
Tonix Pharmaceuticals Holding Corp. 8-K
Exhibit 99.04

© 2023 Tonix Pharmaceuticals Holding Corp. TNX - 102 SL Fibromyalgia & Long COVID NASDAQ: TNXP Version P0502 November 15 , 2023 (Doc 1344)
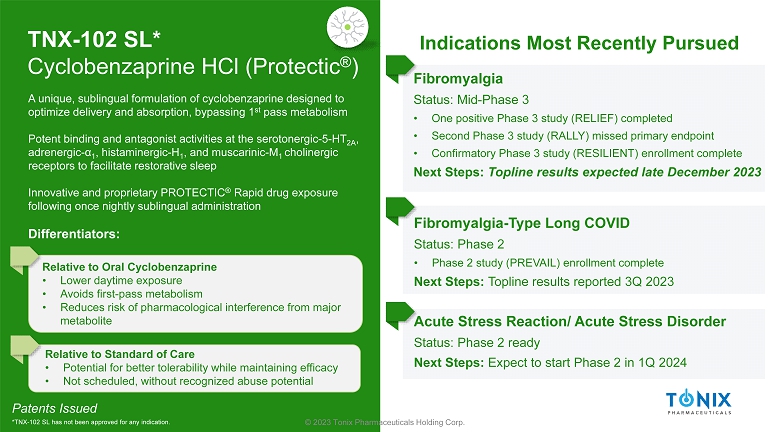
© 2023 Tonix Pharmaceuticals Holding Corp. TNX - 102 SL* Cyclobenzaprine HCl ( Protectic ® ) Fibromyalgia Status: Mid - Phase 3 • One positive Phase 3 study (RELIEF) completed • Second Phase 3 study (RALLY) missed primary endpoint • Confirmatory Phase 3 study (RESILIENT) enrollment complete Next Steps: Topline results expected late December 2023 Fibromyalgia - Type Long COVID Status: Phase 2 • Phase 2 study (PREVAIL) enrollment complete Next Steps: Topline results reported 3Q 2023 Patents Issued *TNX - 102 SL has not been approved for any indication. A unique, sublingual formulation of cyclobenzaprine designed to optimize delivery and absorption, bypassing 1 st pass metabolism Potent binding and antagonist activities at the serotonergic - 5 - HT 2A , adrenergic - α 1 , histaminergic - H 1 , and muscarinic - M 1 cholinergic receptors to facilitate restorative sleep Innovative and proprietary PROTECTIC ® Rapid drug exposure following once nightly sublingual administration Differentiators: Relative to Oral Cyclobenzaprine • Lower daytime exposure • Avoids first - pass metabolism • Reduces risk of pharmacological interference from major metabolite Relative to Standard of Care • Potential for better tolerability while maintaining efficacy • Not scheduled, without recognized abuse potential Indications Most Recently Pursued Acute Stress Reaction/ Acute Stress Disorder Status: Phase 2 ready Next Steps: Expect to start Phase 2 in 1Q 2024

3 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO PROFILE DEVELOPMENT PROGRAM Patents Issued TNX - 102 SL*: Fibromyalgia Cyclobenzaprine Protectic ® Sublingual Tablets CNS PORTFOLIO Fibromyalgia (FM) is a chronic pain disorder resulting from amplified sensory and pain signaling within the CNS • A fflicts an estimated 6 - 12 million adults in the U.S., approximately 90% of whom are women 1 • Symptoms include chronic widespread pain, nonrestorative sleep, fatigue, and cognitive dysfunction • Patients struggle with daily activities, have impaired quality of life, and frequently are disabled • Physicians and patients report common dissatisfaction with currently marketed products Market Entry: Fibromyalgia Additional Indications: Long COVID, PTSD, Agitation in Alzheimer’s, Alcohol Use Disorder Status: One Positive Phase 3 study RELIEF completed 2 Second Phase 3 study RALLY missed primary endpoint Confirmatory Phase 3 study RESILIENT enrollment complete Next Steps: Topline results expected late Dece mber 2023 *TNX - 102 SL has not been approved for any indication. 1 American Chronic Pain Association (www.theacpa.org, 2019) 2 Lederman et al., (2023) Arthritis Care & Research "Efficacy and Safety of TNX - 102 SL (Sublingual Cyclobenzaprine) for the Treatment of Fibromyalgia: Results From the RELIEF Trial ", doi : 10.1002/acr.25142. Epub ahead of print. PMID: 37165930. When the check engine light malfunctions, the light is on even though the car is not malfunctioning

4 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 102 SL: Fibromyalgia Program Update CNS PORTFOLIO Phase 3 Study, RESILIENT, will compare TNX - 102 SL 5.6 mg and placebo • First patient enrolled in April 2022 • Parallel design, double - blind, randomized placebo - controlled study, all US sites • Primary endpoint is pain at Week 14 analyzed by MMRM with MI • Projecting adverse event - related discontinuations to decrease towards rates in RELIEF and PTSD Studies Phase 3 Study, RALLY, comparison of TNX - 102 SL 5.6 mg and placebo • As expected from interim analysis results published in July 2021, RALLY Study missed primary endpoint • Unexpected ~80% increase in adverse event - related discontinuations in both drug and placebo arms • Multiple imputation approach on 'Missing Data' attenuated statistical significance of efficacy endpoints’ • TNX - 102 SL was generally well tolerated with overall adverse event profile comparable to prior studies; no new safety signals observed

5 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 102 SL: Phase 3 RESILIENT Study Design General s tudy c haracteristics: • Randomized, double - blind, placebo - controlle d study in fibromyalgia • U.S. sites only, completed enrollment of 457 patients • Clinical stage complete as of November 15, 2023 • P reliminary unaudited rate of adverse - event related discontinuations was 4.8% • C ompares favorably with prior FM studies RELIEF, 6.0% and RALLY, 10.7% Primary Endpoint: • Daily diary pain severity score change from baseline to Week 14 (TNX - 102 SL vs. placebo) • Weekly averages of the daily numerical rating scale scores • Threshold for potential NDA - enabling study is p < 0.05 Key Secondary Endpoints: • Patient Global Impression of Change responder analysis • Fibromyalgia Impact Questionnaire - Revised (FIQ - R) Symptom Domain score • FIQ - R Function Domain score • PROMIS Sleep Disturbance instrument • PROMIS Fatigue instrument • Weekly average of the daily diary assessment of sleep quality Placebo once - daily at bedtime TNX - 102 SL once - daily at bedtime 5.6 mg (2 x 2.8 mg tablets) * * Two week run in at 2.8 mg dose at bedtime, followed by 12 weeks at 5.6 mg dose ClinicalTrials.gov Identifier: NCT05273749 A Phase 3 Study to Evaluate the Efficacy and Safety of TNX - 102 SL Taken Daily in Patients With Fibromyalgia (RESILIENT) 14 weeks
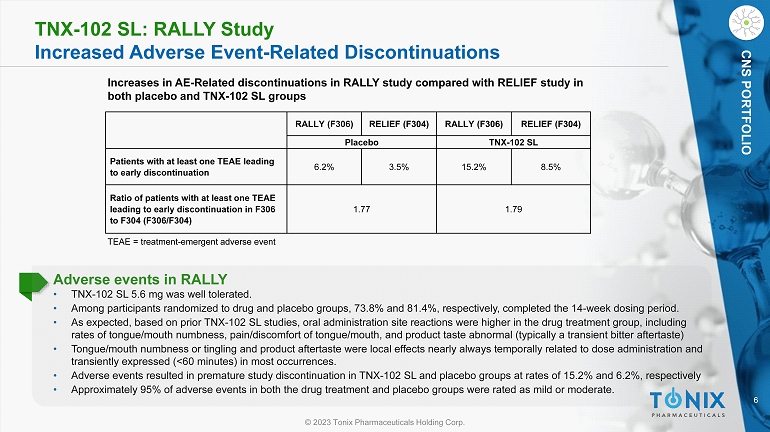
6 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 102 SL: RALLY Study Increased Adverse Event - Related Discontinuations TEAE = treatment - emergent adverse event Increases in AE - Related discontinuations in RALLY study compared with RELIEF study in both placebo and TNX - 102 SL groups Adverse events in RALLY • TNX - 102 SL 5.6 mg was well tolerated. • Among participants randomized to drug and placebo groups, 73.8% and 81.4%, respectively, completed the 14 - week dosing period. • As expected, based on prior TNX - 102 SL studies, oral administration site reactions were higher in the drug treatment group, incl uding rates of tongue/mouth numbness, pain/discomfort of tongue/mouth, and product taste abnormal (typically a transient bitter aft ert aste) • Tongue/mouth numbness or tingling and product aftertaste were local effects nearly always temporally related to dose administ rat ion and transiently expressed (<60 minutes) in most occurrences. • Adverse events resulted in premature study discontinuation in TNX - 102 SL and placebo groups at rates of 15.2% and 6.2%, respecti vely • Approximately 95% of adverse events in both the drug treatment and placebo groups were rated as mild or moderate. RELIEF (F304) RALLY (F306) RELIEF (F304) RALLY (F306) TNX - 102 SL Placebo 8.5% 15.2% 3.5% 6.2% Patients with at least one TEAE leading to early discontinuation 1.79 1.77 Ratio of patients with at least one TEAE leading to early discontinuation in F306 to F304 (F306/F304)
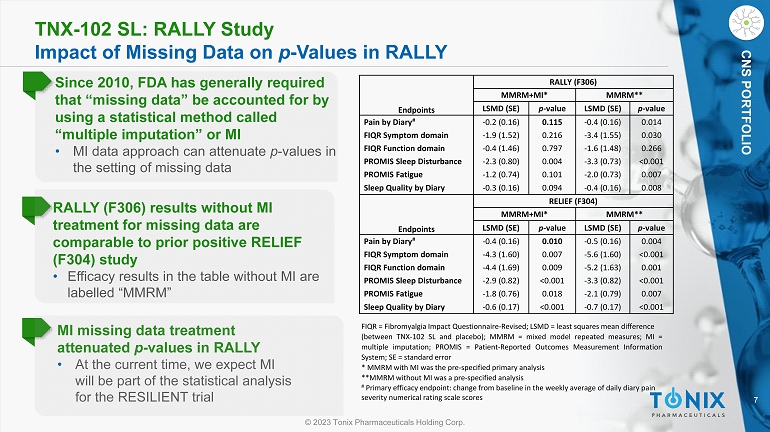
7 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 102 SL: RALLY Study Impact of Missing Data on p - Values in RALLY MI missing data treatment attenuated p - values in RALLY • At the current time, we expect MI will be part of the statistical analysis for the RESILIENT trial FIQR = Fibromyalgia Impact Questionnaire - Revised ; LSMD = least squares mean difference (between TNX - 102 SL and placebo) ; MMRM = mixed model repeated measures ; MI = multiple imputation ; PROMIS = Patient - Reported Outcomes Measurement Information System ; SE = standard error * MMRM with MI was the pre - specified primary analysis **MMRM without MI was a pre - specified analysis # Primary efficacy endpoint: change from baseline in the weekly average of daily diary pain severity numerical rating scale scores Since 2010, FDA has generally required that “missing data” be accounted for by using a statistical method called “multiple imputation” or MI • MI data approach can attenuate p - values in the setting of missing data RALLY (F306) results without MI treatment for missing data are comparable to prior positive RELIEF (F304) study • Efficacy results in the table without MI are labelled “MMRM” RALLY (F306) MMRM+MI* MMRM** Endpoints LSMD (SE) p - value LSMD (SE) p - value Pain by Diary # - 0.2 (0.16) 0.115 - 0.4 (0.16) 0.014 FIQR Symptom domain - 1.9 (1.52) 0.216 - 3.4 (1.55) 0.030 FIQR Function domain - 0.4 (1.46) 0.797 - 1.6 (1.48) 0.266 PROMIS Sleep Disturbance - 2.3 (0.80) 0.004 - 3.3 (0.73) <0.001 PROMIS Fatigue - 1.2 (0.74) 0.101 - 2.0 (0.73) 0.007 Sleep Quality by Diary - 0.3 (0.16) 0.094 - 0.4 (0.16) 0.008 RELIEF (F304) MMRM+MI* MMRM** Endpoints LSMD (SE) p - value LSMD (SE) p - value Pain by Diary # - 0.4 (0.16) 0.010 - 0.5 (0.16) 0.004 FIQR Symptom domain - 4.3 (1.60) 0.007 - 5.6 (1.60) <0.001 FIQR Function domain - 4.4 (1.69) 0.009 - 5.2 (1.63) 0.001 PROMIS Sleep Disturbance - 2.9 (0.82) <0.001 - 3.3 (0.82) <0.001 PROMIS Fatigue - 1.8 (0.76) 0.018 - 2.1 (0.79) 0.007 Sleep Quality by Diary - 0.6 (0.17) <0.001 - 0.7 (0.17) <0.001
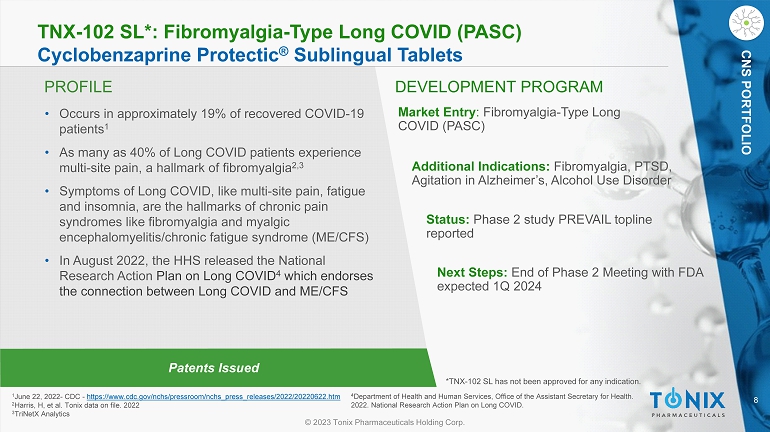
8 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO PROFILE DEVELOPMENT PROGRAM Patents Issued TNX - 102 SL*: Fibromyalgia - Type Long COVID (PASC) Cyclobenzaprine Protectic ® Sublingual Tablets • Occurs in approximately 19% of recovered COVID - 19 patients 1 • As many as 40% of Long COVID patients experience multi - site pain, a hallmark of fibromyalgia 2,3 • Symptoms of Long COVID, like multi - site pain, fatigue and insomnia, are the hallmarks of chronic pain syndromes like fibromyalgia and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) • In August 2022, the HHS released the National Research Action Plan on Long COVID 4 which endorses the connection between Long COVID and ME/CFS Market Entry : Fibromyalgia - Type Long COVID (PASC) Status: Phase 2 study PREVAIL topline reported Next Steps: End of Phase 2 Meeting with FDA expected 1Q 2024 1 June 22, 2022 - CDC - https://www.cdc.gov/nchs/pressroom/nchs_press_releases/2022/20220622.htm 2 Harris, H, et al. Tonix data on file. 2022 3 TriNetX Analytics *TNX - 102 SL has not been approved for any indication. CNS PORTFOLIO Additional Indications: Fibromyalgia, PTSD, Agitation in Alzheimer’s, Alcohol Use Disorder 4 Department of Health and Human Services, Office of the Assistant Secretary for Health. 2022. National Research Action Plan on Long COVID.
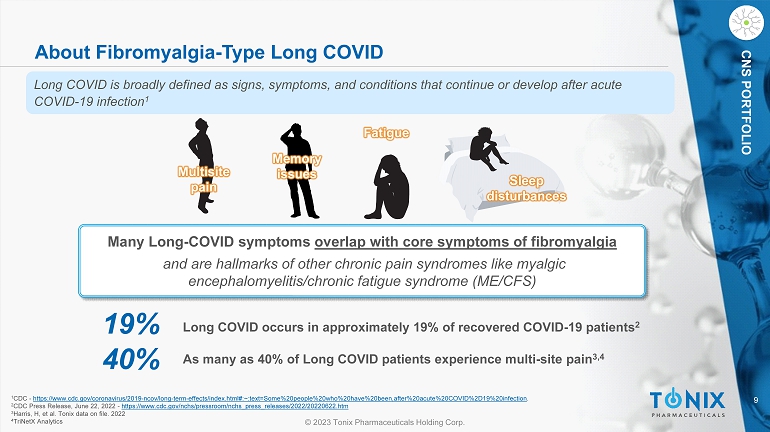
9 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO About Fibromyalgia - Type Long COVID Many Long - COVID symptoms overlap with core symptoms of fibromyalgia and are hallmarks of other chronic pain syndromes like myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) Multisite pain Memory issues Fatigue Sleep disturbances 19% Long COVID occurs in approximately 19% of recovered COVID - 19 patients 2 40% As many as 40% of Long COVID patients experience multi - site pain 3,4 1 CDC - https://www.cdc.gov/coronavirus/2019 - ncov/long - term - effects/index.html#:~:text=Some%20people%20who%20have%20been,after%20acute%2 0COVID%2D19%20infection . 2 CDC Press Release, June 22, 2022 - https://www.cdc.gov/nchs/pressroom/nchs_press_releases/2022/20220622.htm 3 Harris, H, et al. Tonix data on file. 2022 4 TriNetX Analytics Long COVID is broadly defined as signs, symptoms, and conditions that continue or develop after acute COVID - 19 infection 1
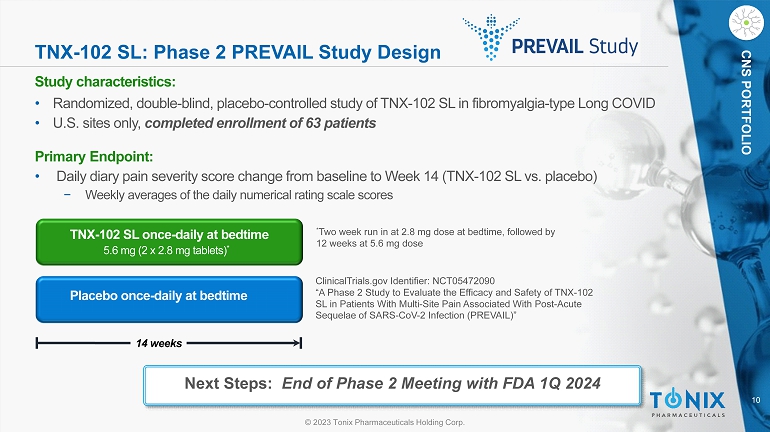
10 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 102 SL: Phase 2 PREVAIL Study Design Study c haracteristics: • Randomized, double - blind, placebo - controlle d study of TNX - 102 SL in fibromyalgia - type Long COVID • U.S. sites only, completed enrollment of 63 patients Primary Endpoint: • Daily diary pain severity score change from baseline to Week 14 (TNX - 102 SL vs. placebo) − Weekly averages of the daily numerical rating scale scores Placebo once - daily at bedtime 14 weeks TNX - 102 SL once - daily at bedtime 5.6 mg (2 x 2.8 mg tablets) * * Two week run in at 2.8 mg dose at bedtime, followed by 12 weeks at 5.6 mg dose ClinicalTrials.gov Identifier: NCT05472090 “A Phase 2 Study to Evaluate the Efficacy and Safety of TNX - 102 SL in Patients With Multi - Site Pain Associated With Post - Acute Sequelae of SARS - CoV - 2 Infection (PREVAIL)” Next Steps: End of Phase 2 Meeting with FDA 1Q 2024
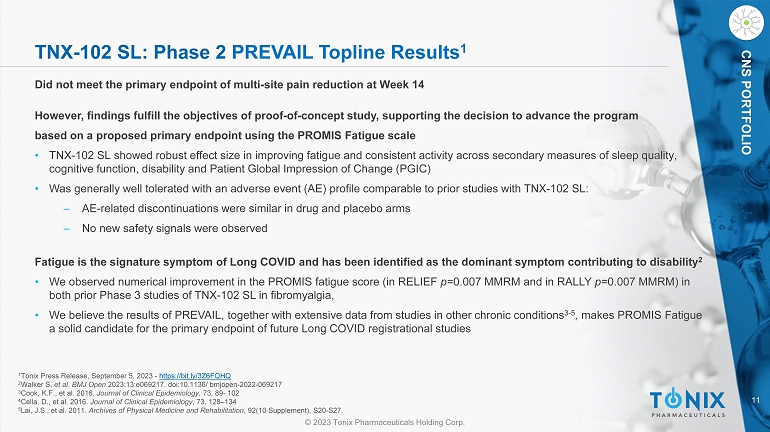
11 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 102 SL: Phase 2 PREVAIL Topline Results 1 Did not meet the primary endpoint of multi - site pain reduction at Week 14 However, f indings fulfill the objectives of proof - of - concept study, supporting the decision to advance the program b ased on a proposed primary endpoint using the PROMIS Fatigue scale • TNX - 102 SL showed robust effect size in improving fatigue and consistent activity across secondary measures of sleep quality, cognitive function, disability and Patient Global Impression of Change (PGIC) • Was g enerally well tolerated with an adverse event (AE) profile comparable to prior studies with TNX - 102 SL: ‒ AE - related discontinuations were similar in drug and placebo arms ‒ No new safety signals were observed Fatigue is the signature symptom of Long COVID and has been identified as the dominant symptom contributing to disability 2 • We observed numerical improvement in the PROMIS fatigue score (in RELIEF p= 0.007 MMRM and in RALLY p= 0.007 MMRM) in both prior Phase 3 studies of TNX - 102 SL in fibromyalgia, • We believe the results of PREVAIL, toge ther with extensive data from studies in other chronic conditions 3 - 5 , makes PROMIS Fatigue a solid candidate for the primary endpoint of future Long COVID registrational studies 1 Tonix Press Release, September 5, 2023 - https://bit.ly/3Z6FQHQ 2 Walker S, et al . BMJ Open 2023;13:e069217. doi:10.1136/ bmjopen - 2022 - 069217 3 Cook, K.F., et al. 2016. Journal of Clinical Epidemiology , 73, 89 - 102 4 Cella, D., et al. 2016. Journal of Clinical Epidemiology , 73, 128 – 134 5 Lai, J.S., et al. 2011. Archives of Physical Medicine and Rehabilitation , 92(10 Supplement), S20 - S27.

12 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Next Steps Tonix plans to meet with FDA to discuss a path to registration ‒ Expected date of End of Phase 2 meeting is 1 st Quarter 2024 F atigue is the principal symptom overlapping with chronic fatigue syndrome/ myalgic encephalomyelitis (CFS/ME) and fibromyalgia syndromes ‒ Expected date of fibromyalgia topline is late December 2023

13 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO CNS PORTFOLIO Acute Stress Reaction (ASR)/ Acute Stress Disorder (ASD) ASR/ASD are acute stress conditions resulting from trauma which c an affect both civilian and military populations. Large unmet need: • According to National Center for PTSD, about 60% of men and 50% of women in the US are exposed least one traumatic experience in their lives 1 • In the US alone, one - third of emergency department visits (40 - 50 million patients per year) are for evaluation after trauma exposures 2 Current standard of care: • No medications are currently available at or near the point of care to treat patients suffering from acute traumatic events and support long - term health 1 National Center for PTSD. How Common is PTSD in Adults? https://www.ptsd.va.gov/understand/common/common_adults.asp 2 Wisco et al. J Clin Psychiatry . 2014.75(12):1338 - 46
14 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO ASR/ASD Program Status Status: Expect to start Phase 2 in 1Q 2024 Phase 2 Trial Funded by DoD grant to University of North Carolina (UNC) • UNC Institute for Trauma Recovery awarded a $3M grant from the Department of Defense (DoD) • OASIS trial will build upon infrastructure developed through the UNC - led, $40M AURORA initiative ‒ AURORA study is a major national research initiative to improve the understanding, prevention, and recovery of individuals who have experienced a traumatic event ‒ Supported in part by funding from the National Institutes of Health (NIH) and the health care arm of Google’s parent company Alphabet • Opportunity to investigate the correlation between motor vehicle collisions and the emergence of ASD and PTSD • Supported by multiple clinical trials: • Phase 2 trial in military - related PTSD ( AtEase or NCT02277704) • Phase 3 trial in military - related PTSD (HONOR or NCT03062540) • Phase 3 trial in primarily civilian PTSD (RECOVERY or NCT03841773) • In each of these studies, early and sustained improvements in sleep were associated with TNX - 102 SL treatment by the PROMIS sleep disturbance (SD) scale and the Clinician Administered PTSD Scale (CAPS - 5) “sleep disturbance” item. Together these studies provide preliminary evidence that TNX - 102 SL is well - tolerated and may promote recovery from PTSD via a pharmacodynamic facilitation of sleep - dependent emotional memory processing

15 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 102 SL: Phase 2 OASIS Study Design General s tudy c haracteristics: • Randomized, double - blind, placebo - controlle d study in Acute Stress Reaction (ASR) / Acute Stress Disorder (ASD) • The proposed O ptimizing A cute S tress reaction I nterventions with TNX - 102 S L (OASIS) trial will examine the safety and efficacy of TNX - 102 SL to reduce adverse posttraumatic neuropsychiatric sequelae among patients presenting to the emergency department after a motor ve hicle collision (MVC) • The trial will enroll approximately 180 individuals who acutely experienced trauma at study sites across the US • Participants will be randomized in the emergency department to receive a two - week course of either TNX - 102 SL or placebo • Investigator - initiated IND Objective: • Investigate the potential of Tonix’s TNX - 102 SL (cyclobenzaprine HCl sublingual tablets) to reduce the frequency and severity of the adverse effects of traumatic exposure, including acute stress reaction (ASR), acute stress disorder (ASD), and posttraumatic stress d iso rder (PTSD). • ASR refers to the body’s immediate response to trauma, whereas ASD is the short - term effects of trauma (within 1 month), and PTS D is the long - term effects of trauma (beyond 1 month) * First dose of TNX - 102 SL 5.6 mg versus placebo taken in the emergency department, and then daily at bedtime to finish 2 weeks of treatment A Phase 2 Study to Evaluate the Efficacy and Safety of TNX - 102 SL Taken Daily in Patients With ASR/ ASD (OASIS) • Primary outcome measure: Acute Stress Disorder Scale (ASDS) assessed at 7 and 21 days post MVC • Posttraumatic stress symptom severity assessed at 6 and 12 weeks post MVC using the PTSD Checklist for DSM - 5 (PCL - 5) • Standardized survey instruments of sleep disturbances, anxiety and depression symptoms, general physical and mental health, and clinical global improvement also employed • Detailed and brief neurocognitive assessments are performed from baseline to 12 weeks after MVC at specific timepoints throughout study participation period Placebo once - daily at bedtime 2 weeks TNX - 102 SL once - daily at bedtime 5.6 mg (2 x 2.8 mg tablets) *

© 2023 Tonix Pharmaceuticals Holding Corp. THANK YOU

© 2023 Tonix Pharmaceuticals Holding Corp. APPENDIX
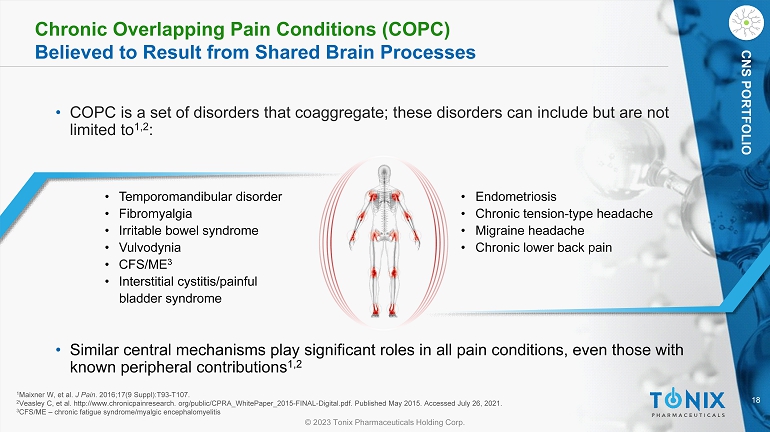
18 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Chronic Overlapping Pain Conditions (COPC) Believed to Result from Shared Brain Processes • COPC is a set of disorders that coaggregate ; these disorders can include but are not limited to 1,2 : • Temporomandibular disorder • Fibromyalgia • Irritable bowel syndrome • Vulvodynia • CFS/ME 3 • Interstitial cystitis/painful bladder syndrome • Endometriosis • Chronic tension - type headache • Migraine headache • Chronic lower back pain 1 Maixner W, et al. J Pain . 2016;17(9 Suppl):T93 - T107. 2 Veasley C, et al. http://www.chronicpainresearch. org/public/CPRA_WhitePaper_2015 - FINAL - Digital.pdf. Published May 2015. Accesse d July 26, 2021. 3 CFS/ME – chronic fatigue syndrome/ myalgic encephalomyelitis • Similar central mechanisms play significant roles in all pain conditions, even those with known peripheral contributions 1,2
19 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Role of Infections in Triggering Fibromyalgia or Chronic fatigue (CFS) - Like Illnesses Infection initiates an autoreactive process, which affects several functions, including brain and energy metabolism 2 - 7 • Infections can trigger any of these conditions in approximately 10% of exposed individuals • The initial location of the infection determines the subsequent pain syndrome • Any type of infectious diarrhea will trigger irritable bowel syndrome (IBS) in 10% to 20% of those exposed 1 Department of Health and Human Services, Office of the Assistant Secretary for Health. 2022. National Research Action Plan on Lo ng COVID, 200 Independence Ave SW, Washington, DC 20201. 2 Blomberg J, et al. Front Immunol. 2018;9:229. Published 2018 Feb 15. 3 Warren JW, et al. Urology. 2008;71(6):1085 - 1090. 4 Buskila D, et al. Autoimmun Rev. 2008;8(1):41 - 43. 5 Hickie I, et al. BMJ. 2006;333(7568):575. 6 Parry SD, et al. Am J Gastroenterol. 2003;98(9):1970 - 1975. 7 Halvorson HA, et al. Am J Gastroenterol. 2006;101(8):1894 - 1942. • Symptoms of Long COVID, like multi - site pain, fatigue and insomnia, are the hallmarks of chronic pain syndromes like fibromyalgia and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). • In August 2022, the HHS released the National Research Action Plan on Long COVID 1 which endorses the connection between Long COVID and chronic fatigue syndrome.
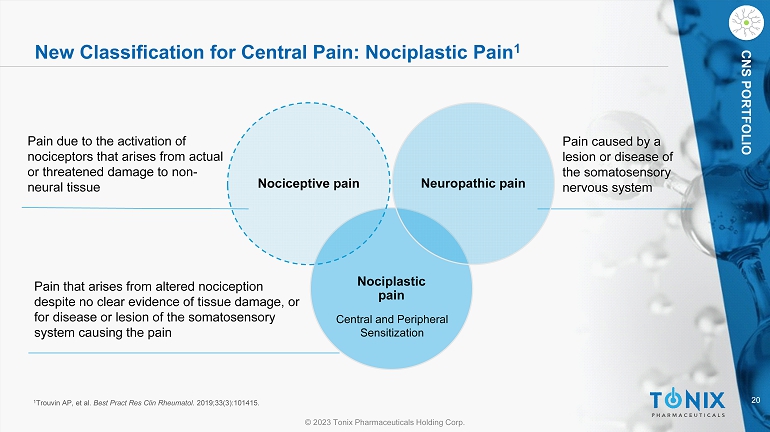
20 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO New Classification for Central Pain: Nociplastic Pain 1 Nociplastic pain Nociceptive pain Neuropathic pain Pain due to the activation of nociceptors that arises from actual or threatened damage to non - neural tissue Pain that arises from altered nociception despite no clear evidence of tissue damage, or for disease or lesion of the somatosensory system causing the pain Pain caused by a lesion or disease of the somatosensory nervous system 1 Trouvin AP, et al. Best Pract Res Clin Rheumatol . 2019;33(3):101415. Central and Peripheral Sensitization
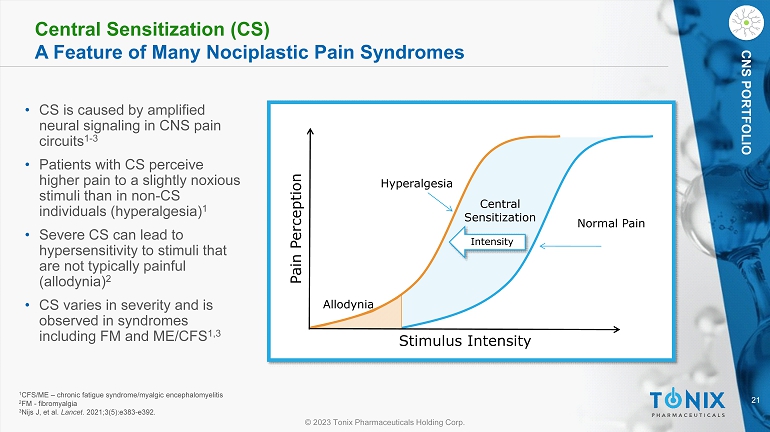
21 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Central Sensitization (CS) A Feature of Many Nociplastic Pain Syndromes • CS is caused by amplified neural signaling in CNS pain circuits 1 - 3 • Patients with CS perceive higher pain to a slightly noxious stimuli than in non - CS individuals (hyperalgesia) 1 • Severe CS can lead to hypersensitivity to stimuli that are not typically painful (allodynia) 2 • CS varies in severity and is observed in syndromes including FM and ME/CFS 1,3 Stimulus Intensity Pain Perception Normal Pain Hyperalgesia Allodynia Central Sensitization Intensity 1 CFS/ME – chronic fatigue syndrome/ myalgic encephalomyelitis 2 FM - fibromyalgia 3 Nijs J, et al. Lancet . 2021;3(5):e383 - e392.
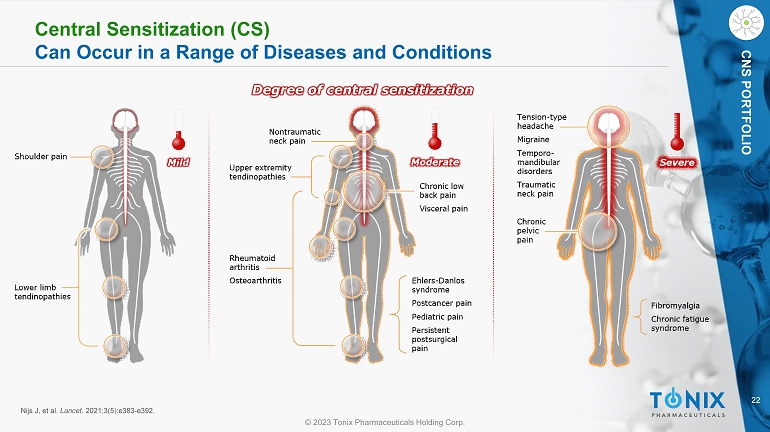
22 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Central Sensitization (CS) Can Occur in a Range of Diseases and Conditions Degree of central sensitization Nijs J, et al. Lancet . 2021;3(5):e383 - e392.