

© 2018 Tonix Pharmaceuticals Holding Corp. December 2018 Investor Presentation Issuer Free Writing Prospectus Filed Pursuant to Rule 433 Registration No. 333 - 227228

© 2018 Tonix Pharmaceuticals Holding Corp. 2 Cautionary Note on Forward - Looking Statements Certain statements in this presentation regarding strategic plans, expectations and objectives for future operations or results are “forward - looking statements” as defined by the Private Securities Litigation Reform Act of 1995 . These statements may be identified by the use of forward - looking words such as “anticipate,” “believe,” “forecast,” “estimate” and “intend,” among others . These forward - looking statements are based on Tonix’s current expectations and actual results could differ materially . There are a number of factors that could cause actual events to differ materially from those indicated by such forward - looking statements . These factors include, but are not limited to, substantial competition ; our need for additional financing ; uncertainties of patent protection and litigation ; uncertainties of government or third party payor reimbursement ; limited research and development efforts and dependence upon third parties ; and risks related to failure to obtain U . S . Food and Drug Administration clearances or approvals and noncompliance with its regulations . As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products . The forward - looking statements in this presentation are made as of the date of this presentation, even if subsequently made available by Tonix on its website or otherwise . Tonix does not undertake an obligation to update or revise any forward - looking statement, except as required by law . Investors should read the risk factors set forth in the Annual Report on Form 10 - K for the year ended December 31 , 2017 , as filed with the Securities and Exchange Commission (the “SEC”) on March 9 , 2018 , and periodic reports and current reports filed with the SEC on or after the date thereof . All of Tonix's forward - looking statements are expressly qualified by all such risk factors and other cautionary statements .

Free Writing Prospectus Statement This presentation highlights basic information about us and the offering to which this communication relates. Because it is a summary, it does not contain all of the information that you should consider before investing in our securities. We have filed a registration statement (including a prospectus, which currently is in preliminary form) with the SEC for the offering to which this presentation relates. The registration has not yet become effective. Before you invest, you should read the preliminary registration statement (including the risk factors described therein) and other documents we have filed with the SEC for more complete information about us and this offering. You may access these documents for free by visiting EDGAR on the SEC Web site at www.sec.gov. The preliminary prospectus, dated December 3, 2018, is available on the SEC Web site at www.sec.gov/Archives/edgar/data/ {}. Alternatively, we or any underwriter participating in the offering will arrange to send you the preliminary prospectus and, when available, the final prospectus and/or any supplements thereto if you contact A.G.P./Alliance Global Partners, 590 Madison Avenue, 36th Floor, New York, NY 10022 or via telephone at 212-624-2006 or email: presentation@allianceg.com. This presentation shall not constitute an offer to sell, or the solicitation of an offer to buy, nor will there be any sale of these securities in any state or other jurisdiction in which such offer, solicitation or sale would be unlawful prior to the registration or qualification under the securities laws of such state or jurisdiction. The offering will only be made by means of a prospectus pursuant to a registration statement that is filed with the SEC after such registration statement becomes effective. © 2018 Tonix Pharmaceuticals Holding Corp.
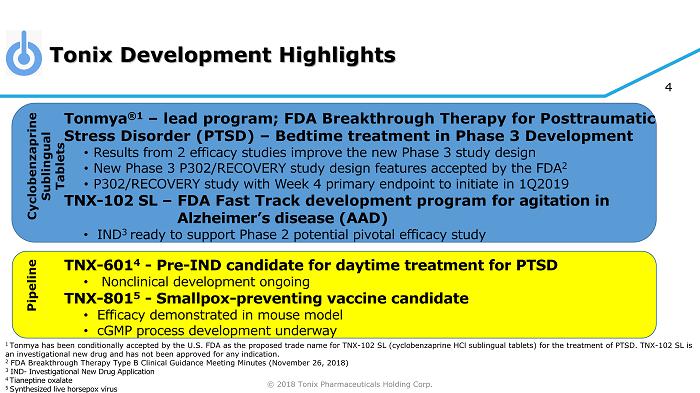
© 2018 Tonix Pharmaceuticals Holding Corp. 4 Tonmya ®1 – lead program; FDA Breakthrough Therapy for Posttraumatic Stress Disorder (PTSD) – Bedtime treatment in Phase 3 Development • Results from 2 efficacy studies improve the new Phase 3 study design • New Phase 3 P302/RECOVERY study design features accepted by the FDA 2 • P302/RECOVERY study with Week 4 primary endpoint to initiate in 1Q2019 TNX - 102 SL – FDA Fast Track development program for agitation in Alzheimer’s disease (AAD) • IND 3 ready to support Phase 2 potential pivotal efficacy study TNX - 601 4 - Pre - IND candidate for daytime treatment for PTSD • Nonclinical development ongoing TNX - 801 5 - Smallpox - preventing vaccine candidate • Efficacy demonstrated in mouse model • cGMP process development underway 1 Tonmya has been conditionally accepted by the U.S. FDA as the proposed trade name for TNX - 102 SL (cyclobenzaprine HCl sublingual tablets) for the treatment of PTSD. TNX - 102 SL is an investigational new drug and has not been approved for any indication. 2 FDA Breakthrough Therapy Type B Clinical Guidance Meeting Minutes (November 26, 2018) 3 IND - Investigational New Drug Application 4 T ianeptine oxalate 5 S ynthesized live horsepox virus Tonix Development Highlights Cyclobenzaprine Sublingual Tablets Pipeline
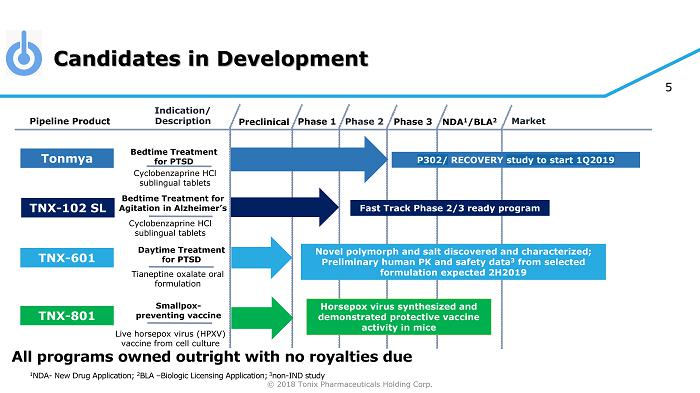
© 2018 Tonix Pharmaceuticals Holding Corp. 5 Candidates in Development Preclinical Phase 2 NDA 1 /BLA 2 Market Pipeline Product Indication/ Description Phase 3 Tonmya Bedtime Treatment for PTSD Daytime Treatment for PTSD TNX - 601 Novel polymorph and salt discovered and characterized; Preliminary human PK and safety data 3 from selected formulation expected 2H2019 TNX - 801 Horsepox virus synthesized and demonstrated protective vaccine activity in mice Smallpox - preventing vaccine Cyclobenzaprine HCl sublingual tablets Tianeptine oxalate oral formulation Live horsepox virus (HPXV) vaccine from cell culture Phase 1 1 NDA - New Drug Application; 2 BLA – Biologic Licensing Application; 3 non - IND study Bedtime Treatment for Agitation in Alzheimer’s Cyclobenzaprine HCl sublingual tablets Fast Track Phase 2/3 ready program TNX - 102 SL All programs owned outright with no royalties due P302/ RECOVERY study to start 1Q2019
© 2018 Tonix Pharmaceuticals Holding Corp. 6 Management Team Seth Lederman, MD President & CEO Jessica Morris Chief Operating Officer Gregory Sullivan, MD Chief Medical Officer Bradley Saenger, CPA Chief Financial Officer
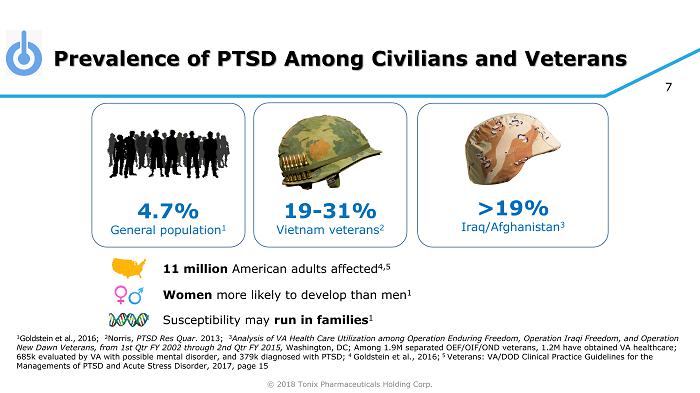
© 2018 Tonix Pharmaceuticals Holding Corp. 7 Prevalence of PTSD Among Civilians and Veterans 1 Goldstein et al., 2016 ; 2 Norris, PTSD Res Quar . 2013; 3 Analysis of VA Health Care Utilization among Operation Enduring Freedom, Operation Iraqi Freedom, and Operation New Dawn Veterans, from 1st Qtr FY 2002 through 2nd Qtr FY 2015, Washington, DC ; Among 1.9M separated OEF/OIF/OND veterans, 1.2M have obtained VA healthcare; 685k evaluated by VA with possible mental disorder, and 379k diagnosed with PTSD; 4 Goldstein et al., 2016; 5 Veterans: VA/DOD Clinical Practice Guidelines for the Managements of PTSD and Acute Stress Disorder, 2017, page 15 >19% Iraq/Afghanistan 3 4.7% General population 1 19 - 31% Vietnam veterans 2 11 million American adults affected 4,5 Women more likely to develop than men 1 Susceptibility may run in families 1
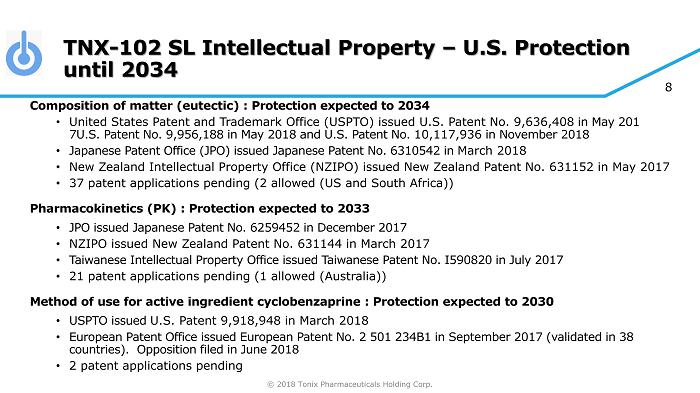
© 2018 Tonix Pharmaceuticals Holding Corp. 8 TNX - 102 SL Intellectual Property – U.S. Protection until 2034 Composition of matter (eutectic) : Protection expected to 2034 • United States Patent and Trademark Office (USPTO) issued U.S. Patent No. 9,636,408 in May 201 7U.S. Patent No. 9,956,188 in May 2018 and U.S. Patent No. 10,117,936 in November 2018 • Japanese Patent Office (JPO) issued Japanese Patent No. 6310542 in March 2018 • New Zealand Intellectual Property Office (NZIPO) issued New Zealand Patent No. 631152 in May 2017 • 37 patent applications pending (2 allowed (US and South Africa)) Pharmacokinetics (PK) : Protection expected to 2033 • JPO issued Japanese Patent No. 6259452 in December 2017 • NZIPO issued New Zealand Patent No. 631144 in March 2017 • Taiwanese Intellectual Property Office issued Taiwanese Patent No. I590820 in July 2017 • 21 patent applications pending (1 allowed (Australia)) Method of use for active ingredient cyclobenzaprine : Protection expected to 2030 • USPTO issued U.S. Patent 9,918,948 in March 2018 • European Patent Office issued European Patent No. 2 501 234B1 in September 2017 (validated in 38 countries). Opposition filed in June 2018 • 2 patent applications pending

© 2018 Tonix Pharmaceuticals Holding Corp. 9 Tonmya® 1 - Breakthrough Therapy for Treatment of PTSD Targets Sleep Disturbance Tonmya - first investigational new drug to show treatment effect in military - related PTSD in two large, 12 - week, multi - center studies Retrospective analyses showed Tonmya reduced PTSD severity in participants who experienced index traumas during military service in 2001 or later • The severity of PTSD symptoms were measured by the CAPS - 5 2 assessment scale, the endpoint required by FDA for marketing approval Breakthrough Therapy Designation from FDA in December 2016 Collaboration with Army: Tonix - USAMMDA CRADA signed 2015 1 Tonmya has been conditionally accepted by the U.S. FDA as the proposed trade name for TNX - 102 SL (cyclobenzaprine HCl sublingual tablets) for the treatment of PTSD. TNX - 102 SL is an investigational new drug and has not been approved for any indication. 2 Clinician Administered PTSD Scale for DSM - 5

© 2018 Tonix Pharmaceuticals Holding Corp. 10 Unmet Need for Effective and Safe Therapies for Treatment of Military PTSD PTSD is signature wound of last 25 years of war • Affects servicemember health and performance, force readiness, retention • Believed to be the underlying cause of suicide in many cases No FDA - approved products for PTSD since Pfizer’s Zoloft® (sertraline) and GSK’s Paxil® (paroxetine) circa 2000 • Neither has shown efficacy in military - related PTSD • Male PTSD patients often unresponsive or intolerant of current treatments • Side effects relating to sexual dysfunction, sleep and weight gain are commonly reported U.S. Department of Defense (DoD) is working to understand and treat PTSD • Increased scrutiny of PTSD - related discharges for behavioral problems • Wider recognition that PTSD is a service - related disability
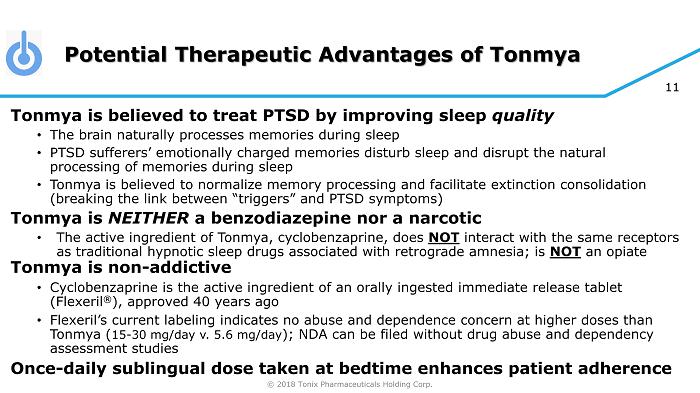
© 2018 Tonix Pharmaceuticals Holding Corp. 11 Potential Therapeutic Advantages of Tonmya Tonmya is believed to treat PTSD by improving sleep quality • The brain naturally processes memories during sleep • PTSD sufferers’ emotionally charged memories disturb sleep and disrupt the natural processing of memories during sleep • Tonmya is believed to normalize memory processing and facilitate extinction consolidation (breaking the link between “triggers” and PTSD symptoms) Tonmya is NEITHER a benzodiazepine nor a narcotic • The active ingredient of Tonmya , cyclobenzaprine, does NOT interact with the same receptors as traditional hypnotic sleep drugs associated with retrograde amnesia; is NOT an opiate Tonmya is non - addictive • Cyclobenzaprine is the active ingredient of an orally ingested immediate release tablet (Flexeril ® ), approved 40 years ago • Flexeril’s current labeling indicates no abuse and dependence concern at higher doses than Tonmya ( 15 - 30 mg/day v. 5.6 mg/day ); NDA can be filed without drug abuse and dependency assessment studies Once - daily sublingual dose taken at bedtime enhances patient adherence
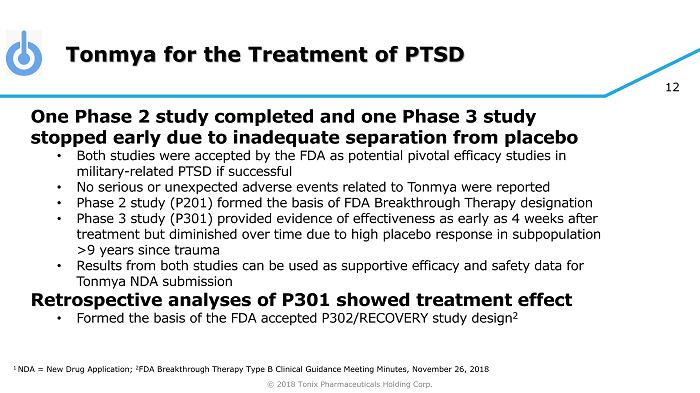
© 2018 Tonix Pharmaceuticals Holding Corp. 12 Tonmya for the Treatment of PTSD One Phase 2 study completed and one Phase 3 study stopped early due to inadequate separation from placebo • Both studies were accepted by the FDA as potential pivotal efficacy studies in military - related PTSD if successful • No serious or unexpected adverse events related to Tonmya were reported • Phase 2 study (P201) formed the basis of FDA Breakthrough Therapy designation • Phase 3 study (P301) provided evidence of effectiveness as early as 4 weeks after treatment but diminished over time due to high placebo response in subpopulation >9 years since trauma • Results from both studies can be used as supportive efficacy and safety data for Tonmya NDA submission Retrospective analyses of P301 showed treatment effect • Formed the basis of the FDA accepted P302/RECOVERY study design 2 1 NDA = New Drug Application; 2 FDA Breakthrough Therapy Type B Clinical Guidance Meeting Minutes, November 26, 2018
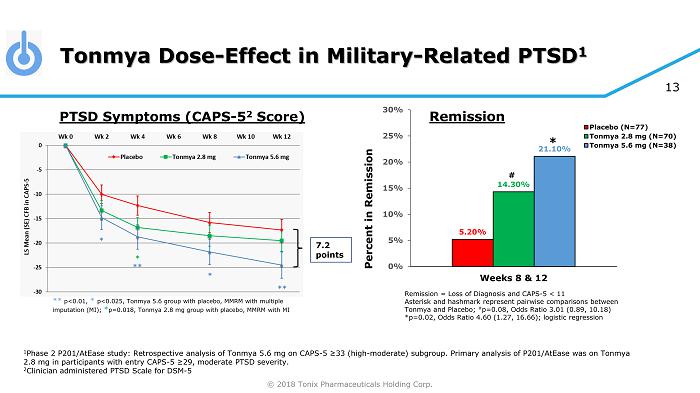
© 2018 Tonix Pharmaceuticals Holding Corp. 13 Tonmya Dose - Effect in Military - Related PTSD 1 1 Phase 2 P201/AtEase study: Retrospective analysis of Tonmya 5.6 mg on CAPS - 5 ≥33 (high - moderate) subgroup. Primary analysis of P 201/AtEase was on Tonmya 2.8 mg in participants with entry CAPS - 5 ≥29 , moderate PTSD severity. 2 Clinician administered PTSD Scale for DSM - 5 7.2 points ** p<0.01, * p<0.025, Tonmya 5.6 group with placebo, MMRM with multiple imputation (MI); * p=0.018, Tonmya 2.8 mg group with placebo, MMRM with MI PTSD Symptoms (CAPS - 5 2 Score) Remission = Loss of Diagnosis and CAPS - 5 < 11 Asterisk and hashmark represent pairwise comparisons between Tonmya and Placebo; # p=0.08, Odds Ratio 3.01 (0.89, 10.18) *p=0.02, Odds Ratio 4.60 (1.27, 16.66); logistic regression 5.20% 14.30% 21.10% 0% 5% 10% 15% 20% 25% 30% Weeks 8 & 12 Percent in Remission Placebo (N=77) Tonmya 2.8 mg (N=70) Tonmya 5.6 mg (N=38) # * Remission
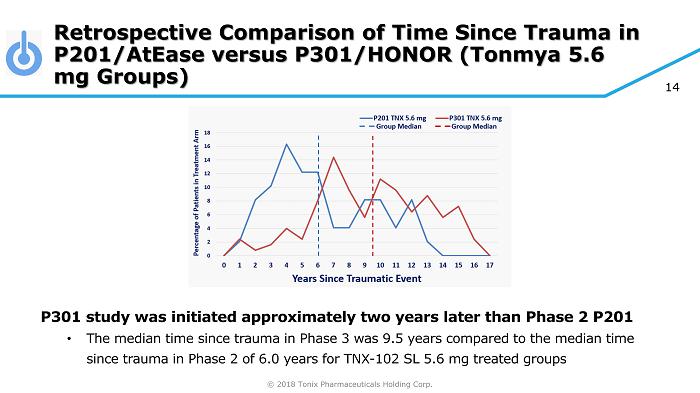
© 2018 Tonix Pharmaceuticals Holding Corp. 14 Retrospective Comparison of Time Since Trauma in P201/AtEase versus P301/HONOR (Tonmya 5.6 mg Groups) P301 study was initiated approximately two years later than Phase 2 P201 • The median time since trauma in Phase 3 was 9.5 years compared to the median time since trauma in Phase 2 of 6.0 years for TNX - 102 SL 5.6 mg treated groups
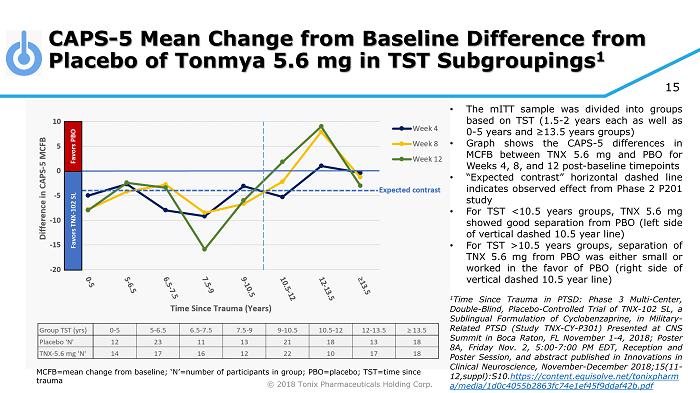
© 2018 Tonix Pharmaceuticals Holding Corp. 15 CAPS - 5 Mean Change from Baseline Difference from Placebo of Tonmya 5.6 mg in TST Subgroupings 1 MCFB=mean change from baseline; ‘N’=number of participants in group; PBO=placebo; TST=time since trauma • The mITT sample was divided into groups based on TST ( 1 . 5 - 2 years each as well as 0 - 5 years and ≥ 13 . 5 years groups) • Graph shows the CAPS - 5 differences in MCFB between TNX 5 . 6 mg and PBO for Weeks 4 , 8 , and 12 post - baseline timepoints • “Expected contrast” horizontal dashed line indicates observed effect from Phase 2 P 201 study • For TST < 10 . 5 years groups, TNX 5 . 6 mg showed good separation from PBO (left side of vertical dashed 10 . 5 year line) • For TST > 10 . 5 years groups, separation of TNX 5 . 6 mg from PBO was either small or worked in the favor of PBO (right side of vertical dashed 10 . 5 year line) 1 Time Since Trauma in PTSD : Phase 3 Multi - Center, Double - Blind, Placebo - Controlled Trial of TNX - 102 SL, a Sublingual Formulation of Cyclobenzaprine, in Military - Related PTSD (Study TNX - CY - P 301 ) Presented at CNS Summit in Boca Raton, FL November 1 - 4 , 2018 ; Poster 8 A, Friday Nov . 2 , 5 : 00 - 7 : 00 PM EDT, Reception and Poster Session, and abstract published in Innovations in Clinical Neuroscience, November - December 2018 ; 15 ( 11 - 12 ,suppl) : S 10 . https : //content . equisolve . net/ tonixpharm a /media/ 1 d 0 c 4055 b 2863 fc 74 e 1 ef 45 f 9 ddaf 42 b . pdf
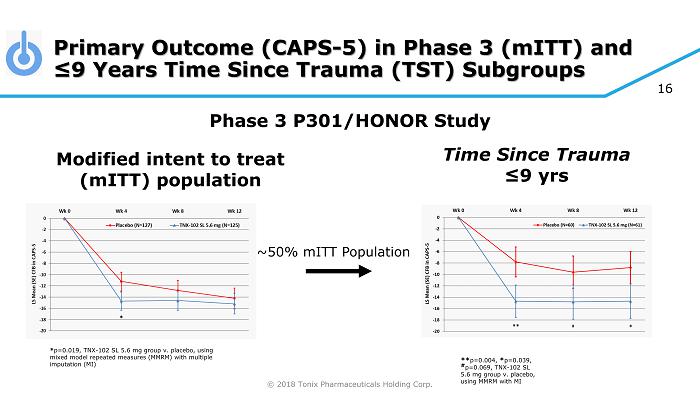
© 2018 Tonix Pharmaceuticals Holding Corp. Primary Outcome (CAPS - 5) in Phase 3 ( mITT ) and ≤9 Years Time Since Trauma (TST) Subgroups Modified intent to treat ( mITT ) population Phase 3 P301/HONOR Study ** p=0.004, * p=0.039, # p=0.069, TNX - 102 SL 5.6 mg group v. placebo, using MMRM with MI ~50% mITT Population 16 * p=0.019, TNX - 102 SL 5.6 mg group v. placebo, using mixed model repeated measures (MMRM) with multiple imputation (MI) Time Since Trauma ≤9 yrs
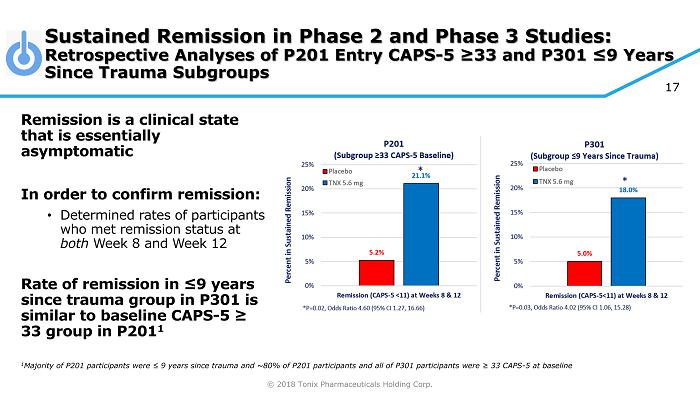
© 2018 Tonix Pharmaceuticals Holding Corp. 17 Sustained Remission in Phase 2 and Phase 3 Studies: Retrospective Analyses of P201 Entry CAPS - 5 ≥33 and P301 ≤9 Years Since Trauma Subgroups Remission is a clinical state that is essentially asymptomatic In order to confirm remission: • Determined rates of participants who met remission status at both Week 8 and Week 12 Rate of remission in ≤9 years since trauma group in P301 is similar to baseline CAPS - 5 ≥ 33 group in P201 1 1 Majority of P201 participants were ≤ 9 years since trauma and ~80% of P201 participants and all of P301 participants were ≥ 33 CAPS - 5 at baseline
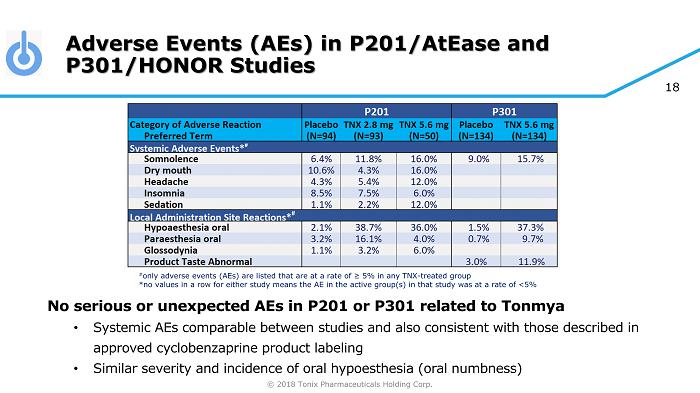
© 2018 Tonix Pharmaceuticals Holding Corp. 18 Adverse Events (AEs) in P201/AtEase and P301/HONOR Studies No serious or unexpected AEs in P201 or P301 related to Tonmya • Systemic AEs comparable between studies and also consistent with those described in approved cyclobenzaprine product labeling • Similar severity and incidence of oral hypoesthesia (oral numbness) # only adverse events (AEs) are listed that are at a rate of ≥ 5% in any TNX - treated group *no values in a row for either study means the AE in the active group(s) in that study was at a rate of <5%
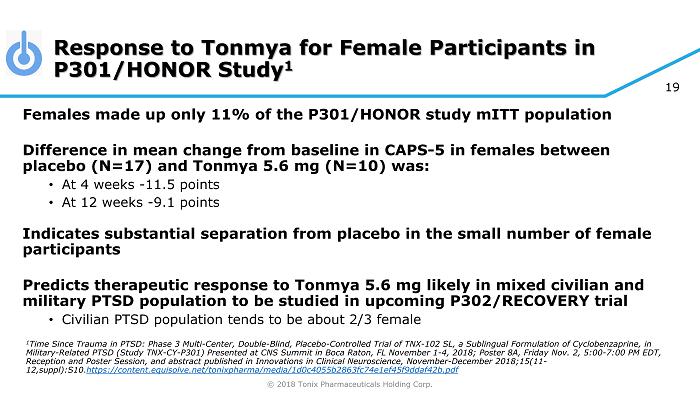
© 2018 Tonix Pharmaceuticals Holding Corp. 19 Response to Tonmya for Female Participants in P301/HONOR Study 1 Females made up only 11% of the P301/HONOR study mITT population Difference in mean change from baseline in CAPS - 5 in females between placebo (N=17) and Tonmya 5.6 mg (N=10) was: • At 4 weeks - 11.5 points • At 12 weeks - 9.1 points Indicates substantial separation from placebo in the small number of female participants Predicts therapeutic response to Tonmya 5.6 mg likely in mixed civilian and military PTSD population to be studied in upcoming P302/RECOVERY trial • Civilian PTSD population tends to be about 2/3 female 1 Time Since Trauma in PTSD: Phase 3 Multi - Center, Double - Blind, Placebo - Controlled Trial of TNX - 102 SL, a Sublingual Formulation of Cyclobenzaprine, in Military - Related PTSD (Study TNX - CY - P301) Presented at CNS Summit in Boca Raton, FL November 1 - 4, 2018; Poster 8A, Friday Nov. 2 , 5:00 - 7:00 PM EDT, Reception and Poster Session, and abstract published in Innovations in Clinical Neuroscience, November - December 2018;15(11 - 12,suppl):S10 . https://content.equisolve.net/ tonixpharma /media/1d0c4055b2863fc74e1ef45f9ddaf42b.pdf
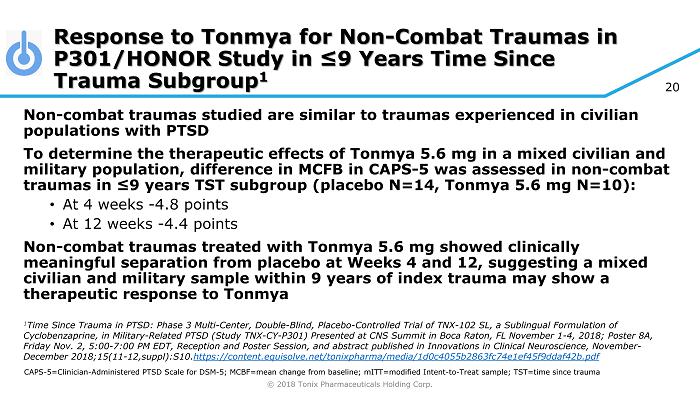
© 2018 Tonix Pharmaceuticals Holding Corp. 20 Non - combat traumas studied are similar to traumas experienced in civilian populations with PTSD To determine the therapeutic effects of Tonmya 5.6 mg in a mixed civilian and military population, difference in MCFB in CAPS - 5 was assessed in non - combat traumas in ≤9 years TST subgroup (placebo N=14, Tonmya 5.6 mg N=10): • At 4 weeks - 4.8 points • At 12 weeks - 4.4 points Non - combat traumas treated with Tonmya 5.6 mg showed clinically meaningful separation from placebo at Weeks 4 and 12, suggesting a mixed civilian and military sample within 9 years of index trauma may show a therapeutic response to Tonmya 1 Time Since Trauma in PTSD: Phase 3 Multi - Center, Double - Blind, Placebo - Controlled Trial of TNX - 102 SL, a Sublingual Formulation of Cyclobenzaprine, in Military - Related PTSD (Study TNX - CY - P301) Presented at CNS Summit in Boca Raton, FL November 1 - 4, 2018; Post er 8A, Friday Nov. 2, 5:00 - 7:00 PM EDT, Reception and Poster Session, and abstract published in Innovations in Clinical Neuroscience, N ovember - December 2018;15(11 - 12,suppl):S10. https://content.equisolve.net/ tonixpharma /media/1d0c4055b2863fc74e1ef45f9ddaf42b.pdf Response to Tonmya for Non - Combat Traumas in P301/HONOR Study in ≤9 Years Time Since Trauma Subgroup 1 CAPS - 5=Clinician - Administered PTSD Scale for DSM - 5; MCBF=mean change from baseline; mITT =modified Intent - to - Treat sample; TST=time since trauma
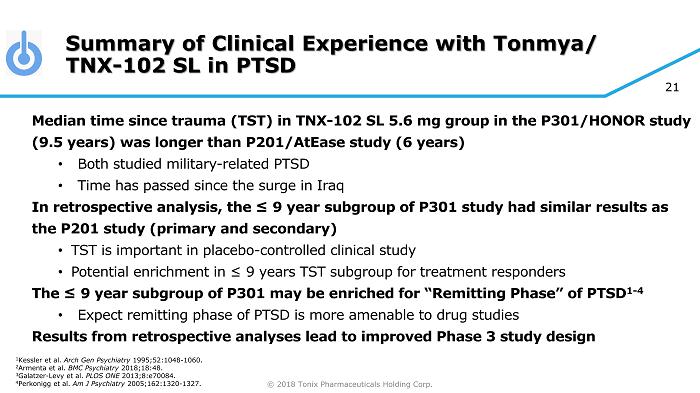
© 2018 Tonix Pharmaceuticals Holding Corp. 21 Summary of Clinical Experience with Tonmya/ TNX - 102 SL in PTSD Median time since trauma (TST) in TNX - 102 SL 5.6 mg group in the P301/HONOR study (9.5 years) was longer than P201/AtEase study (6 years) • Both studied military - related PTSD • Time has passed since the surge in Iraq In retrospective analysis, the ≤ 9 year subgroup of P301 study had similar results as the P201 study (primary and secondary) • TST is important in placebo - controlled clinical study • Potential enrichment in ≤ 9 years TST subgroup for treatment responders The ≤ 9 year subgroup of P301 may be enriched for “Remitting Phase” of PTSD 1 - 4 • Expect remitting phase of PTSD is more amenable to drug studies Results from retrospective analyses lead to improved Phase 3 study design 1 Kessler et al. Arch Gen Psychiatry 1995;52:1048 - 1060. 2 Armenta et al. BMC Psychiatry 2018;18:48. 3 Galatzer - Levy et al. PLOS ONE 2013;8:e70084. 4 Perkonigg et al. Am J Psychiatry 2005;162:1320 - 1327.

© 2018 Tonix Pharmaceuticals Holding Corp. 22 Tonmya/TNX - 102 SL – New Phase 3 Study (P302/RECOVERY) Features In two prior clinical trials, Tonmya 5.6 mg consistently reduced military - related PTSD severity for participants with ≤ 9 years since trauma These data provide the rationale to study Tonmya’s effect in participants with ≤ 9 years since trauma in P302/RECOVERY study Additional design features include: • Civilian and military - related PTSD will be studied (shortening recruitment time) • Primary endpoint: CAPS - 5 at Week 4 (minimizing drop - out) • 12 - week treatment period (providing CAPS - 5 at Week 12 a key secondary endpoint) Targeting study initiation: 1Q 2019
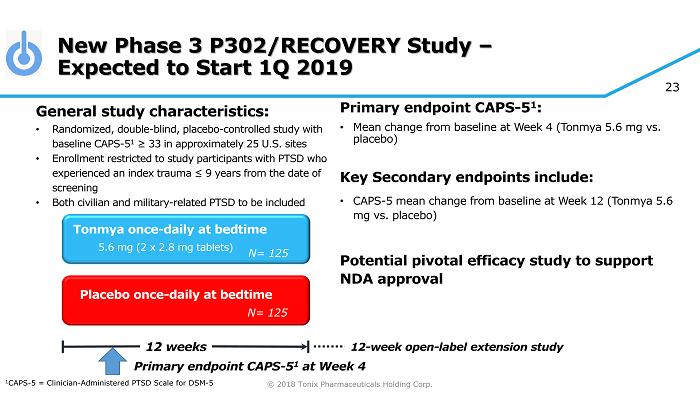
© 2018 Tonix Pharmaceuticals Holding Corp. 23 New Phase 3 P302/RECOVERY Study – Expected to Start 1Q 2019 Primary e ndpoint CAPS - 5 1 : • Mean change from baseline at W eek 4 (Tonmya 5.6 mg vs. placebo ) Key Secondary e ndpoint s include: • CAPS - 5 m ean change from baseline at W eek 12 (Tonmya 5.6 mg vs. placebo ) Potential pivotal efficacy study to support NDA approval Placebo once - daily at bedtime 12 weeks Tonmya once - daily at bedtime 5.6 mg (2 x 2.8 mg tablets) General s tudy c haracteristics: • Randomized, double - blind, placebo - controlle d study with baseline CAPS - 5 1 ≥ 33 in approximately 25 U.S. sites • Enrollment restricted to study participants with PTSD who experienced an index trauma ≤ 9 years from the date of screening • Both civilian and military - related PTSD to be included 12 - week open - label extension study 1 CAPS - 5 = Clinician - Administered PTSD Scale for DSM - 5 Primary e ndpoint CAPS - 5 1 at Week 4 N= 125 N= 125

Late-Stage PTSD Drug Candidates Tonmya • Breakthrough Therapy in Phase 3; development program focused on military-related and civilian PTSD; showed activity in treatment of military-related PTSD in large multi-center trials MDMA-assisted psychotherapy • Breakthrough therapy that is Phase 3-ready; showed activity in a Phase 2 study of PTSD Other drugs currently (or recently) in Phase 2 development • Rexulti® (brexpiprazole) - Otsuka/Lundbeck; atypical antipsychotic; positive clinical results from Phase 2 study reported in November 2018 for brexpiprazole, when used in combination with an approved PTSD medication, sertraline, but not as monotherapy • BNC-201 – Bionomics; nicotinic receptor modulator (program stopped after Phase 2) © 2018 Tonix Pharmaceuticals Holding Corp.
© 2018 Tonix Pharmaceuticals Holding Corp. 25 TNX - 102 SL – Bedtime Treatment for Multiple Potential Indications Ma nagement of Fibromyalgia (FM) – chronic pain condition • TNX - 102 SL studied at low dose (2.8 mg) – half the dose being developed for PTSD – did not separate from placebo on primary endpoint, average pain improvement (responder analysis) • Retrospective analysis showed average pain improvement (secondary endpoint) after 12 weeks of treatment showed statistical significance (P< 0.05, MMRM) • Low dose TNX - 102 SL (2.8 mg) showed an improvement in sleep quality in Phase 2 and Pha se 3 FM trials • Efficacy of TNX - 102 SL 5.6 mg in FM can be studied in a potential pivotal study to support product registration Agitation in Alzheimer’s Disease • Fast Track designation granted July 2018 • Phase 2/ potential pivotal efficacy study protocol received FDA comments in October 2018

© 2018 Tonix Pharmaceuticals Holding Corp. 26 TNX - 102 SL for Agitation in Alzheimer’s – Regulatory Status and Registration Strategy FDA confirmed no additional study is needed prior to IND submission • Pre - IND meeting established open dialogue with the FDA on pivotal clinical study design and efficacy endpoints to support product registration Proposed Phase 2 IND study can potentially serve as a pivotal efficacy study to support NDA approval • FDA comments on final protocol received October 2018 Registration Strategy of TNX - 102 SL for agitation in Alzheimer’s disease • Efficacy Supplement (sNDA 1 ) may be leveraged from the PTSD/FM development program and supported by Initial NDA approval for PTSD/FM 1 Supplemental New Drug Application
© 2018 Tonix Pharmaceuticals Holding Corp. 27 TNX - 601 ( Tianeptine Oxalate): A Potential Clinical Candidate for PTSD Pre - IND Candidate Targeted as a 1 st line monotherapy for PTSD: oral formulation for daytime dosing x Leverages expertise in PTSD (clinical and regulatory experience, market analysis, etc.) x Mechanism of Action (MOA) is different from TNX - 102 SL • Tianeptine sodium (amorphous) has been approved in EU, Russia, Asia and Latin America for depression since 1987 with established post - marketing experience • Identified new oxalate salt polymorph with improved pharmaceutical properties ideal for reformulation • Preliminary human pharmacokinetic and safety data (non - IND study) from selected formulation expected in second half 2019 Filed patent application on novel salt polymorph • Issued patent on steroid - induced cognitive impairment and memory loss issues Targeting a Condition with Significant Unmet Need Clinical evidence for PTSD • Several studies have shown tianeptine to be active in the treatment of PTSD 1 - 4 1 Frančišković T, et al. Psychiatr Danub . 2011 Sep;23(3):257 - 63. PMID: 21963693 2 Rumyantseva GM and, Stepanov AL. Neurosci Behav Physiol. 2008 Jan;38(1):55 - 61. PMID: 18097761 3 Aleksandrovskiĭ IA, et al. Zh Nevrol Psikhiatr Im S S Korsakova . 2005;105(11):24 - 9. PMID: 16329631 [Russian] 4 Onder E, et al. Eur Psychiatry. 2006 (3):174 - 9. PMID: 15964747

© 2018 Tonix Pharmaceuticals Holding Corp. 28 TNX - 801 (Synthesized Live Horsepox Virus): A Smallpox - Preventing Vaccine Candidate Pre - IND Stage Potential improvement over current biodefense tools against smallpox ✓ Leverages Tonix’s government affairs effort ✓ Collaboration with Professor David Evans and Dr. Ryan Noyce at University of Alberta ✓ Demonstrated protective vaccine activity in mice ✓ Patent application on novel vaccine submitted Regulatory strategy • We intend to meet with FDA to discuss the most efficient and appropriate investigational plan to support the licensure, either: ✓ Application of the “Animal Rule”, or ✓ Conducting an active comparator study using ACAM2000 • Good Manufacturing Practice (GMP) viral production process in development Targeting a Potential Public Health Issue Material threat medical countermeasure under 21 st Century Cures Act • Qualifies for Priority Review Voucher (PRV) upon licensure * ✓ PRVs have no expiration date, are transferrable and have sold for ~$125 M *BLA/NDA priority 6 - month review is expected.
© 2018 Tonix Pharmaceuticals Holding Corp. 29 Milestones – Recently Completed and Upcoming □ July 201 8 Completed P301/HONOR study interim analysis - result did not support study continuation but strengthened new Phase 3 study □ August 201 8 Presentation of P301/HONOR study results at Military Health System Scientific Symposium □ October 201 8 Met with FDA and received preliminary agreement on the design of new Phase 3 study of Tonmya for PTSD (P302/RECOVERY study) □ November 201 8 Received FDA minutes confirming agreement on the design of P302/RECOVERY study □ First Quarter 2019 P302/RECOVERY study to be initiated □ Second Half 2019 P reliminary human pharmacokinetic and safety data (non - IND study) from selected TNX - 601 (tianeptine oxalate) formulation expected □ First Half 2020 Topline data from P302/RECOVERY study expected x x x x
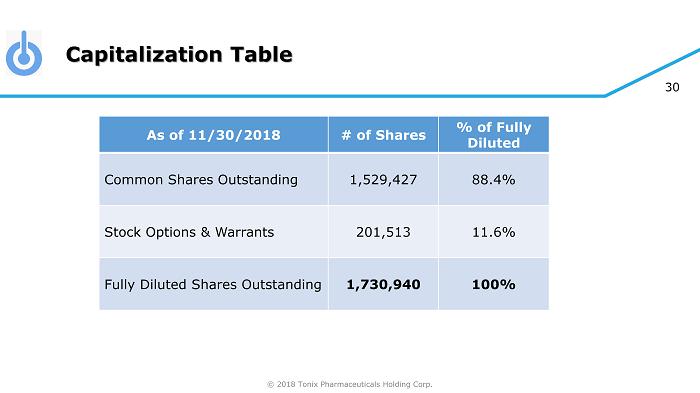
© 2018 Tonix Pharmaceuticals Holding Corp. 30 Capitalization Table As of 11/30/2018 # of Shares % of Fully Diluted Common Shares Outstanding 1,529,427 88.4% Stock Options & Warrants 201,513 11.6% Fully Diluted Shares Outstanding 1,730,940 100%
© 2018 Tonix Pharmaceuticals Holding Corp. 31 Summary Phase 3 development of Breakthrough Therapy treatment for PTSD, including military - related PTSD • Major unmet need; ~11 million Americans affected • Benefited from FDA 505(b)(2) NDA approval requirement New indication in development for agitation in Alzheimer’s Disease • Unmet medical need, no approved drug available • Fast Track Phase 2/3 ready program Complimentary day - time PTSD treatment in development • Leverages development expertise in PTSD, i.e., regulatory, trial recruitment and execution Innovative vaccine in development to prevent Smallpox • Opportunity to supply stockpiling requirement; short development path • Studies in mice suggest improved safety profile

© 2018 Tonix Pharmaceuticals Holding Corp. Thank you ! NASDAQ: TNXP