 points ** p<0.01, * p<0.025, Tonmya 5.6 group with placebo, MMRM with multiple imputation (MI); * p=0.018, Tonmya 2.8 mg group with placebo, MMRM with MI PTSD Symptoms (CAPS - 5 Score) Remission = Loss of Diagnosis and CAPS - 5 < 11 Asterisk and hashmark represent pairwise comparisons between Tonmya and Placebo; # p=0.08, Odds Ratio 3.01 (0.89, 10.18) *p=0.02, Odds Ratio 4.60 (1.27, 16.66); logistic regression 5.20% 14.30% 21.10% 0% 5% 10% 15% 20% 25% 30% Weeks 8 & 12 Percent in Remission Placebo (N=77) Tonmya 2.8 mg (N=70) Tonmya 5.6 mg (N=38) # * Remission
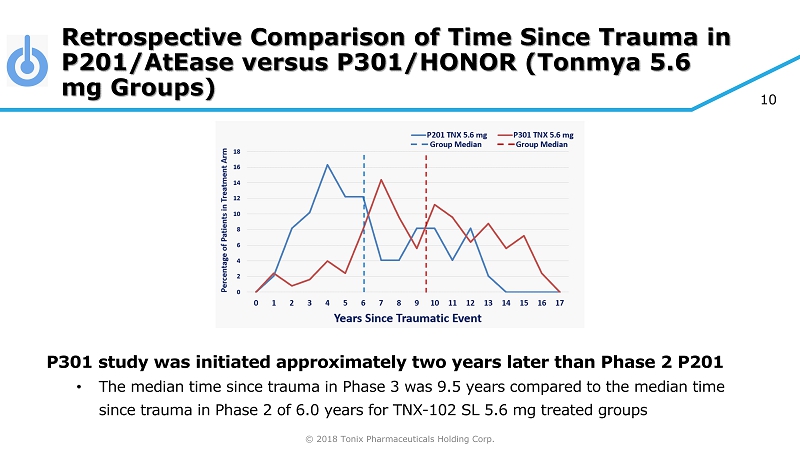
© 2018 Tonix Pharmaceuticals Holding Corp. 10 Retrospective Comparison of Time Since Trauma in P201/AtEase versus P301/HONOR (Tonmya 5.6 mg Groups) P301 study was initiated approximately two years later than Phase 2 P201 • The median time since trauma in Phase 3 was 9.5 years compared to the median time since trauma in Phase 2 of 6.0 years for TNX - 102 SL 5.6 mg treated groups
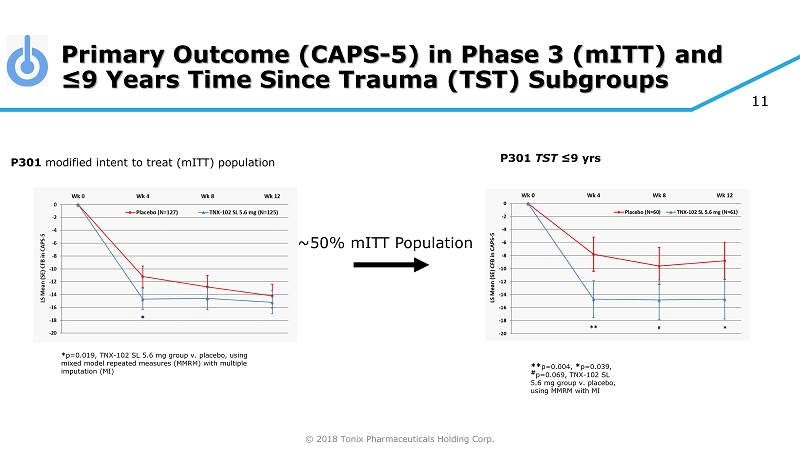
© 2018 Tonix Pharmaceuticals Holding Corp. Primary Outcome (CAPS - 5) in Phase 3 ( mITT ) and ≤9 Years Time Since Trauma (TST) Subgroups P301 modified intent to treat ( mITT ) population P301 TST ≤9 yrs ** p=0.004, * p=0.039, # p=0.069, TNX - 102 SL 5.6 mg group v. placebo, using MMRM with MI ~50% mITT Population 11 * p=0.019, TNX - 102 SL 5.6 mg group v. placebo, using mixed model repeated measures (MMRM) with multiple imputation (MI)
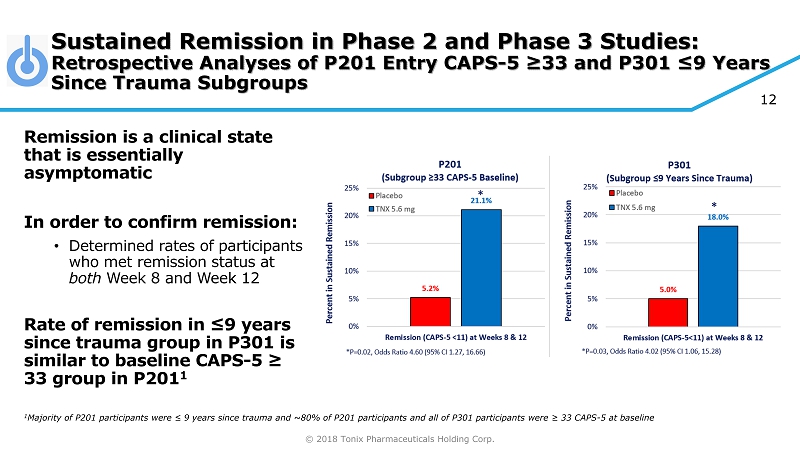
© 2018 Tonix Pharmaceuticals Holding Corp. 12 Sustained Remission in Phase 2 and Phase 3 Studies: Retrospective Analyses of P201 Entry CAPS - 5 ≥33 and P301 ≤9 Years Since Trauma Subgroups Remission is a clinical state that is essentially asymptomatic In order to confirm remission: • Determined rates of participants who met remission status at both Week 8 and Week 12 Rate of remission in ≤9 years since trauma group in P301 is similar to baseline CAPS - 5 ≥ 33 group in P201 1 1 Majority of P201 participants were ≤ 9 years since trauma and ~80% of P201 participants and all of P301 participants were ≥ 33 CAPS - 5 at baseline
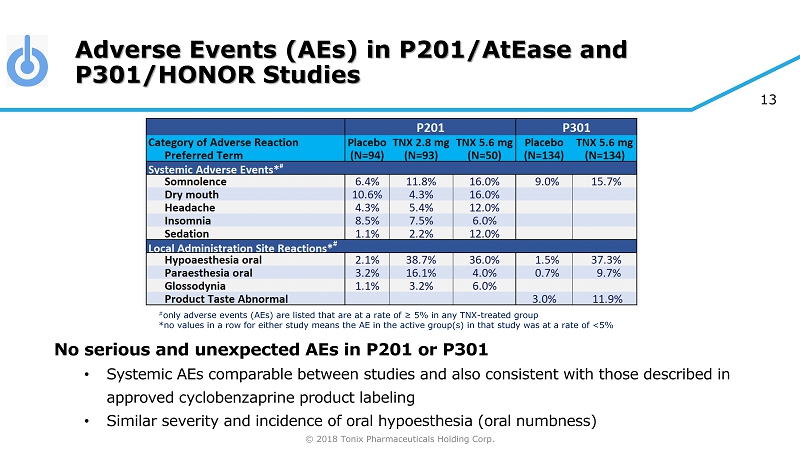
© 2018 Tonix Pharmaceuticals Holding Corp. 13 Adverse Events (AEs) in P201/AtEase and P301/HONOR Studies No serious and unexpected AEs in P201 or P301 • Systemic AEs comparable between studies and also consistent with those described in approved cyclobenzaprine product labeling • Similar severity and incidence of oral hypoesthesia (oral numbness) # only adverse events (AEs) are listed that are at a rate of ≥ 5% in any TNX - treated group *no values in a row for either study means the AE in the active group(s) in that study was at a rate of <5%

© 2018 Tonix Pharmaceuticals Holding Corp. 14 Summary of Clinical Experience with Tonmya/ TNX - 102 SL in PTSD Median time since trauma (TST) in TNX - 102 SL 5.6 mg group in the P301/HONOR study (9.5 years) was longer than P201/AtEase study (6 years) • Both studied military - related PTSD • Time has passed since the surge in Iraq In retrospective analysis, the ≤ 9 year subgroup of P301 study had similar results as the P201 study (primary and secondary) • TST is important in placebo - controlled clinical study • Potential enrichment in ≤ 9 years TST subgroup for treatment responders The ≤ 9 year subgroup of P301 may be enriched for “Remitting Phase” of PTSD 1 - 4 • Expect remitting phase of PTSD is more amenable to drug studies Results from retrospective analyses lead to improved Phase 3 study design 1 Kessler et al. Arch Gen Psychiatry 1995;52:1048 - 1060. 2 Armenta et al. BMC Psychiatry 2018;18:48. 3 Galatzer - Levy et al. PLOS ONE 2013;8:e70084. 4 Perkonigg et al. Am J Psychiatry 2005;162:1320 - 1327.

© 2018 Tonix Pharmaceuticals Holding Corp. 15 Tonmya/TNX - 102 SL – New Phase 3 Study In two prior clinical trials, Tonmya 5.6 mg consistently reduced military - related PTSD severity for participants with ≤ 9 years since trauma These data provide the rationale to study Tonmya’s effect in participants with ≤ 9 years since trauma in a new Phase 3 study • Civilian - related PTSD will be studied along with military - related PTSD • Primary endpoint will be CAPS - 5 at Week 4 to minimize drop - out • Treatment duration will be 12 weeks • Plans to initiate in 1Q 2019
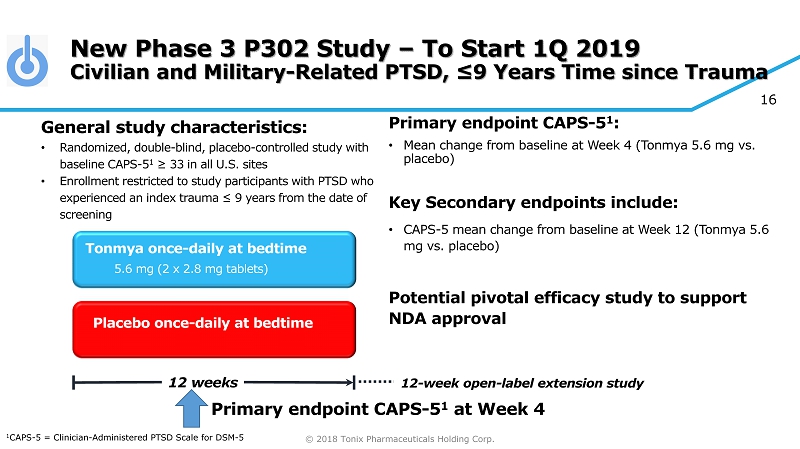
© 2018 Tonix Pharmaceuticals Holding Corp. 16 New Phase 3 P302 Study – To Start 1Q 2019 Civilian and Military - Related PTSD, ≤9 Years Time since Trauma Primary e ndpoint CAPS - 5 1 : • Mean change from baseline at W eek 4 (Tonmya 5.6 mg vs. placebo ) Key Secondary e ndpoint s include: • CAPS - 5 m ean change from baseline at W eek 12 (Tonmya 5.6 mg vs. placebo ) Potential pivotal efficacy study to support NDA approval Placebo once - daily at bedtime 12 weeks Tonmya once - daily at bedtime 5.6 mg (2 x 2.8 mg tablets) General s tudy c haracteristics: • Randomized, double - blind, placebo - controlle d study with baseline CAPS - 5 1 ≥ 33 in all U.S. sites • Enrollment restricted to study participants with PTSD who experienced an index trauma ≤ 9 years from the date of screening 12 - week open - label extension study 1 CAPS - 5 = Clinician - Administered PTSD Scale for DSM - 5 Primary e ndpoint CAPS - 5 1 at Week 4

© 2018 Tonix Pharmaceuticals Holding Corp. 17 Late - Stage PTSD Drug Candidates Tonmya • Breakthrough Therapy in Phase 3; only development program focused on military - related and civilian PTSD, only drug to show activity in treatment of military - related PTSD in large multi - center trials MDMA - assisted psychotherapy • Breakthrough therapy that is Phase 3 - ready; showed activity in a Phase 2 study of PTSD Other drugs currently (or recently) in Phase 2 development • Rexulti ® ( brexpiprazole ) - Otsuka/Lundbeck; atypical antipsychotic • BNC - 201 – Bionomics; nicotinic receptor modulator (program stopped after Phase 2)
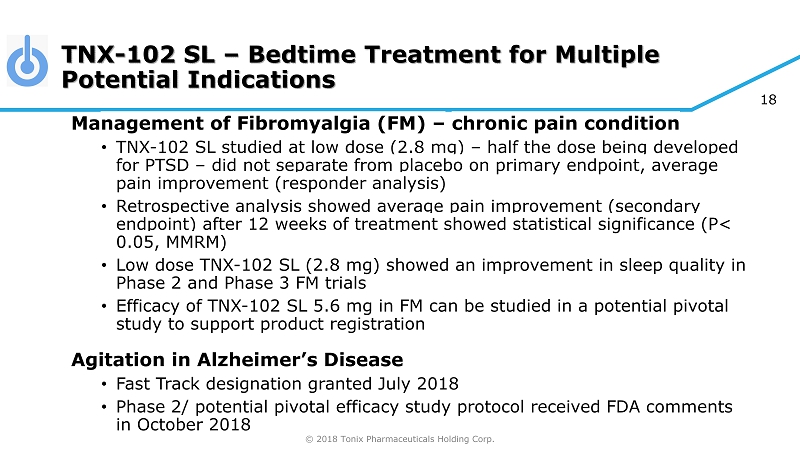
© 2018 Tonix Pharmaceuticals Holding Corp. 18 TNX - 102 SL – Bedtime Treatment for Multiple Potential Indications Ma nagement of Fibromyalgia (FM) – chronic pain condition • TNX - 102 SL studied at low dose (2.8 mg) – half the dose being developed for PTSD – did not separate from placebo on primary endpoint, average pain improvement (responder analysis) • Retrospective analysis showed average pain improvement (secondary endpoint) after 12 weeks of treatment showed statistical significance (P< 0.05, MMRM) • Low dose TNX - 102 SL (2.8 mg) showed an improvement in sleep quality in Phase 2 and Pha se 3 FM trials • Efficacy of TNX - 102 SL 5.6 mg in FM can be studied in a potential pivotal study to support product registration Agitation in Alzheimer’s Disease • Fast Track designation granted July 2018 • Phase 2/ potential pivotal efficacy study protocol received FDA comments in October 2018

© 2018 Tonix Pharmaceuticals Holding Corp. 19 TNX - 102 SL for Agitation in Alzheimer’s – Regulatory Status and Registration Strategy FDA confirmed no additional study is needed prior to IND submission • Pre - IND meeting established open dialogue with the FDA on pivotal clinical study design and efficacy endpoints to support product registration Proposed Phase 2 IND study can potentially serve as a pivotal efficacy study to support NDA approval • FDA comments on final protocol received October 2018 Registration Strategy of TNX - 102 SL for agitation in Alzheimer’s disease • Efficacy Supplement (sNDA 1 ) may be leveraged from the PTSD/FM development program and supported by Initial NDA approval for PTSD/FM 1 Supplemental New Drug Application
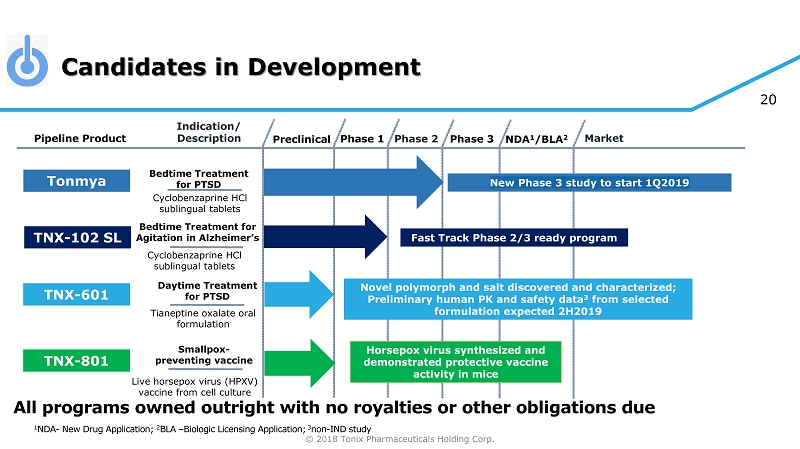
© 2018 Tonix Pharmaceuticals Holding Corp. 20 Candidates in Development Preclinical Phase 2 NDA 1 /BLA 2 Market Pipeline Product Indication/ Description Phase 3 Tonmya Bedtime Treatment for PTSD Daytime Treatment for PTSD TNX - 601 Novel polymorph and salt discovered and characterized; Preliminary human PK and safety data 3 from selected formulation expected 2H2019 TNX - 801 Horsepox virus synthesized and demonstrated protective vaccine activity in mice Smallpox - preventing vaccine Cyclobenzaprine HCl sublingual tablets Tianeptine oxalate oral formulation Live horsepox virus (HPXV) vaccine from cell culture Phase 1 1 NDA - New Drug Application; 2 BLA – Biologic Licensing Application; 3 non - IND study Bedtime Treatment for Agitation in Alzheimer’s Cyclobenzaprine HCl sublingual tablets Fast Track Phase 2/3 ready program TNX - 102 SL All programs owned outright with no royalties or other obligations due New Phase 3 study to start 1Q2019
© 2018 Tonix Pharmaceuticals Holding Corp. 21 TNX - 601 ( Tianeptine Oxalate): A Potential Clinical Candidate for PTSD Pre - IND Candidate Targeted as a 1 st line monotherapy for PTSD: oral formulation for daytime dosing x Leverages expertise in PTSD (clinical and regulatory experience, market analysis, etc.) x Mechanism of Action (MOA) is different from TNX - 102 SL • Tianeptine sodium (amorphous) has been approved in EU, Russia, Asia and Latin America for depression since 1987 with established post - marketing experience • Identified new oxalate salt polymorph with improved pharmaceutical properties ideal for reformulation • Preliminary human pharmacokinetic and safety data (non - IND study) from selected formulation expected in second half 2019 Filed patent application on novel salt polymorph • Issued patent on steroid - induced cognitive impairment and memory loss issues Targeting a Condition with Significant Unmet Need Clinical evidence for PTSD • Several studies have shown tianeptine to be active in the treatment of PTSD 1 - 4 1 Frančišković T, et al. Psychiatr Danub . 2011 Sep;23(3):257 - 63. PMID: 21963693 2 Rumyantseva GM and, Stepanov AL. Neurosci Behav Physiol. 2008 Jan;38(1):55 - 61. PMID: 18097761 3 Aleksandrovskiĭ IA, et al. Zh Nevrol Psikhiatr Im S S Korsakova . 2005;105(11):24 - 9. PMID: 16329631 [Russian] 4 Onder E, et al. Eur Psychiatry. 2006 (3):174 - 9. PMID: 15964747

© 2018 Tonix Pharmaceuticals Holding Corp. 22 TNX - 801 (Synthesized Live Horsepox Virus): A Smallpox - Preventing Vaccine Candidate Pre - IND Stage Potential improvement over current biodefense tools against smallpox ✓ Leverages Tonix’s government affairs effort ✓ Collaboration with Professor David Evans and Dr. Ryan Noyce at University of Alberta ✓ Demonstrated protective vaccine activity in mice ✓ Patent application on novel vaccine submitted Regulatory strategy • We intend to meet with FDA to discuss the most efficient and appropriate investigational plan to support the licensure, either: ✓ Application of the “Animal Rule”, or ✓ Conducting an active comparator study using ACAM2000 • Good Manufacturing Practice (GMP) viral production process in development Targeting a Potential Public Health Issue Material threat medical countermeasure under 21 st Century Cures Act • Qualifies for Priority Review Voucher (PRV) upon licensure * ✓ PRVs have no expiration date, are transferrable and have sold for ~$125 M *BLA/NDA priority 6 - month review is expected.

© 2018 Tonix Pharmaceuticals Holding Corp. 23 Management Team Seth Lederman, MD President & CEO Jessica Morris Chief Operating Officer Gregory Sullivan, MD Chief Medical Officer Bradley Saenger, CPA Chief Financial Officer
© 2018 Tonix Pharmaceuticals Holding Corp. 24 Milestones – Recently Completed and Upcoming □ July 201 8 Completed P301/HONOR study interim analysis - result did not support study continuation but strengthened new Phase 3 study □ August 201 8 Presentation of P301/HONOR study results at Military Health System Scientific Symposium □ October 201 8 Met with FDA and received preliminary agreement on the design of new Phase 3 study of Tonmya for PTSD □ First Quarter 2019 Initiate new Phase 3 study of Tonmya for PTSD (civilian and military) □ Second Half 2019 Preliminary human pharmacokinetic and safety data (non - IND study) from selected TNX - 601 (tianeptine oxalate) formulation x x x
© 2018 Tonix Pharmaceuticals Holding Corp. 25 Summary Phase 3 development of Breakthrough Therapy treatment for PTSD, including military - related PTSD • Major unmet need; ~11 million Americans affected • Benefited from FDA 505(b)(2) NDA approval requirement New indication in development for agitation in Alzheimer’s Disease • Unmet medical need, no approved drug available • Fast Track Phase 2/3 ready program Complimentary day - time PTSD treatment in development • Leverages development expertise in PTSD, i.e., regulatory, trial recruitment and execution Innovative vaccine in development to prevent Smallpox • Opportunity to supply stockpiling requirement; short development path • Studies in mice suggest improved safety profile

© 2018 Tonix Pharmaceuticals Holding Corp. Thank you ! NASDAQ: TNXP