
Tonix Pharmaceuticals Holding Corp. 8-K
Exhibit 99.03

© 2023 Tonix Pharmaceuticals Holding Corp. TNX - 1900 Chronic Migraine NASDAQ: TNXP Version P0500 November 13, 2023 (Doc 1341)

2 © 2022 Tonix Pharmaceuticals Holding Corp. Cautionary Note on Forward - Looking Statements Certain statements in this presentation regarding strategic plans, expectations and objectives for future operations or results are “forward - looking statements” as defined by the Private Securities Litigation Reform Act of 1995. These statements may be identified by the use of forward - looking words such as “anticipate,” “believe,” “forecast,” “estimate” and “intend,” among others. These forward - looking statements are based on Tonix’s current expectations and actual results could differ materially. There are a number of factors that could cause actual events to differ materially from those indicated by such forward - looking statements. These factors include, but are not limited to, the risks related to failure to obtain FDA clearances or approvals and noncompliance with FDA regulations; delays and uncertainties caused by the global COVID - 19 pandemic; risks related to the timing and progress of clinical development of our product candidates; our need for additional financing; uncertainties of patent protection and litigation; uncertainties of government or third party payor reimbursement; limited research and development efforts and dependence upon third parties; and substantial competition. As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products. The forward - looking statements in this presentation are made as of the date of this presentation, even if subsequently made available by Tonix on its website or otherwise. Tonix does not undertake an obligation to update or revise any forward - looking statement, except as required by law. Investors should read the risk factors set forth in the Annual Report on Form 10 - K for the year ended December 31 , 2022, as filed with the Securities and Exchange Commission (the “SEC”) on March 13, 2023, and periodic reports and current reports filed with the SEC on or after the date thereof. All of Tonix's forward - looking statements are expressly qualified by all such risk factors and other cautionary statements.
3 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 1900 for the Treatment of Migraine: Prevalence One billion individuals worldwide suffer from migraines (~14% of population) 1 Migraine is the second leading cause of years lived with disability 1 In U.S., the estimated cost of all migraine headaches was $78 billion in 2014 2 • Approximately 30% of those costs ($23 billion) were direct medical costs Chronic migraine (≥ 15 headaches / month ) effects about 1 - 2% of individuals 3 • 75 - 150 million individuals worldwide • 3 - 7 million in the U.S. CGRP antibodies are the only migraine specific prophylaxis drugs approved in decades • Requires parenteral administration (systemic effects on peripheral CGRP pathways) • Long term safety concerns with prolonged systemic blockade of CGRP receptor 4 1 GBD 2016 Headache Collaborators, Global, regional, and national burden of migraine and tension - type headache, 1990 – 2016: a syste matic analysis for the Global Burden of Disease Study 2016, Lancet Neurol 2018; 17: 954 – 76 2 Gooch, C. L., et al., The Burden of Neurological Disease in the United States: A Summary Report and Call to Action. Ann Neuro l. 2017; 81:479 - 484 3 Natoli et al., Global prevalence of chronic migraine: a systematic review, Cephalagia , 2010, 30:599 - 609 4 Robbins, At Stake: The Possible Long - Term Side Effects of CGRP Antagonists, https://www.practicalpainmanagement.com/pain/headache/stake - possible - long - term - side - effects - cgrp - antagonists , accessed November 8, 2020.
4 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 1900 for the Treatment of Migraine and Craniofacial Pain: Overview Novel intranasal oxytocin formulation being developed as a prophylactic treatment for chronic migraine • Based on a propriety formulation of oxytocin*, a naturally occurring human hormone that acts as a neurotransmitter in the brain Clinical and preliminary research has shown that low oxytocin levels in the body can lead to increase in headache frequency, and that increased oxytocin levels can relieve headaches • Certain other chronic pain conditions are also associated with decreased oxytocin levels Oxytocin when delivered via the nasal route, results in enhanced binding of oxytocin to receptors on neurons in the trigeminal system, inhibiting transmission of pain signals Intranasal oxytocin has been shown in animals that it can also block CGRP release, a pathway known to be critical to the pathogenesis of migraine attacks. * Oxytocin is approved by the U.S. Food and Drug Administration (FDA) as Pitocin ® , an intravenous infusion or intramuscular injection drug, for use in pregnant women to induce labor. An intranasal form of oxytocin was marketed by Novartis to assist in nursing as Syntocinon ® , but the product was withdrawn and the New Drug Application (NDA) has been discontinued.
5 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 1900 for the Treatment of Migraine: Mechanism of Action Preclinical research showed that nasally applied TNX - 1900 selectively inhibits the activity of trigeminal pain - sensing nerve cells and blocks the release of CGRP • TNX - 1900 is believed to interrupt pain signals at the trigeminal ganglia by suppressing electrical impulses, a potentially different activity than drugs that just block CGRP Migraine attacks are caused, in part, by the release of CGRP from pain - sensing nerve cells that are part of the trigeminal system • The CGRP binds to receptors on other nerve cells and starts a cascade of events that eventually results in a severe headache. This, in turn, reduces various kinds of trigeminal nerve associated pain and prevents CGRP from acting at receptors in the central nervous system that are involved in migraine. We believe targeted delivery of oxytocin could translate into selective blockade of CGRP release in the trigeminal ganglion and not throughout the body, which could be a potential safety advantage over systemic CGRP inhibition • In addition, daily dosing is more quickly reversible, in contrast to monthly or quarterly dosing, giving physicians and their patients greater control
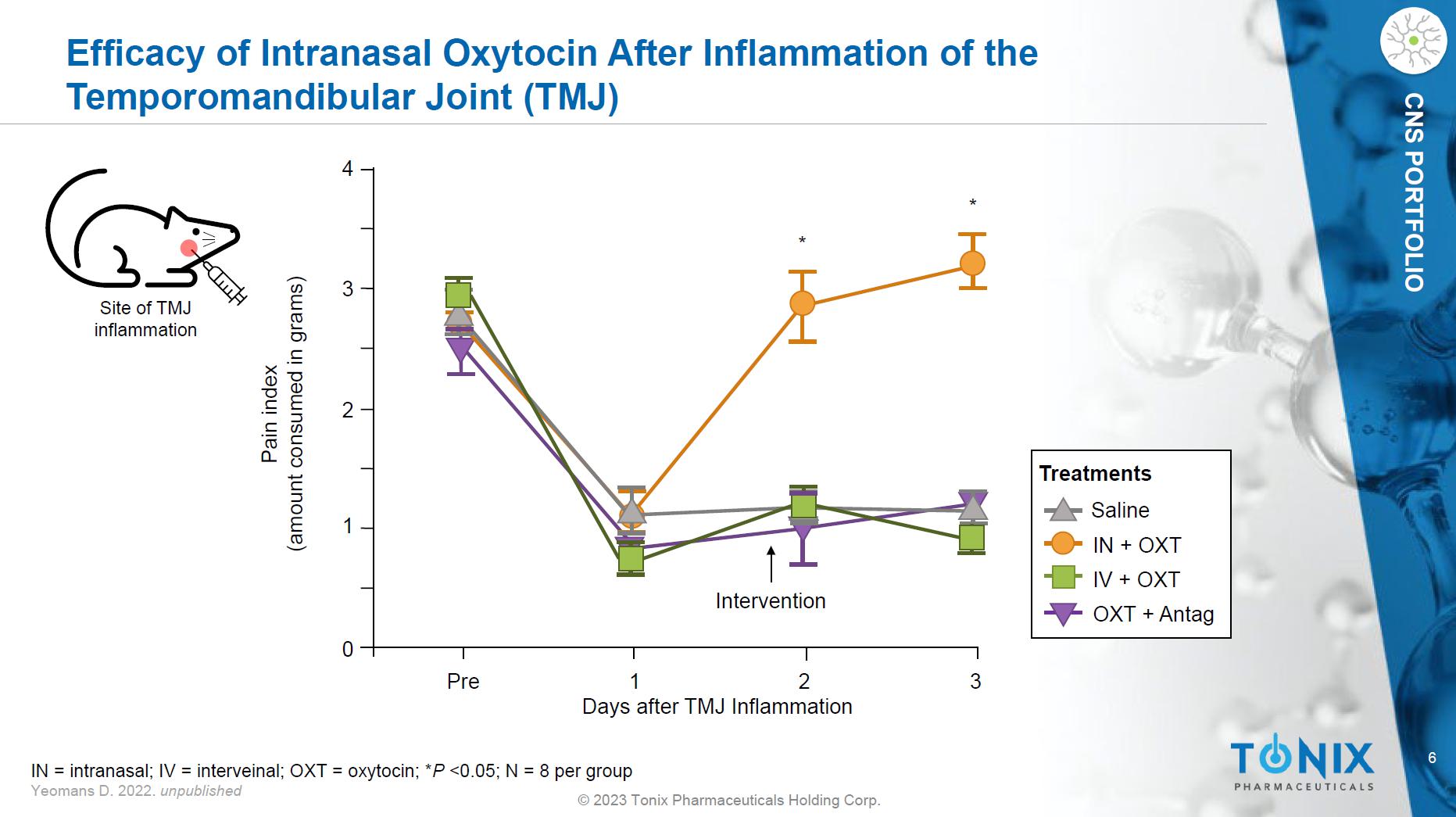
6 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Efficacy of Intranasal Oxytocin After Inflammation of the Temporomandibular Joint (TMJ) Pre 1 2 3 0 1 2 3 4 * * Intervention Days after TMJ Inflammation Pain index (amount consumed in grams) IN = intranasal; IV = interveinal; OXT = oxytocin; * P <0.05; N = 8 per group Yeomans D. 2022. unpublished Saline IN + OXT OXT + Antag IV + OXT Treatments Site of TMJ inflammation
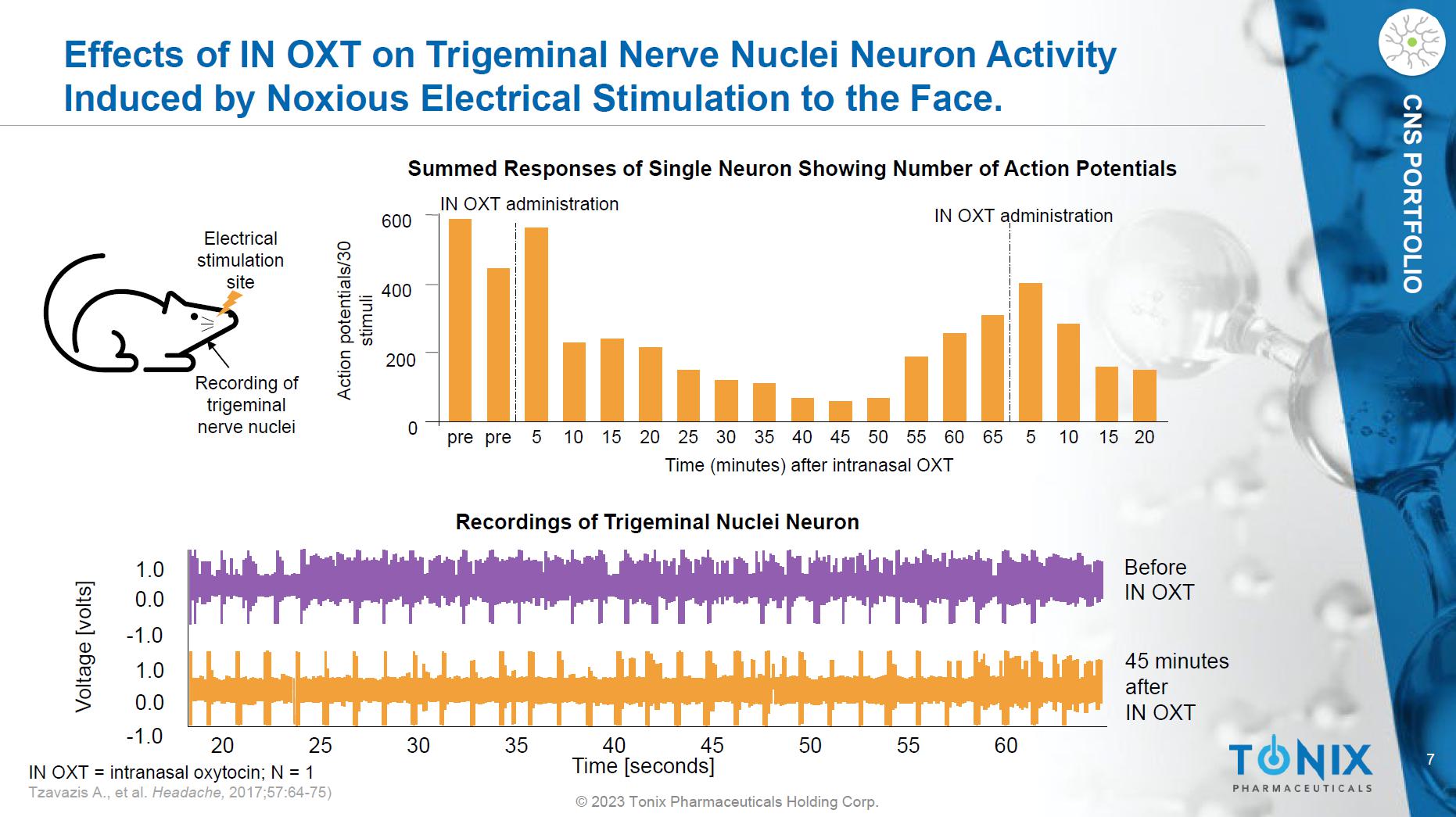
7 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Effects of IN OXT on Trigeminal Nerve Nuclei Neuron Activity Induced by Noxious Electrical Stimulation to the Face. 5 0 200 400 600 Action potentials/30 stimuli Time (minutes) after intranasal OXT pre pre 5 10 15 20 25 30 35 40 45 50 55 60 65 10 15 20 IN OXT administration IN OXT administration Electrical stimulation site Recording of trigeminal nerve nuclei 20 - 1.0 - 1.0 0.0 0.0 1.0 1.0 25 30 35 40 45 50 55 60 Before IN OXT 45 minutes after IN OXT Time [seconds] Voltage [volts] Summed Responses of Single Neuron Showing Number of Action Potentials Recordings of Trigeminal Nuclei Neuron IN OXT = intranasal oxytocin; N = 1 Tzavazis A., et al. Headache, 2017;57:64 - 75)
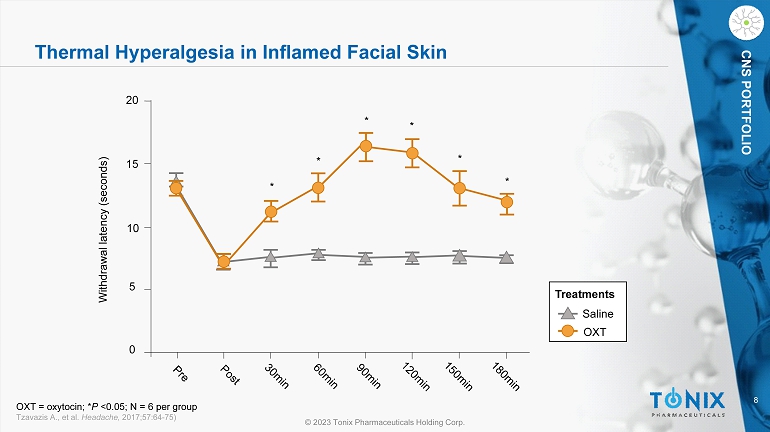
8 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Thermal Hyperalgesia in Inflamed Facial Skin OXT = oxytocin; * P <0.05; N = 6 per group Tzavazis A., et al. Headache, 2017;57:64 - 75) Saline OXT Treatments 0 5 10 15 20 Withdrawal latency (seconds) * * * * * *
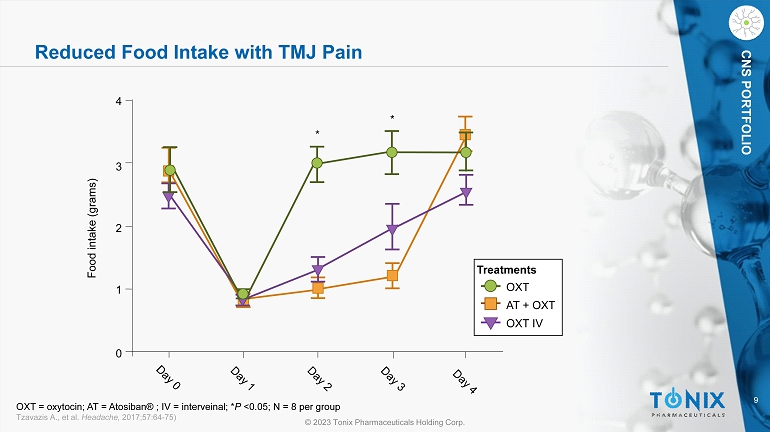
9 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Reduced Food Intake with TMJ Pain OXT OXT IV AT + OXT Treatments 0 1 2 3 4 Food intake (grams) * * OXT = oxytocin; AT = Atosiban ® ; IV = interveinal; * P <0.05; N = 8 per group Tzavazis A., et al. Headache, 2017;57:64 - 75)
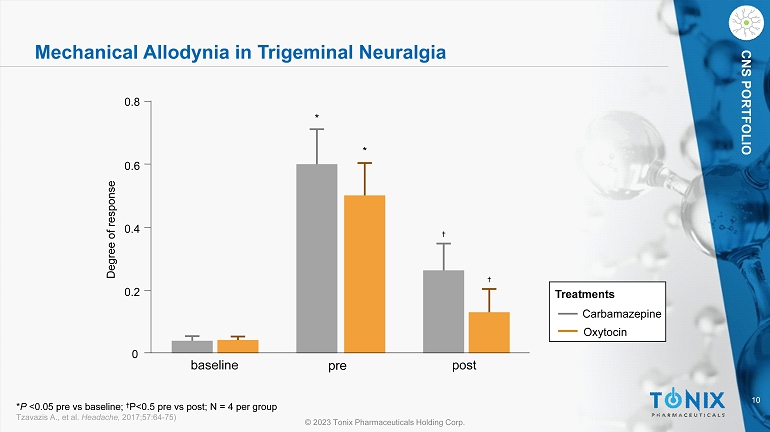
10 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Mechanical Allodynia in Trigeminal Neuralgia baseline pre 0 0.2 0.4 0.6 0.8 Degree of response post * * † † Carbamazepine Oxytocin Treatments * P <0.05 pre vs baseline; † P<0.5 pre vs post; N = 4 per group Tzavazis A., et al. Headache, 2017;57:64 - 75)
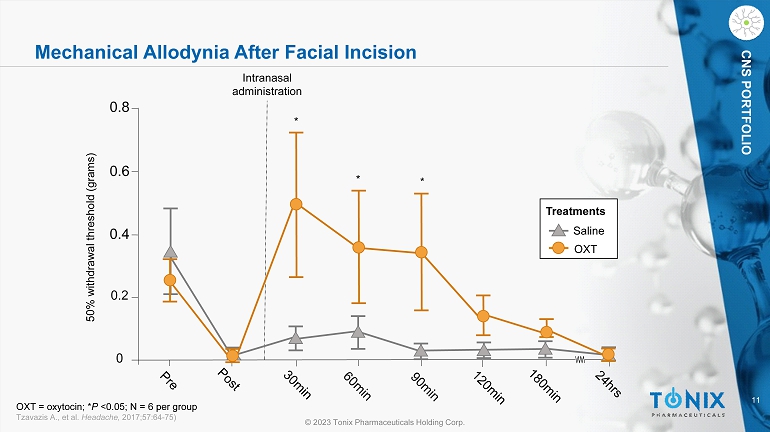
11 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Mechanical Allodynia After Facial Incision 0 0.2 0.4 0.6 0.8 50% withdrawal threshold (grams) Saline OXT Treatments Intranasal administration * * * OXT = oxytocin; * P <0.05; N = 6 per group Tzavazis A., et al. Headache, 2017;57:64 - 75)
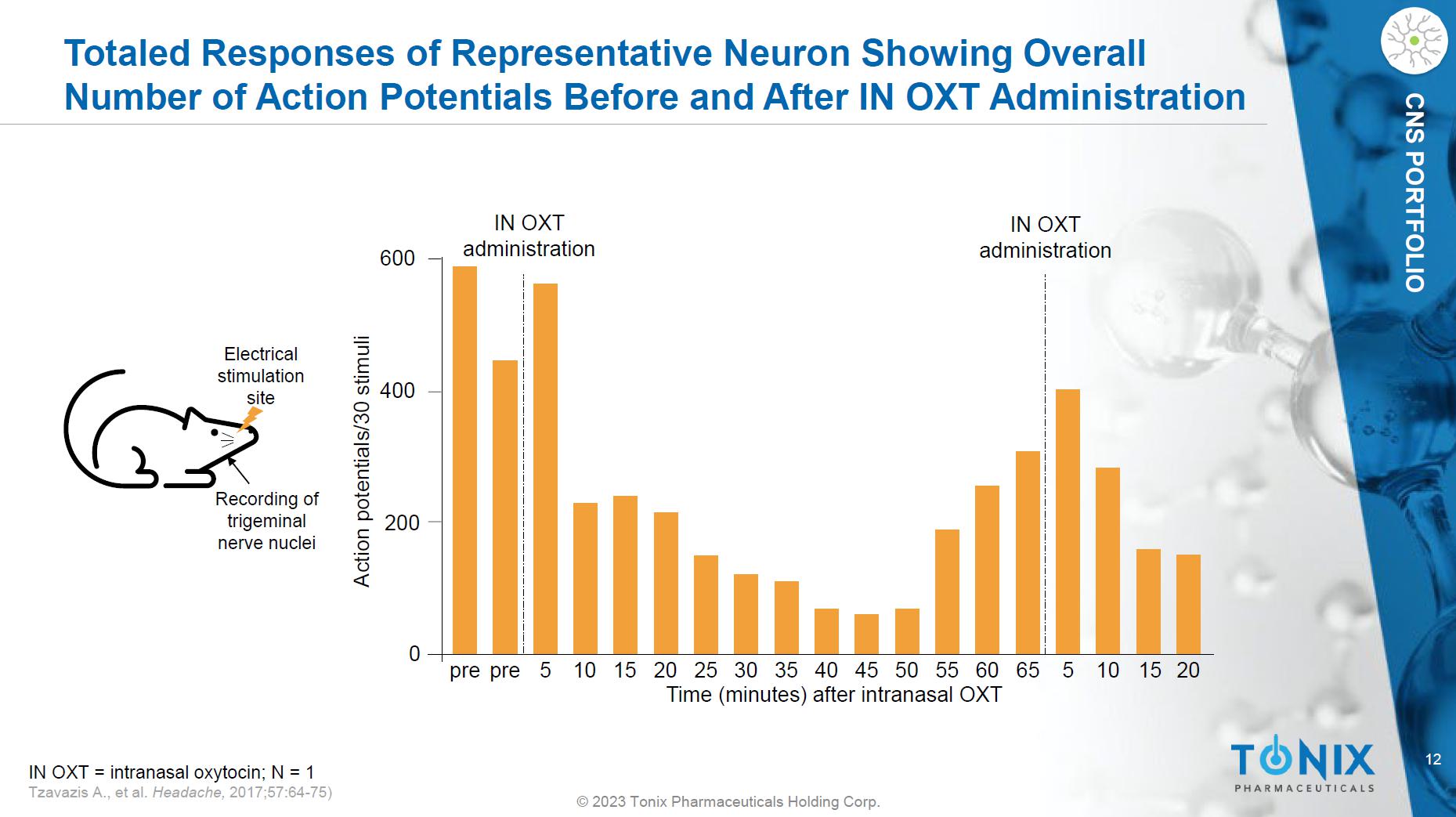
12 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Totaled Responses of Representative Neuron Showing Overall Number of Action Potentials Before and After IN OXT Administration 5 0 200 400 600 Action potentials/30 stimuli Time (minutes) after intranasal OXT pre pre 5 10 15 20 25 30 35 40 45 50 55 60 65 10 15 20 IN OXT administration IN OXT administration IN OXT = intranasal oxytocin; N = 1 Tzavazis A., et al. Headache, 2017;57:64 - 75) Electrical stimulation site Recording of trigeminal nerve nuclei
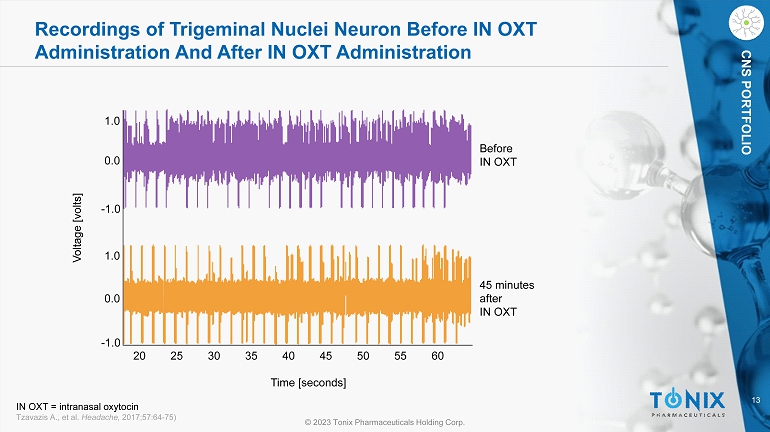
13 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Recordings of Trigeminal Nuclei Neuron Before IN OXT Administration And After IN OXT Administration 20 - 1.0 - 1.0 0.0 0.0 1.0 1.0 25 30 35 40 45 50 55 60 Before IN OXT 45 minutes after IN OXT Time [seconds] Voltage [volts] IN OXT = intranasal oxytocin Tzavazis A., et al. Headache, 2017;57:64 - 75)
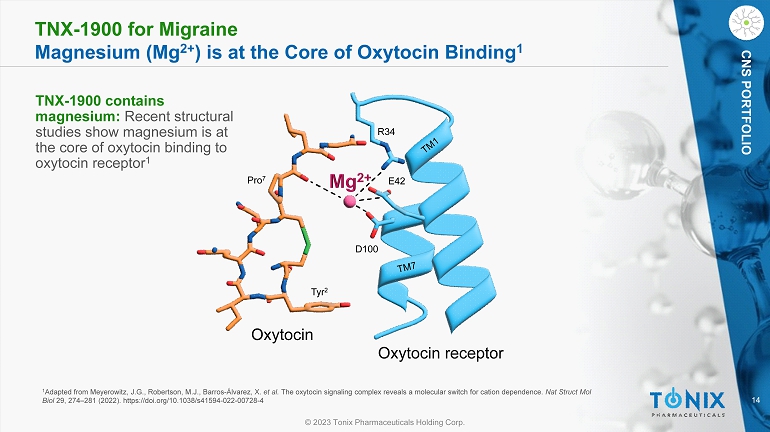
14 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 1900 for Migraine Magnesium (Mg 2+ ) is at the Core of Oxytocin Binding 1 1 Adapted from Meyerowitz, J.G., Robertson, M.J., Barros - Álvarez, X. et al. The oxytocin signaling complex reveals a molecular switch for cation dependence. Nat Struct Mol Biol 29, 274 – 281 (2022). https://doi.org/10.1038/s41594 - 022 - 00728 - 4 R34 E42 D100 Tyr 2 Pro 7 Oxytocin receptor Oxytocin TNX - 1900 contains magnesium: Recent structural studies show magnesium is at the core of oxytocin binding to oxytocin receptor 1
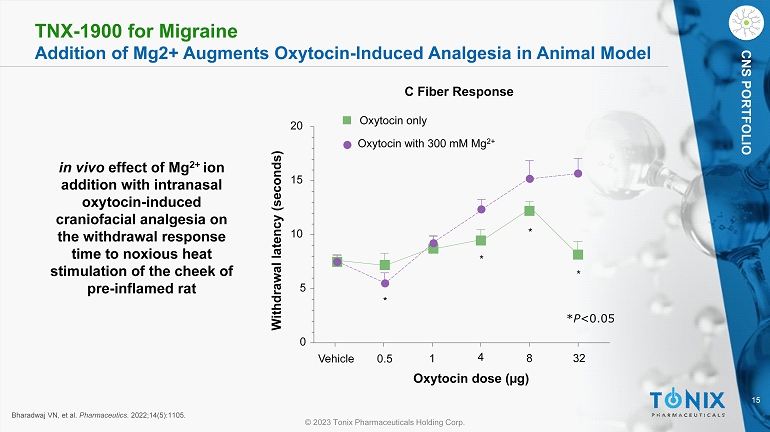
15 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Bharadwaj VN, et al. Pharmaceutics . 2022;14(5):1105. in vivo effect of Mg 2+ ion addition with intranasal oxytocin - induced craniofacial analgesia on the withdrawal response time to noxious heat stimulation of the cheek of pre - inflamed rat Vehicle 0.5 1 4 8 32 0 5 10 15 20 Oxytocin only Oxytocin with 300 mM Mg 2+ * * * * Oxytocin dose (µg) Withdrawal latency (seconds) C Fiber Response * P <0.05 TNX - 1900 for Migraine Addition of Mg2+ Augments Oxytocin - Induced Analgesia in Animal Model
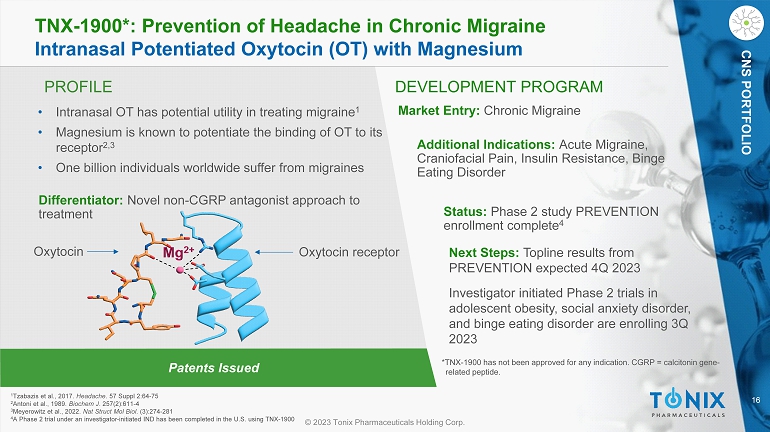
16 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO PROFILE DEVELOPMENT PROGRAM Patents Issued TNX - 1900*: Prevention of Headache in Chronic Migraine Intranasal Potentiated Oxytocin (OT) with Magnesium CNS PORTFOLIO • Intranasal OT has potential utility in treating migraine 1 • Magnesium is known to potentiate the binding of OT to its receptor 2,3 • One billion individuals worldwide suffer from migraines Differentiator: Novel non - CGRP antagonist approach to treatment Market Entry: Chronic Migraine Additional Indications: Acute Migraine, Craniofacial Pain, Insulin Resistance, Binge Eating Disorder Status: Phase 2 study PREVENTION enrollment complete 4 Next Steps: Topline results from PREVENTION expected 4Q 2023 Investigator initiated Phase 2 trials in adolescent obesity, social anxiety disorder, and binge eating disorder are enrolling 3Q 2023 1 Tzabazis et al., 2017. Headache . 57 Suppl 2:64 - 75 2 Antoni et al., 1989. Biochem J . 257(2):611 - 4 3 Meyerowitz et al., 2022. Nat Struct Mol Biol . (3):274 - 281 4 A Phase 2 trial under an investigator - initiated IND has been completed in the U.S. using TNX - 1900 *TNX - 1900 has not been approved for any indication. CGRP = calcitonin gene - related peptide. Oxytocin receptor Oxytocin
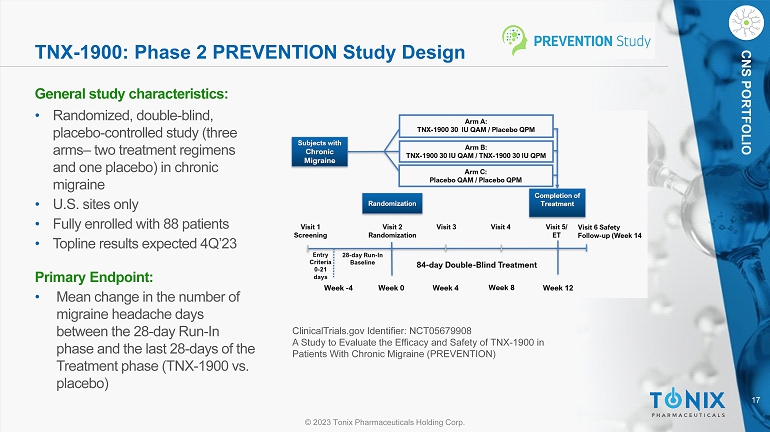
17 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 1900: Phase 2 PREVENTION Study Design General s tudy c haracteristics: • Randomized, double - blind, placebo - controlle d study (three arms – two treatment regimens and one placebo) in chronic migraine • U.S. sites only • Fully enrolled with 88 patients • Topline results expected 4Q’23 Primary Endpoint: • M ean change in the number of migraine headache days between the 28 - day Run - In phase and the last 28 - days of the Treatment phase (TNX - 1900 vs. placebo) ClinicalTrials.gov Identifier: NCT05679908 A Study to Evaluate the Efficacy and Safety of TNX - 1900 in Patients With Chronic Migraine (PREVENTION) N = 50 N = 50 N = 50
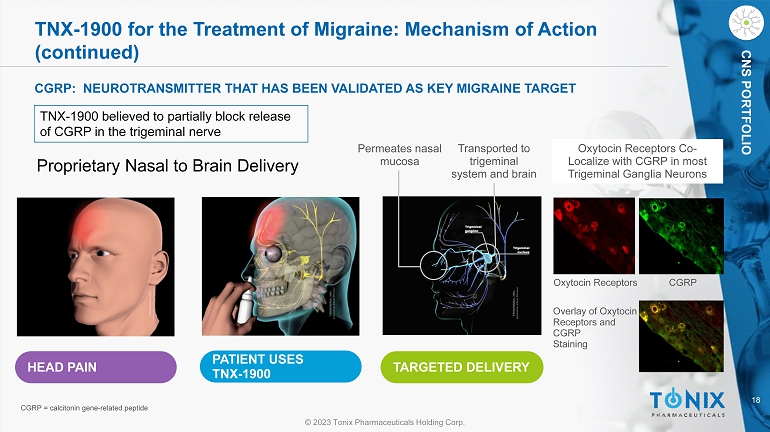
18 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 1900 for the Treatment of Migraine: Mechanism of Action (continued) HEAD PAIN PATIENT USES TNX - 1900 TARGETED DELIVERY Permeates nasal mucosa Transported to trigeminal system and brain Proprietary Nasal to Brain Delivery CGRP: NEUROTRANSMITTER THAT HAS BEEN VALIDATED AS KEY MIGRAINE TARGET TNX - 1900 believed to partially block release of CGRP in the trigeminal nerve Oxytocin Receptors CGRP Oxytocin Receptors Co - Localize with CGRP in most Trigeminal Ganglia Neurons Overlay of Oxytocin Receptors and CGRP Staining CGRP = calcitonin gene - related peptide
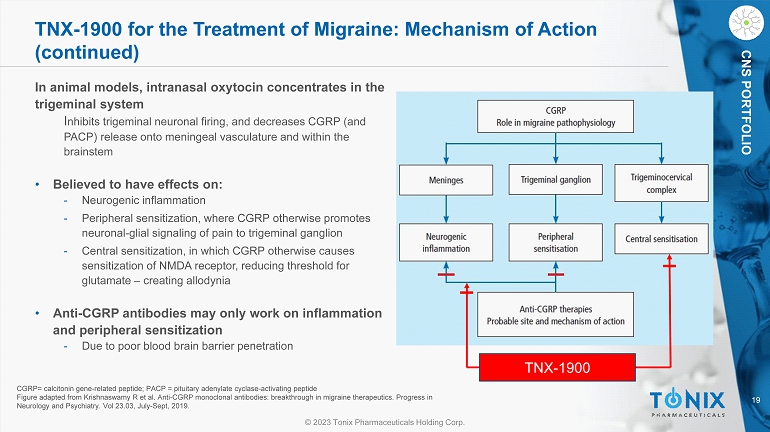
19 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO In animal models, intranasal oxytocin concentrates in the trigeminal system I nhibits trigeminal neuronal firing, and decreases CGRP (and PACP) release onto meningeal vasculature and within the brainstem • Believed to have effects on: - Neurogenic inflammation - Peripheral sensitization, where CGRP otherwise promotes neuronal - glial signaling of pain to trigeminal ganglion - Central sensitization, in which CGRP otherwise causes sensitization of NMDA receptor, reducing threshold for glutamate – creating allodynia • Anti - CGRP antibodies may only work on inflammation and peripheral sensitization - Due to poor blood brain barrier penetration CGRP= calcitonin gene - related peptide; PACP = pituitary adenylate cyclase - activating peptide Figure adapted from Krishnaswamy R et al. Anti - CGRP monoclonal antibodies: breakthrough in migraine therapeutics. Progress in Neurology and Psychiatry. Vol 23.03, July - Sept, 2019. TNX - 1900 TNX - 1900 for the Treatment of Migraine: Mechanism of Action (continued)
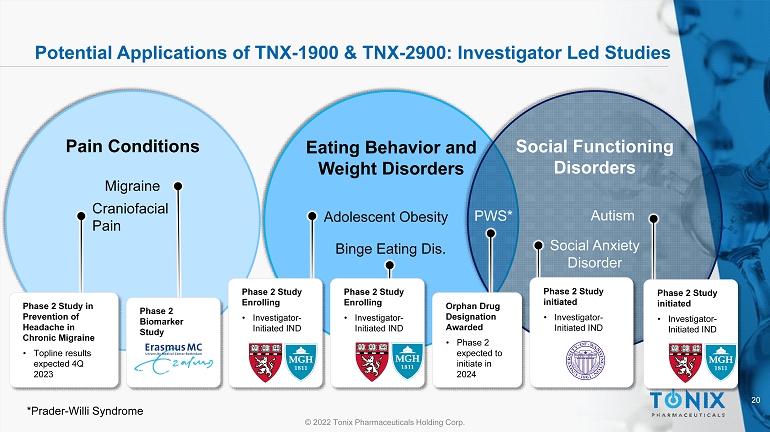
20 © 2022 Tonix Pharmaceuticals Holding Corp. Potential Applications of TNX - 1900 & TNX - 2900: Investigator Led Studies Adolescent Obesity Binge Eating Dis. Eating Behavior and Weight Disorders Autism Social Functioning Disorders Social Anxiety Disorder Migraine Craniofacial Pain Pain Conditions Phase 2 Study in Prevention of Headache in Chronic Migraine • Topline results expected 4Q 2023 Phase 2 Biomarker Study Phase 2 Study Enrolling • Investigator - Initiated IND Phase 2 Study Enrolling • Investigator - Initiated IND Orphan Drug Designation Awarded • Phase 2 expected to initiate in 2024 Phase 2 Study initiated • Investigator - Initiated IND PWS* *Prader - Willi Syndrome Phase 2 Study initiated • Investigator - Initiated IND

21 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 1900 – Other Studies in Collaboration with Academic Investigators Pharmacodynamic biomarker study related to headache 1 ‒ Testing TNX - 1900 effects on capsaicin - or electrical stimulation - induced forehead dermal blood flow in healthy female human volu nteers ‒ Forehead dermal blood flow is considered a trigeminovascular biomarker for antimigraine drugs. ▪ Both a CGRP inhibitor and a triptan have been successfully tested in the model and have been found to inhibit the forehead de rma l blood flow response to capsaicin in migraineurs and healthy volunteers, respectively. 2,3 ‒ Erasmus University Medical Center, Dr. Antoinette Maassen van den Brink, Principal Investigator ( P.I.) Pediatric Obesity 4 ‒ Phase 2 double - blind ‘POWER’ study testing TNX - 1900 as a novel therapeutic agent to induce weight loss and improve indicators of cardiometabolic risk in adolescent patients with obesity ‒ Massachusetts General Hospital (MGH), Dr. Elizabeth Lawson, P.I. Social Anxiety 5 ‒ Study effects of TNX - 1900 on social safety learning in social anxiety disorder (SAD) ‒ Univ. of Washington, Dr. Angela Fang, P.I. Binge Eating Disorder 6 ‒ Phase 2 double - blind STROBE’ study testing TNX - 1900 as a novel therapeutic agent to s tudy effects of TNX - 1900 on social safety learning in Binge Eating ‘STROBE’ study ‒ Massachusetts General Hospital (MGH), Dr. Elizabeth Lawson, P.I. 1 Tonix Press Release May 22, 202 3: https://ir.tonixpharma.com/news - events/press - releases/detail/1391/tonix - pharmaceuticals - announces - clinical - proof - of - concept 2 de Vries Lentsch S, et al. 2022 “CGRP - mediated trigeminovascular reactivity in migraine patients treated with erenumab .” J Neurol Neurosurg Psychiatry . Aug;93(8):911 - 912. 3 Ibrahimi K, et al. 2017 “A human trigeminovascular biomarker for antimigraine drugs: A randomized double - blind, placebo - controlled, crossover trial with sumatriptan.” Cephalalgia . Jan;37(1):94 - 98. 4 Tonix Press Release July 10 2023 – https://ir.tonixpharma.com/news - events/press - releases/detail/1404/tonix - pharmaceuticals - announces - initiation - of - enrollment - in 5 Tonix Press Release July 17, 2023 – https://ir.tonixpharma.com/news - events/press - releases/detail/1405/tonix - pharmaceuticals - announces - agreement - and - initiation - o 6 Tonix Press Release July 31, 2023 – https://ir.tonixpharma.com/news - events/press - releases/detail/1410/tonix - pharmaceuticals - announces - enrollment - initiated - in - the
22 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 1900 for Pediatric Autism • Children with Autism Spectrum Disorder (ASD) are at risk for low bone density • Preliminary data s uggest that administration of oxytocin may f avorably impact bone f ormation and strength • Recent meta - analysis reported that plasma o xytocin levels tend to be lower in children with Autism Spectrum Disorder than controls 1 “BOX” Investigator - Initiated Study in Pediatric Autism at MGH • Randomized, placebo - controlled study to evaluate the effects of twice daily administration of TNX - 1900 on bone measures in children with ASD • Study subjects, ages six to 18 years old, will be randomized 1:1 to receive TNX - 1900 twice per day or placebo for 12 months in the double - blind phase, followed by a six - month open label phase during which all study subjects will receive TNX - 1900 twice daily • Primary endpoint: difference between TNX - 1900 compared to placebo groups in 12 - month change in whole body less head bone mineral density Z - scores o Z - score compares one’s bone density to the average bone density of age and gender matched controls 1 John S and Jaeggi , AV . Autism . 2021 . 25 : 2152 - 2161
23 © 2022 Tonix Pharmaceuticals Holding Corp. RARE DISEASE PORTFOLIO Rare genetic diseas e that afflicts 10 - 20 thousand individuals in the US TNX - 2900 for Prader - Willi Syndrome Prader - Willi Syndrome (PWS) is the most common genetic cause of life - threatening childhood obesity. PWS causes unhealthy behaviors around food 1 - 4 , c onsequences such as obesity, type 2 diabetes, and cardiovascular disease 1 - 5 , and creates significant caretaker burden 1 - 4 10 - 20 thousand individuals *TNX - 2900 has been granted FDA Orphan Drug Designation 1 Miller et al., 2011. Am J Med Genet A . 1 55A(5):1040 - 1049 2 Butler et al., 2017. Genet Med. 19(6):635 - 642 3 Butler MG. NORD. Updated 2018. Accessed May 25, 2022. https://rarediseases.org/rare - diseases/prader - willi - syndrome/ 4 Prader - Willi Syndrome Association USA. Accessed May 25, 2022. https://www.pwsausa.org/what - is - prader - willi - syndrome/ 5 Muscogiuri et al., 2021. J Endocrinol Invest . 44(10):2057 - 2070 Current standard of care: • Human growth hormone treatment is FDA - approved for growth failure in PWS children Large unmet need: • Currently no cure, and no treatment for PWS - related hyperphagia • Consequences can be life threatening - obesity and cardiovascular disease are leading cause of death

© 2023 Tonix Pharmaceuticals Holding Corp. THANK YOU