
Tonix Pharmaceuticals Holding Corp. 8-K
Exhibit 99.02

© 2024 Tonix Pharmaceuticals Holding Corp. Corporate Presentation Focus on: Tonmya Œ * (TNX - 102 SL) in Development for the Management of Fibromyalgia February 2024 NASDAQ: TNXP *Tonmya is conditionally accepted by FDA as the tradename for TNX - 102 SL for the management of fibromyalgia Version P0527 February 12 , 2024 (Doc 1377 )

2 © 2024 Tonix Pharmaceuticals Holding Corp. Cautionary Note on Forward - Looking Statements Certain statements in this presentation regarding strategic plans, expectations and objectives for future operations or results are “forward - looking statements” as defined by the Private Securities Litigation Reform Act of 1995. These statements may be identified by the use of forward - looking words such as “anticipate,” “believe,” “forecast,” “estimate” and “intend,” among others. These forward - looking statements are based on Tonix’s current expectations and actual results could differ materially. There are a number of factors that could cause actual events to differ materially from those indicated by such forward - looking statements. These factors include, but are not limited to, the risks related to failure to obtain FDA clearances or approvals and noncompliance with FDA regulations; risks related to the failure to successfully market any of our products; risks related to the timing and progress of clinical development of our product candidates; our need for additional financing; uncertainties of patent protection and litigation; uncertainties of government or third party payor reimbursement; limited research and development efforts and dependence upon third parties; and substantial competition. As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products. The forward - looking statements in this presentation are made as of the date of this presentation, even if subsequently made available by Tonix on its website or otherwise. Tonix does not undertake an obligation to update or revise any forward - looking statement, except as required by law. Investors should read the risk factors set forth in the Annual Report on Form 10 - K for the year ended December 31, 2022, as filed with the Securities and Exchange Commission (the “SEC”) on March 13, 2023, and periodic reports and current reports filed with the SEC on or after the date thereof. All of Tonix's forward - looking statements are expressly qualified by all such risk factors and other cautionary statements.

3 © 2024 Tonix Pharmaceuticals Holding Corp. Tonix is committed to developing and marketing therapeutics to treat pain, neurologic, psychiatric and addiction conditions through our central nervous system portfolio and within other areas of high unmet need , including immunology, infectious disease, and rare disease With a Focus on: Filing a New Drug Application (NDA) with the US Food and Drug Administration (FDA) for Tonmya Œ (TNX - 102 SL) for the management of Fibromyalgia Who We Are

4 © 2024 Tonix Pharmaceuticals Holding Corp. CNS - Focused Biopharma with Preclinical, Clinical and Commercial Stage Products Marketed Products • Zembrace ® and Tosymra ® indicated for the treatment of acute migraine Tonmya Œ fo r Fibromyalgia: Preparing New Drug Application (NDA) • Two positive Phase 3 trials completed • NDA filing expected 2H’24 • FDA decision on NDA approval expected 2H’25 Internal Capabilities • Commercial prescription drug sales • R&D and clinical - trial scale manufacturing Strategic Partnerships • With government institutions, world - class academic & research organizations Pipeline • Phase 2 biologic cocaine antidote, FDA “Breakthrough Therapy Designation” • Phase 1 anti - CD40L monoclonal antibody to prevent organ transplant rejection *Tonmya Œ is conditionally accepted by the U.S. Food and Drug Administration (FDA) as the tradename for TNX - 102 SL for the management of fibromyalgia
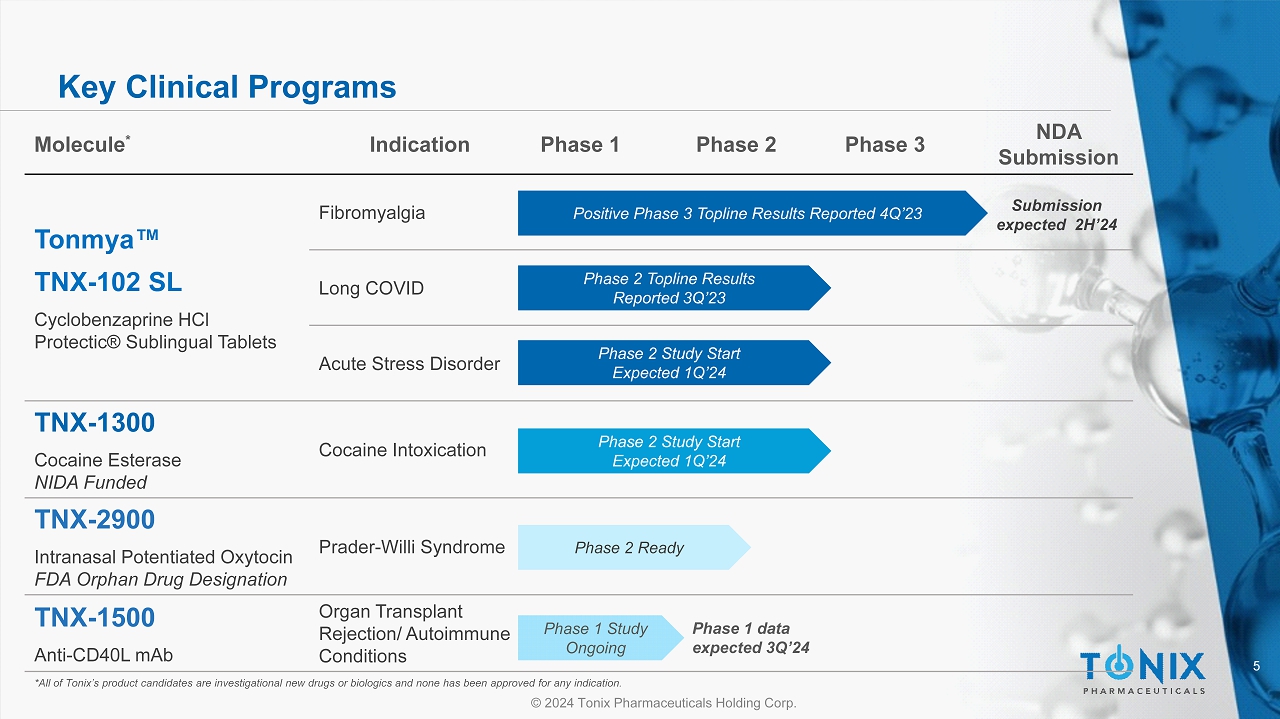
5 © 2024 Tonix Pharmaceuticals Holding Corp. Key Clinical Programs *All of Tonix’s product candidates are investigational new drugs or biologics and none has been approved for any indication. Molecule * Indication Phase 1 Phase 2 Phase 3 NDA Submission Tonmya Œ TNX - 102 SL Cyclobenzaprine HCl Protectic ® Sublingual Tablets Fibromyalgia Long COVID Acute Stress Disorder TNX - 1300 Cocaine Esterase NIDA Funded Cocaine Intoxication TNX - 2900 Intranasal Potentiated Oxytocin FDA Orphan Drug Designation Prader - Willi Syndrome TNX - 1500 Anti - CD40L mAb Organ Transplant Rejection/ Autoimmune Conditions Positive Phase 3 Topline Results Reported 4Q’23 Phase 2 Study Start Expected 1Q’24 Phase 1 Study Ongoing Submission expected 2H’24 Phase 2 Ready Phase 2 Topline Results Reported 3Q’23 Phase 2 Study Start Expected 1Q’24 Phase 1 data expected 3Q’24

© 2024 Tonix Pharmaceuticals Holding Corp. CNS: KEY DEVELOPMENT CANDIDATES

7 © 2024 Tonix Pharmaceuticals Holding Corp. Tonmya Œ TNX - 102 SL Cyclobenzaprine ( Protectic ® ) A unique, sublingual formulation of cyclobenzaprine designed to optimize delivery and absorption

TNX - 102 SL: Unique MOA Facilitates Restorative Sleep Centrally Acting Analgesic 8 Potent binding and antagonist activities at four key receptors facilitate restorative sleep • serotonergic - 5 - HT2A • adrenergic - α1 • histaminergic - H1 • muscarinic - M1 Relative to Oral Cyclobenzaprine o Lower daytime exposure o Avoids first - pass metabolism o Reduces risk of pharmacological interference from major metabolite Relative to Standard of Care o Potential for better tolerability while maintaining efficacy o Not scheduled nor with recognized abuse potential Key Differentiators © 2024 Tonix Pharmaceuticals Holding Corp. *TNX - 102 SL has not been approved for any indication.
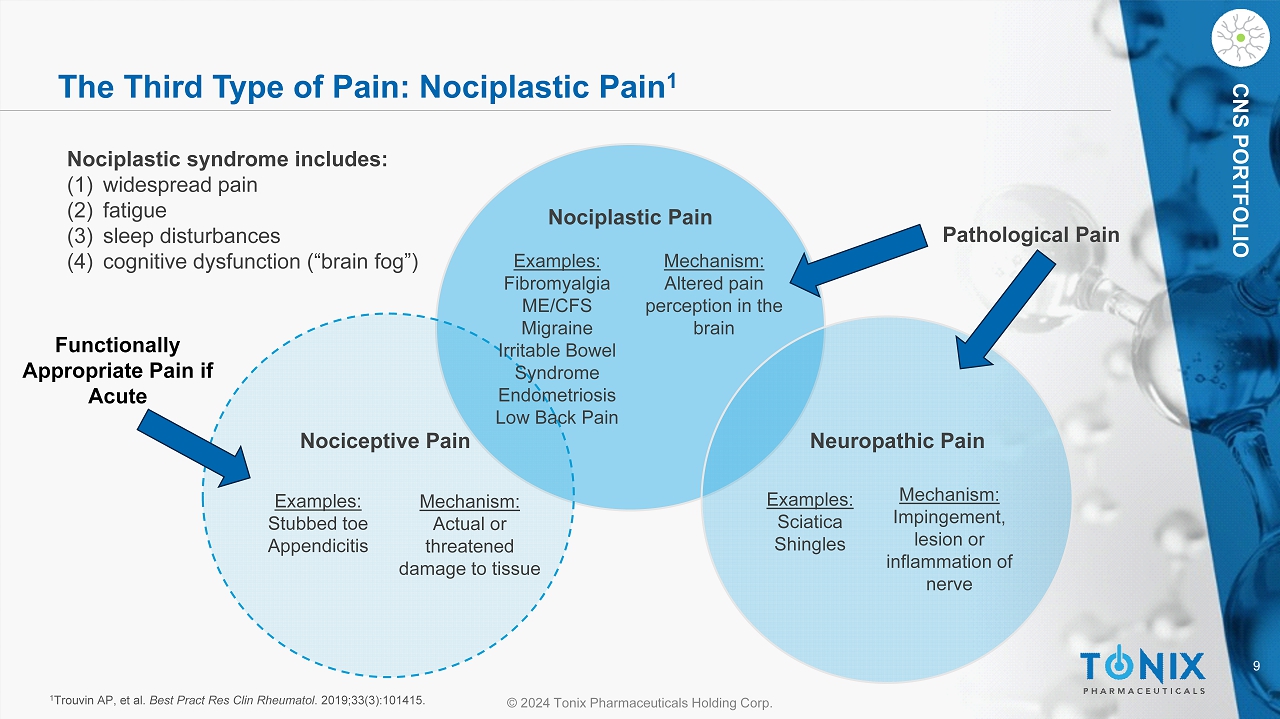
9 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO The Third Type of Pain: Nociplastic Pain 1 1 Trouvin AP, et al. Best Pract Res Clin Rheumatol . 2019;33(3):101415. Nociplastic syndrome includes : (1) widespread pain (2) fatigue (3) sleep disturbances (4) cognitive dysfunction (“brain fog”) Nociplastic Pain Examples: Fibromyalgia ME/CFS Migraine Irritable Bowel Syndrome Endometriosis Low Back Pain Examples: Stubbed toe Appendicitis Examples: Sciatica Shingles Mechanism: Altered pain perception in the brain Mechanism: Impingement, lesion or inflammation of nerve Mechanism: Actual or threatened damage to tissue Nociceptive Pain Neuropathic Pain Pathological Pain Functionally Appropriate Pain if Acute

10 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Fibromyalgia is Believed to Result from Chronic Pain or Prior Stress Experiences Stresses that may precede or precipitate FM include : Chronic nociceptive pain • e.g. , osteoarthritis Chronic neuropathic pain • e.g. , diabetic neuropathy Infectious • e.g. , viral illness Cancer • e.g. , breast cancer Chemical • e.g., cancer chemotherapy Traumatic • e.g. , motor vehicle accident Physiologic • e.g. , disturbed sleep The pain system evolved to detect acute pain • The body’s “check engine” light Chronic pain breaks down the system that determines whether a sensory experience is painful • Chronic pain results in nociplastic syndromes • Nociplastic syndrome was formerly known as “Central and Peripheral Sensitization” Chronic Overlapping Pain Conditions (COPCs) are Nociplastic Syndromes : • Fibromyalgia • ME/CFS • Migraine • Irritable Bowel Syndrome • Endometriosis • Low Back Pain
11 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Common Chronic Conditions are a Challenge for Pharma Fibromyalgia is a common chronic disease 1 • Chronic pain syndrome that persists for years or decades No animal model is recognized for nociplastic syndromes or its component symptoms • Widespread pain • Fatigue • Sleep disturbance • Cognitive impairment Nociplastic symptoms are subjective • Humans need to report symptoms using scales Clinical trials measuring subjective symptoms are challenging • Placebo response is typically observed • Long - term therapy means requires long - term tolerability 1 The U.S. Centers for Disease Control defines chronic diseases as “conditions that last 1 year or more and require ongoing med ica l attention or limit activities of daily living or both.” www.cdc.gov/chronicdisease/about/index.htm . (accessed Jan 28, 2024)

12 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Common Chronic Conditions are a Challenge for Society The Opiate Crisis in the U.S. was driven by mistreatment of chronic pain, which was often nociplastic pain • The e pidemic of prescription pain killers was addressed by regulations which limited the availability of opiates • Mandy individuals who are opiate dependent have transitioned to illegal street heroin and fentanyl • Illegal drugs contribute to homelessness There is an unmet need for non - opiate analgesics that address nociplastic pain • No new drug for fibromyalgia has been approved since 2009
13 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO CNS PORTFOLIO Fibromyalgia a fflicts an estimated 6 - 12 million adults in the US, predominantly women 1 1 American Chronic Pain Association (www.theacpa.org, 2019) 2 Robinson et al, Pain Medicine 2013;14:1400 3 The three drugs with FDA approval for the treatment of fibromyalgia: Pregabalin (Lyrica); Duloxetine (Cymbalta); Milnacipran ( Savella ) 4 Market research by Frost & Sullivan, commissioned by Tonix About Fibromyalgia Fibromyalgia (FM) is a chronic pain disorder resulting from amplified sensory and pain signaling within the CNS. Symptoms include chronic widespread pain, nonrestorative sleep , fatigue, and cognitive dysfunction 6 - 12 million adults Large unmet need: • Patients struggle with daily activities, have impaired quality of life, and frequently are disabled • Physicians and patients report common dissatisfaction with currently marketed products • Average patient has 20 physician office visits per year 2 Current standard of care: • FDA - approved products include Lyrica®, Cymbalta®, and Savella ® - each approved 10 or more years ago • Fewer than half of those treated for fibromyalgia receive sustained benefit from the approved drugs 3 • Majority (60%) fail therapy due to lack of a response (25%) or poor tolerability (35%) 4 • Opioid usage is not uncommon

14 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Fibromyalgia: Market Characteristics Prevalence • One of the more common chronic pain disorders (2 - 4% of US Population) 1 Diagnosed population • Large population but underdiagnosed 2 relative to prevalence rate • Majority receive drug treatment 3 Treatment Pattern • Polypharmacy the norm - average 2.6 drugs/patient 3 • Rotation through therapy common: average ~5 drugs/year 3 • Estimated that >22 million prescriptions are issued for the treatment of fibromyalgia (on - and off - label usage) each year 4,5 Unmet Need • Majority of patients do not respond or cannot tolerate therapy 6 1 American College of Rheumatology ( www.ACRPatientInfo.org accessed May 7, 2019) – prevalence rate of 2 - 4% for U.S. adult population (~250 million) 2 Vincent et al., 2013; diagnosed prevalence rate was 1.1% of adult population or 50% of the prevalent population 3 Robinson, et al., 2012; 85% received drug treatment 4 Vincent et al, Arthritis Care Res 2013;65:786 5 Product sales derived from IMS MIDAS; IMS NDTI used to factor usage for fibromyalgia; data accessed April 2015. 6 Market research by Frost & Sullivan, commissioned by Tonix , 2011
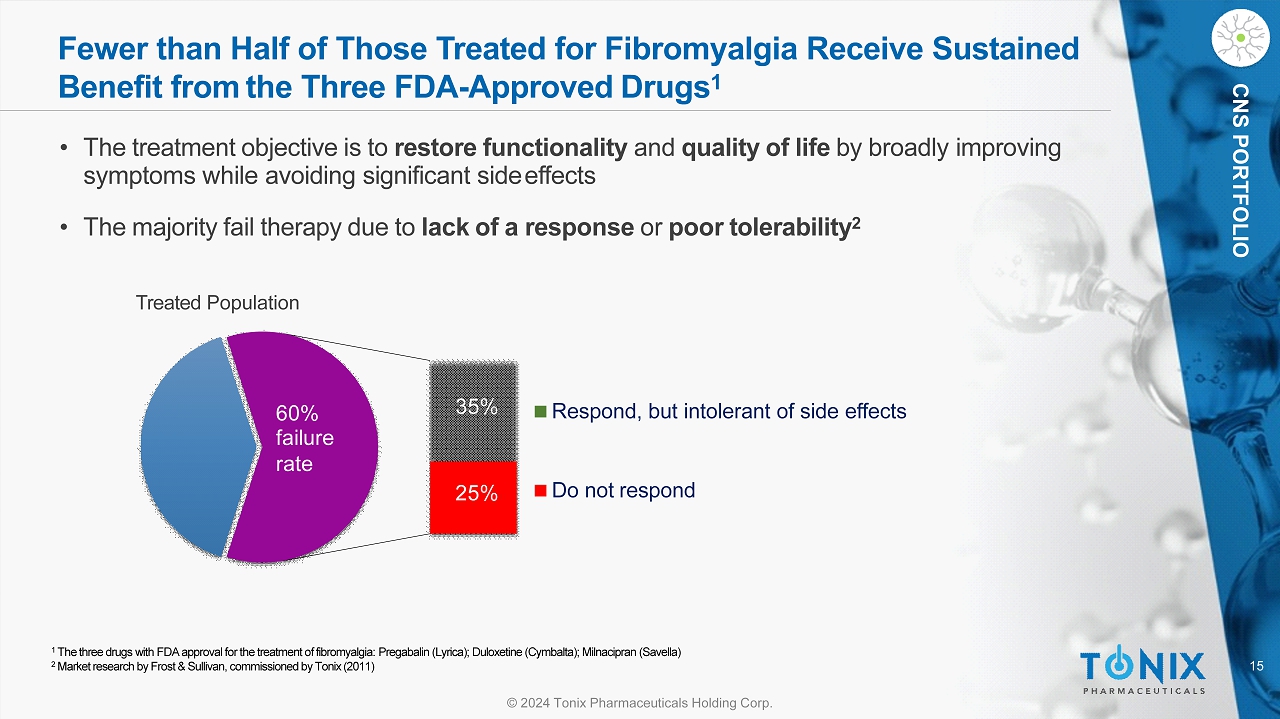
15 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Fewer than Half of Those Treated for Fibromyalgia Receive Sustained Benefit from the Three FDA - Approved Drugs 1 Respond, but intolerant of side effects Do not respond 25% 35% 60% fa il ure rate 1 The three drugs with FDA approval for the treatment of fibromyalgia: Pregabalin (Lyrica); Duloxetine (Cymbalta); Milnacipran (Savella) 2 Market research by Frost & Sullivan, commissioned by Tonix (2011) • The treatment objective is to restore functionality and quality of life by broadly improving symptoms while avoiding significant side effects • The majority fail therapy due to lack of a response or poor tolerability 2 Treated Population
16 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Large Need for New Fibromyalgia Therapies that Provide Broad Symptom Improvement with Better Tolerability • Currently - approved medications may have side effects that limit long - term use 1 • Many patients skip doses or discontinue altogether within months of treatment initiation • Medication - related side effects may be similar to fibromyalgia symptoms • High rates of discontinuation, switching and augmentation • Attempt to treat multiple symptoms and/or avoid intolerable side effects • Average of 2 - 3 medications used simultaneously 2 • The typical patient has tried six different medications 3 • Substantial off - label use of narcotic painkillers and prescription sleep aids 3 • Among those diagnosed, more than one - third have used prescription opioids as a means of treatment 4 • Tonmya Œ ( TNX - 102 SL ) is a non - opioid, centrally - acting analgesic that could provide a new therapeutic option for fibromyalgia patients 1 Nuesch et al, Ann Rheum Dis 2013;72:955 - 62. 2 Robinson RL et al, Pain Medicine 2012;13:1366. 3 Patient Trends: Fibromyalgia”, Decision Resources, 2011. 4 Berger A, Dukes E, Martin S, Edelsberg J, Oster G, Int J Clin Pract , 2007; 61(9):1498 – 1508.
17 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Fibromyalgia Program Status Tonmya Œ (TNX - 102 SL) Cyclobenzaprine Protectic ® Sublingual Tablets Fibromyalgia Positive 2 nd Phase 3 Topline Results Reported 4Q’23 1) P ositive Phase 3 study ( RELIEF ) reported – December 2020 1 2) Second Phase 3 study ( RALLY ) missed primary endpoint – July 2021 • Unexpected increase in adverse event - related discontinuations in both drug and placebo arms, potentially due to recruiting during COVID - 19 3) Positive 2 nd (c onfirmatory ) Phase 3 study ( RESILIENT ) reported – December 2023 1 Lederman et al., (2023) Arthritis Care & Research "Efficacy and Safety of TNX - 102 SL (Sublingual Cyclobenzaprine) for the Treatment of Fibromyalgia: Results From the RELIEF Trial ", doi : 10.1002/acr.25142. Epub ahead of print. PMID: 37165930. *Tonmya Œ is conditionally accepted by the U.S. Food and Drug Administration (FDA) as the tradename for TNX - 102 SL for the management of fibromyalgia. *Tonmya has not been approved for any indication. Next Steps: Pre - NDA meeting with FDA expected 1H’24 NDA filing expected 2H’24 FDA decision on NDA approval expected 2H’25

18 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Tonmya Œ (TNX - 102 SL): P hase 3 RESILIENT Study Design General s tudy c haracteristics: • Randomized, double - blind, multicenter, placebo - controlle d study in fibromyalgia • 33 U.S. sites enrolled 457 participants with fibromyalgia as defined by 2016 Revisions to the 2010/2011 FM Diagnostic C riteria 1 Primary Endpoint: • Change from baseline to Week 14 (TNX - 102 SL vs. placebo) in weekly averages of daily diary average pain severity score • Primary Endpoint, p - value = 0.00005 Placebo once - daily at bedtime TNX - 102 SL once - daily at bedtime 5.6 mg (2 x 2.8 mg tablets) * * Two - week run - in at 2.8 mg dose at bedtime followed by 12 weeks at 5.6 mg dose ClinicalTrials.gov Identifier: NCT05273749 Study Title: A Phase 3 Study to Evaluate the Efficacy and Safety of TNX - 102 SL Taken Daily in Patients With Fibromyalgia (RESILIENT) Trial ID: TNY - CY - F307 (‘RESILIENT’) 14 weeks 1 Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Häuser W, Katz RL, Mease PJ, Russell AS, Russell IJ, Walitt B. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin Arthritis Rheum. 2016; 46(3):319 - 329.
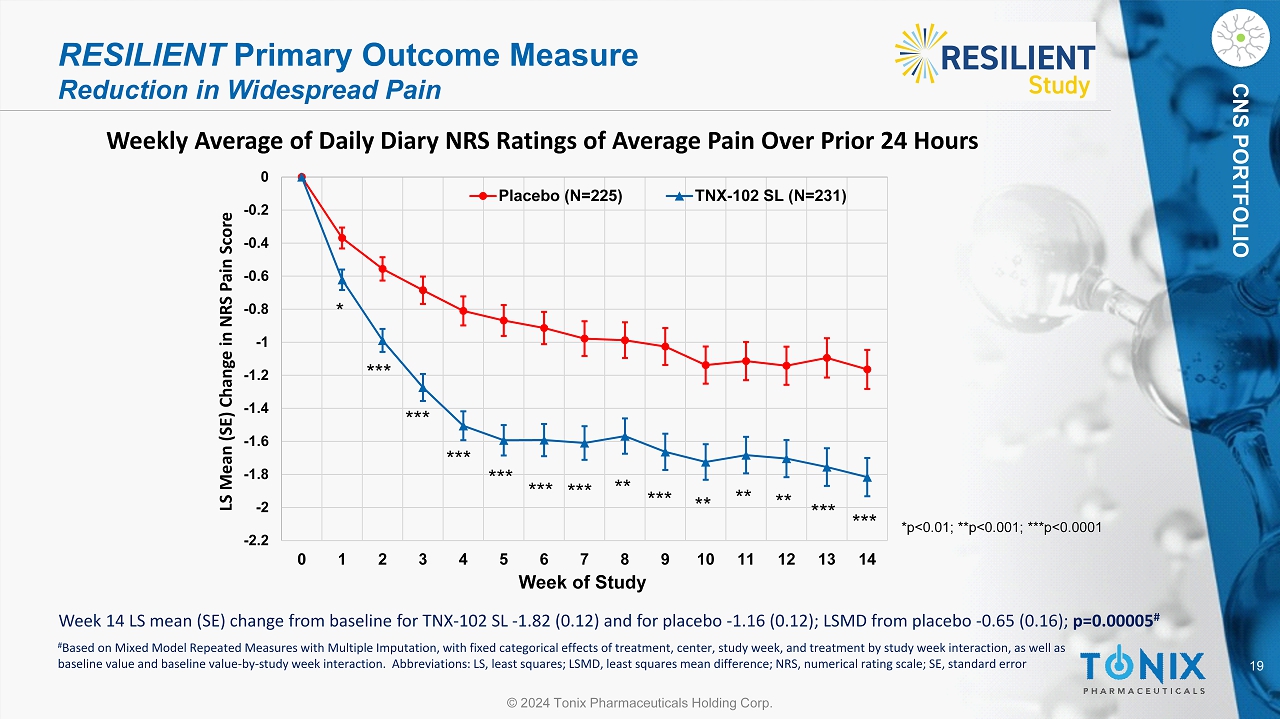
19 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Weekly Average of Daily Diary NRS Ratings of Average Pain Over Prior 24 Hours RESILIENT Primary Outcome Measure Reduction in Widespread Pain -2.2 -2 -1.8 -1.6 -1.4 -1.2 -1 -0.8 -0.6 -0.4 -0.2 0 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 LS Mean (SE) Change in NRS Pain Score Week of Study Placebo (N=225) TNX-102 SL (N=231) * *** *** ** ** ** *** *** *** *** *** *** *** ** *p<0.01; **p<0.001; ***p<0.0001 Week 14 LS mean (SE) change from baseline for TNX - 102 SL - 1.82 (0.12) and for placebo - 1.16 (0.12); LSMD from placebo - 0.65 (0.1 6); p=0.00005 # # Based on Mixed Model Repeated Measures with Multiple Imputation, with fixed categorical effects of treatment, center, study w eek , and treatment by study week interaction, as well as baseline value and baseline value - by - study week interaction. Abbreviations: LS, least squares; LSMD, least squares mean differe nce; NRS, numerical rating scale; SE, standard error
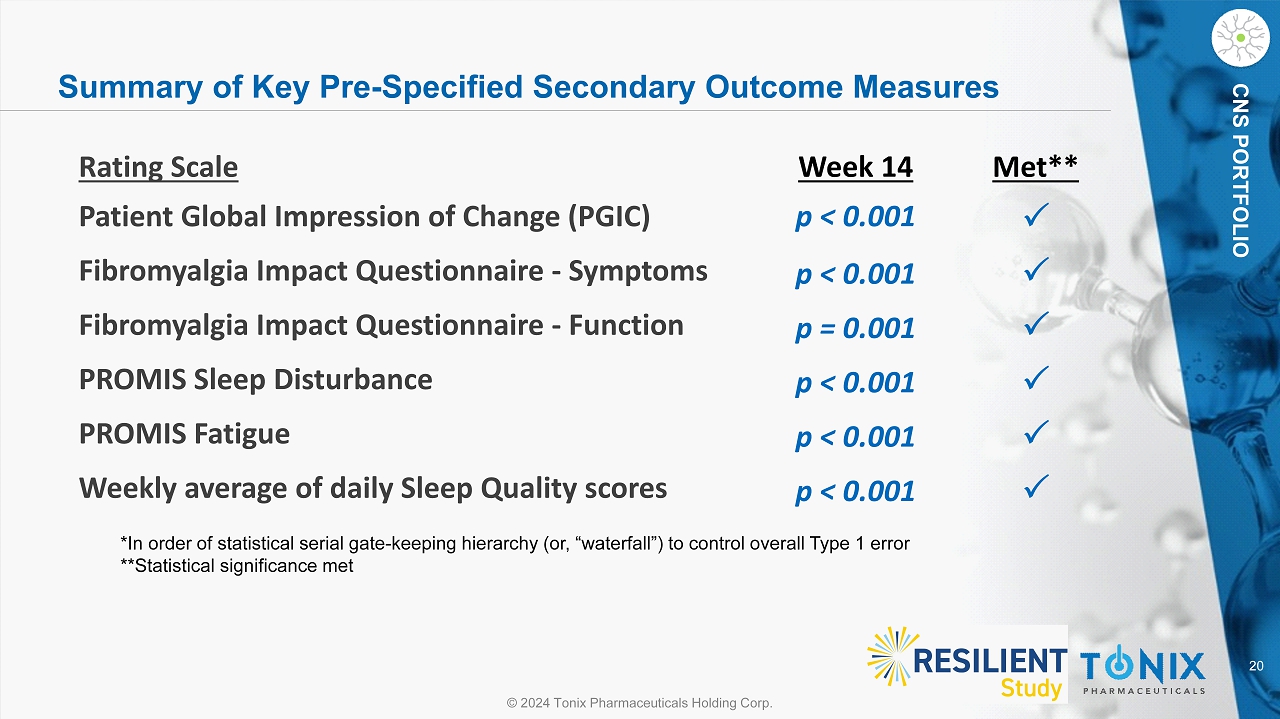
20 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Summary of Key Pre - Specified Secondary Outcome Measures *In order of statistical serial gate - keeping hierarchy (or, “waterfall”) to control overall Type 1 error **Statistical significance met Rating Scale Week 14 Met** Patient Global Impression of Change (PGIC) p < 0.001 Fibromyalgia Impact Questionnaire - Symptoms p < 0.001 Fibromyalgia Impact Questionnaire - Function p = 0.001 PROMIS Sleep Disturbance p < 0.001 PROMIS Fatigue p < 0.001 Weekly average of daily Sleep Quality scores p < 0.001
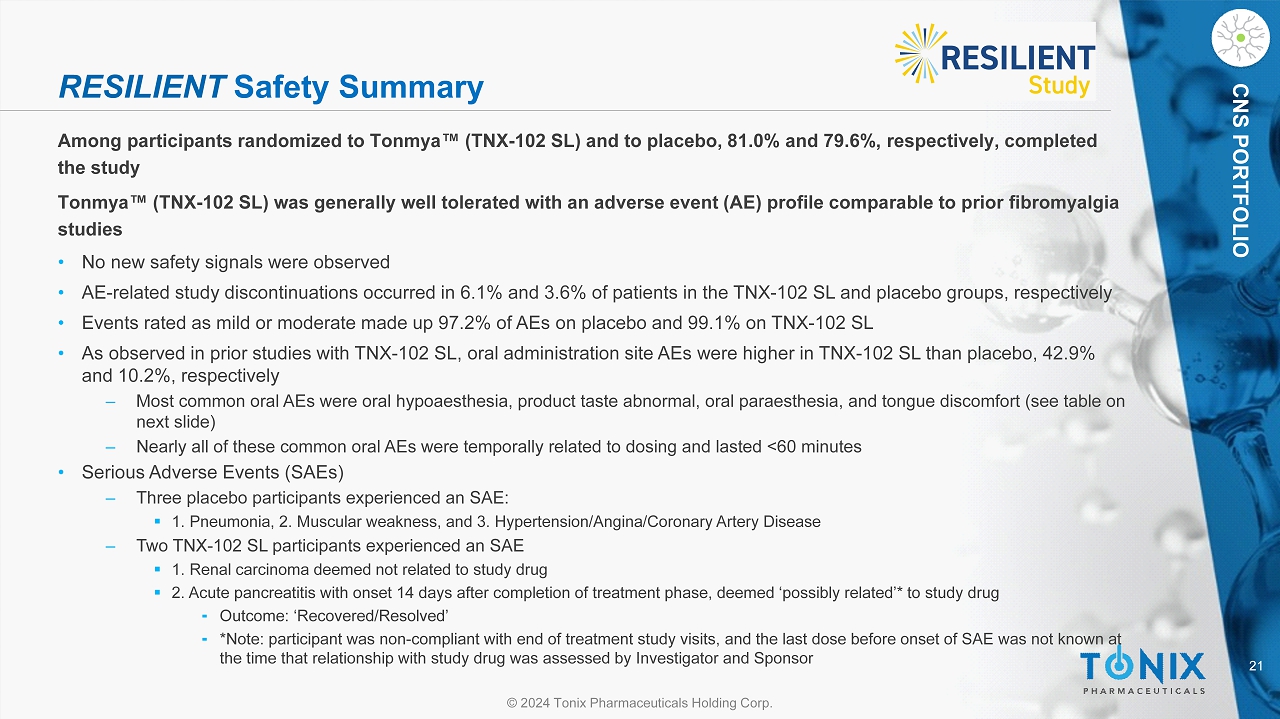
21 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO RESILIENT Safety Summary Among participants randomized to Tonmya Œ (TNX - 102 SL) and to placebo, 81.0% and 79.6%, respectively, completed the study Tonmya Œ (TNX - 102 SL) was generally well tolerated with an adverse event (AE) profile comparable to prior fibromyalgia studies • No new safety signals were observed • AE - related study discontinuations occurred in 6.1% and 3.6% of patients in the TNX - 102 SL and placebo groups, respectively • Events rated as mild or moderate made up 97.2% of AEs on placebo and 99.1% on TNX - 102 SL • As observed in prior studies with TNX - 102 SL, oral administration site AEs were higher in TNX - 102 SL than placebo, 42.9% and 10.2%, respectively ‒ Most common oral AEs were oral hypoaesthesia, product taste abnormal, oral paraesthesia, and tongue discomfort (see table on next slide) ‒ Nearly all of these common oral AEs were temporally related to dosing and lasted <60 minutes • Serious Adverse Events (SAEs) ‒ Three placebo participants experienced an SAE: ▪ 1. Pneumonia, 2. Muscular weakness, and 3. Hypertension/Angina/Coronary Artery Disease ‒ Two TNX - 102 SL participants experienced an SAE ▪ 1. Renal carcinoma deemed not related to study drug ▪ 2. Acute pancreatitis with onset 14 days after completion of treatment phase, deemed ‘possibly related’* to study drug ⁃ Outcome: ‘Recovered/Resolved’ ⁃ *Note: participant was non - compliant with end of treatment study visits, and the last dose before onset of SAE was not known at the time that relationship with study drug was assessed by Investigator and Sponsor
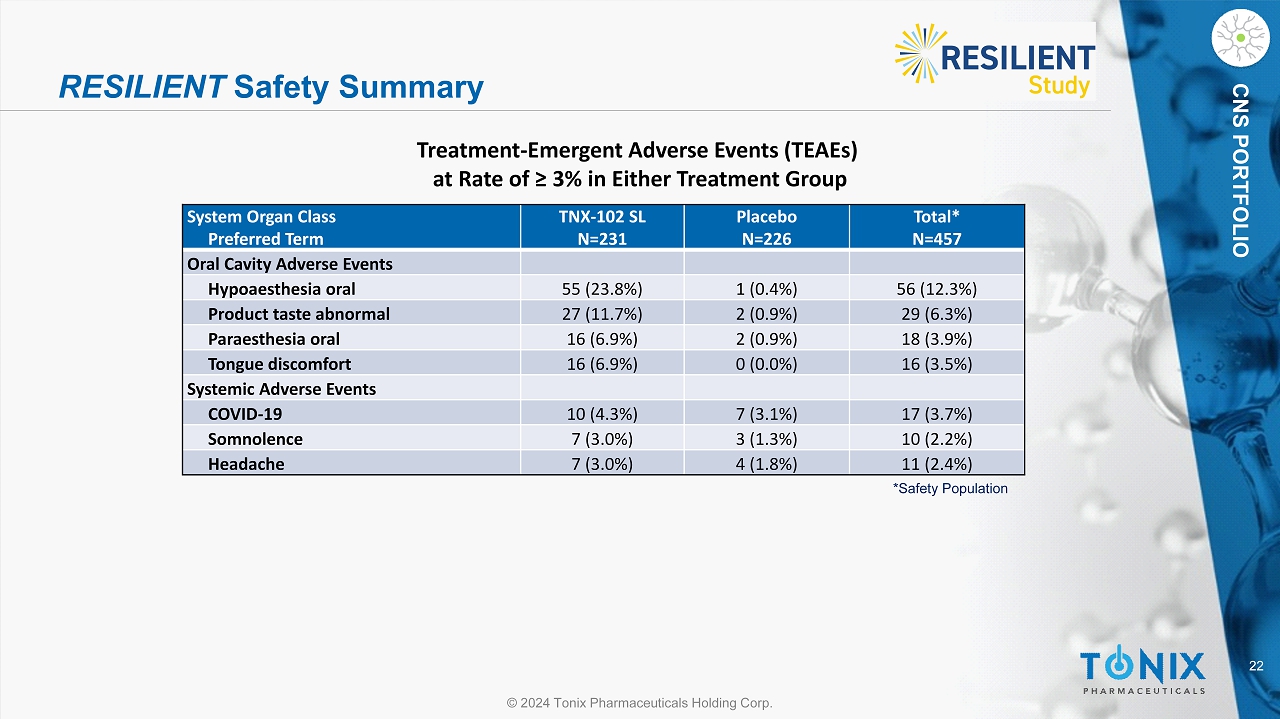
22 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO RESILIENT Safety Summary System Organ Class Preferred Term TNX - 102 SL N=231 Placebo N=226 Total* N=457 Oral Cavity Adverse Events Hypoaesthesia oral 55 (23.8%) 1 (0.4%) 56 (12.3%) Product taste abnormal 27 (11.7%) 2 (0.9%) 29 (6.3%) Paraesthesia oral 16 (6.9%) 2 (0.9%) 18 (3.9%) Tongue discomfort 16 (6.9%) 0 (0.0%) 16 (3.5%) Systemic Adverse Events COVID - 19 10 (4.3%) 7 (3.1%) 17 (3.7%) Somnolence 7 (3.0%) 3 (1.3%) 10 (2.2%) Headache 7 (3.0%) 4 (1.8%) 11 (2.4%) *Safety Population Treatment - Emergent Adverse Events (TEAEs) at Rate of ≥ 3% in Either Treatment Group

23 © 2024 Tonix Pharmaceuticals Holding Corp. PROFILE DEVELOPMENT PROGRAM Patents Issued CNS PORTFOLIO Tonmya Œ (TNX - 102 SL): Fibromyalgia Cyclobenzaprine Protectic ® Sublingual Tablets Fibromyalgia (FM) is a chronic pain disorder resulting from amplified sensory and pain signaling within the CNS • Afflicts an estimated 6 - 12 million adults in the U.S., the majority of whom are women 1 • Symptoms include chronic widespread pain, nonrestorative sleep, fatigue, and cognitive dysfunction • Patients struggle with daily activities, have impaired quality of life, and frequently are disabled • Physicians and patients report common dissatisfaction with currently marketed products Market Entry: Fibromyalgia Additional Indications: Fibromyalgia - type Long COVID, Acute Stress Disorder (ASD), PTSD, Agitation in Alzheimer’s, Alcohol Use Disorder Status: One Positive Phase 3 study RELIEF completed, p - value = 0.01 2 Second Phase 3 study RALLY missed primary endpoint Confirmatory Phase 3 study RESILIENT positive, p - value = 0.00005 Next Steps: Pre - NDA meeting with FDA *TNX - 102 SL has not been approved for any indication. 1 American Chronic Pain Association (www.theacpa.org, 2019) 2 Lederman et al., (2023) Arthritis Care & Research "Efficacy and Safety of TNX - 102 SL (Sublingual Cyclobenzaprine) for the Treatment of Fibromyalgia: Results From the RELIEF Trial ", doi : 10.1002/acr.25142. Epub ahead of print. PMID: 37165930. When the check engine light malfunctions, the light is on even though the car is not malfunctioning
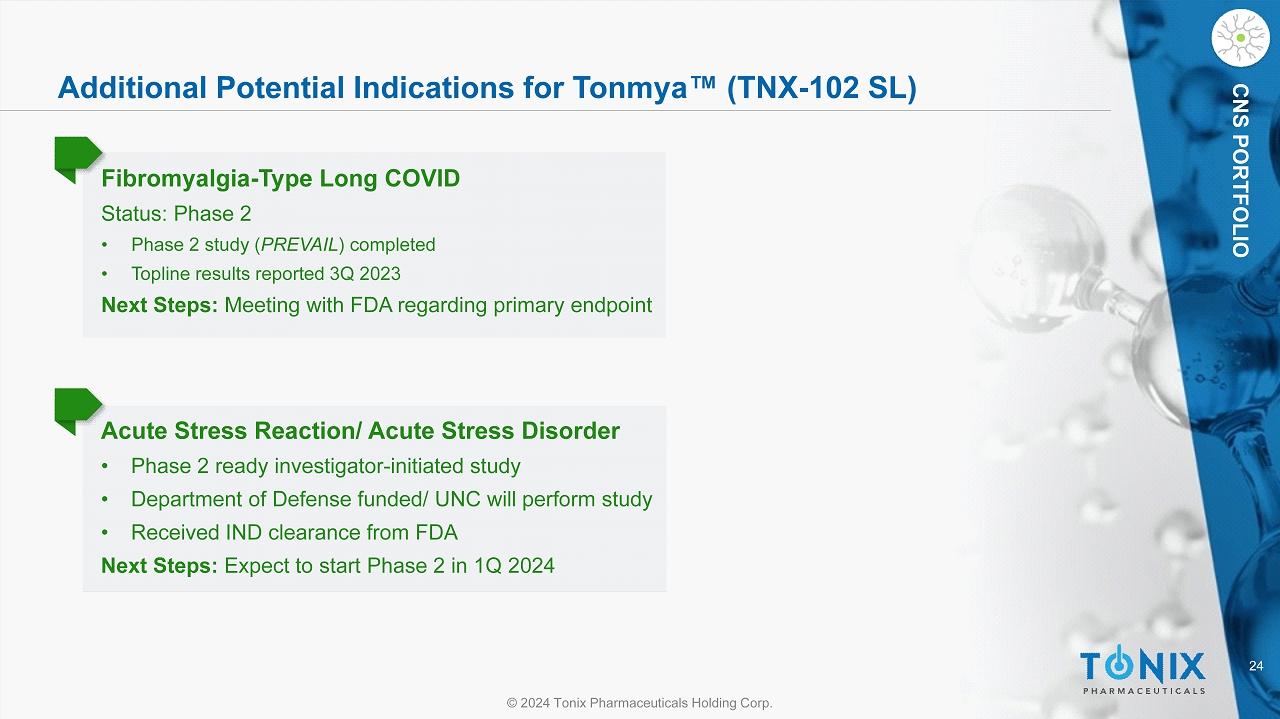
24 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Additional Potential Indications for Tonmya Œ (TNX - 102 SL) Fibromyalgia - Type Long COVID Status: Phase 2 • Phase 2 study ( PREVAIL ) completed • Topline results reported 3Q 2023 Next Steps: Meeting with FDA regarding primary endpoint Acute Stress Reaction/ Acute Stress Disorder • Phase 2 ready investigator - initiated study • Department of Defense funded/ UNC will perform study • Received IND clearance from FDA Next Steps: Expect to start Phase 2 in 1Q 2024
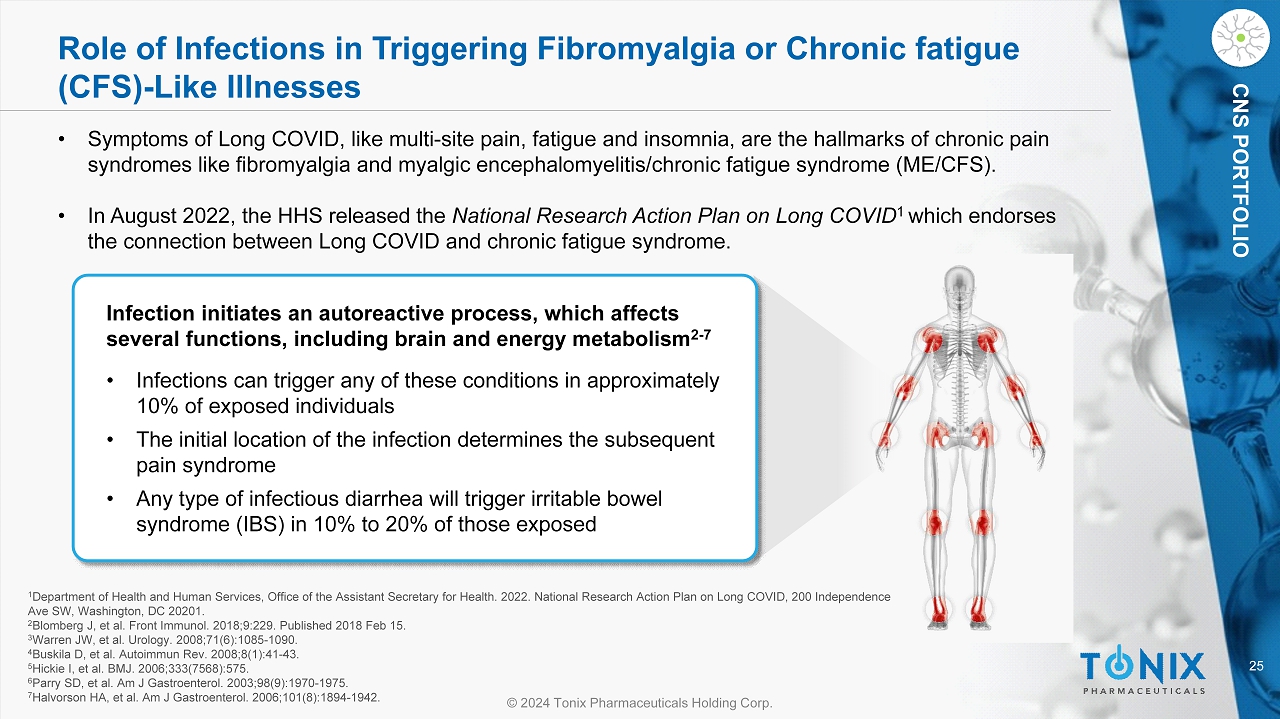
25 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Role of Infections in Triggering Fibromyalgia or Chronic fatigue (CFS) - Like Illnesses Infection initiates an autoreactive process, which affects several functions, including brain and energy metabolism 2 - 7 • Infections can trigger any of these conditions in approximately 10% of exposed individuals • The initial location of the infection determines the subsequent pain syndrome • Any type of infectious diarrhea will trigger irritable bowel syndrome (IBS) in 10% to 20% of those exposed 1 Department of Health and Human Services, Office of the Assistant Secretary for Health. 2022. National Research Action Plan on Lo ng COVID, 200 Independence Ave SW, Washington, DC 20201. 2 Blomberg J, et al. Front Immunol. 2018;9:229. Published 2018 Feb 15. 3 Warren JW, et al. Urology. 2008;71(6):1085 - 1090. 4 Buskila D, et al. Autoimmun Rev. 2008;8(1):41 - 43. 5 Hickie I, et al. BMJ. 2006;333(7568):575. 6 Parry SD, et al. Am J Gastroenterol. 2003;98(9):1970 - 1975. 7 Halvorson HA, et al. Am J Gastroenterol. 2006;101(8):1894 - 1942. • Symptoms of Long COVID, like multi - site pain, fatigue and insomnia, are the hallmarks of chronic pain syndromes like fibromyalgia and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). • In August 2022, the HHS released the National Research Action Plan on Long COVID 1 which endorses the connection between Long COVID and chronic fatigue syndrome.
26 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO About Fibromyalgia - Type Long COVID Many Long - COVID symptoms overlap with core symptoms of fibromyalgia and are hallmarks of other chronic pain syndromes like myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) Multisite pain Memory issues Fatigue Sleep disturbances 19% Long COVID occurs in approximately 19% of recovered COVID - 19 patients 2 40 % As many as 40% of Long COVID patients experience multi - site pain 3,4 1 CDC - https://www.cdc.gov/coronavirus/2019 - ncov/long - term - effects/index.html#:~:text=Some%20people%20who%20have%20been,after%20acute%2 0COVID%2D19%20infection . 2 CDC Press Release, June 22, 2022 - https://www.cdc.gov/nchs/pressroom/nchs_press_releases/2022/20220622.htm 3 Harris, H, et al. Tonix data on file. 2022 4 TriNetX Analytics Long COVID is broadly defined as signs, symptoms, and conditions that continue or develop after acute COVID - 19 infection 1
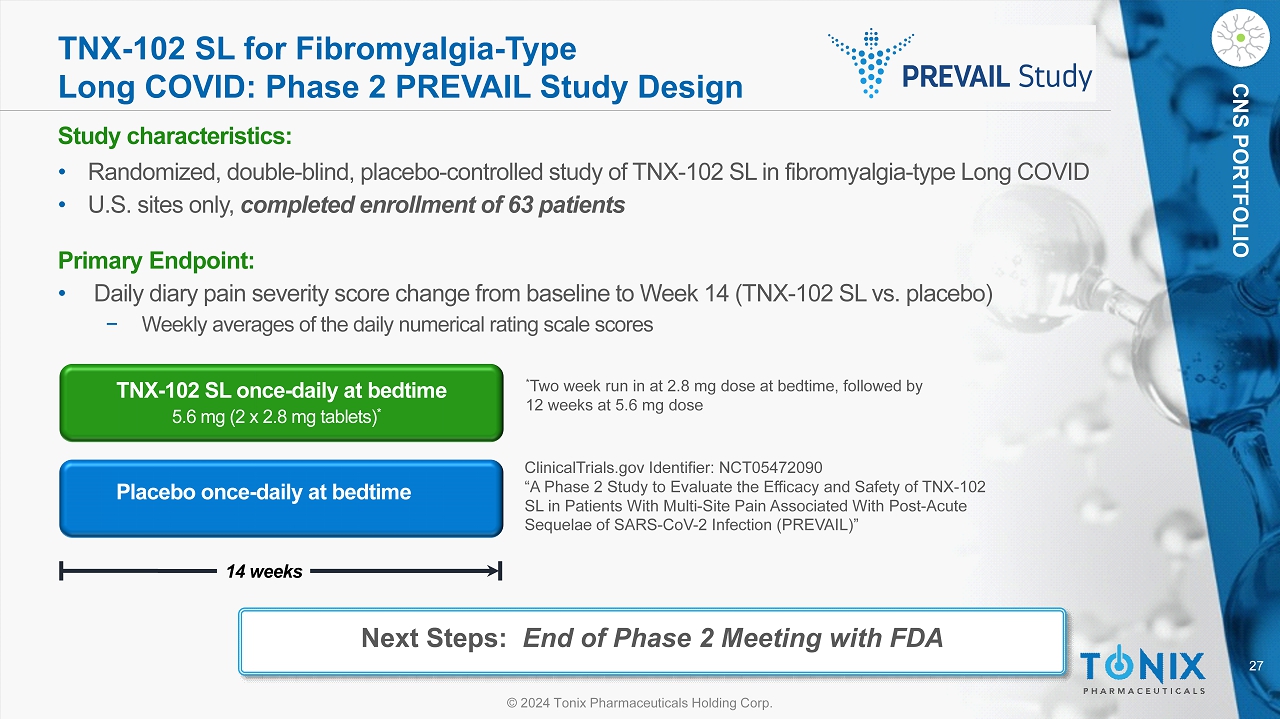
27 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 102 SL for Fibromyalgia - Type Long COVID: Phase 2 PREVAIL Study Design Study c haracteristics: • Randomized, double - blind, placebo - controlle d study of TNX - 102 SL in fibromyalgia - type Long COVID • U.S. sites only, completed enrollment of 63 patients Primary Endpoint: • Daily diary pain severity score change from baseline to Week 14 (TNX - 102 SL vs. placebo) − Weekly averages of the daily numerical rating scale scores Placebo once - daily at bedtime 14 weeks TNX - 102 SL once - daily at bedtime 5.6 mg (2 x 2.8 mg tablets) * * Two week run in at 2.8 mg dose at bedtime, followed by 12 weeks at 5.6 mg dose ClinicalTrials.gov Identifier: NCT05472090 “A Phase 2 Study to Evaluate the Efficacy and Safety of TNX - 102 SL in Patients With Multi - Site Pain Associated With Post - Acute Sequelae of SARS - CoV - 2 Infection (PREVAIL)” Next Steps: End of Phase 2 Meeting with FDA
28 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 102 SL: Phase 2 PREVAIL Topline Results 1 D id not meet the primary endpoint of multi - site pain reduction at W eek 14 However, f indings fulfill the objectives of proof - of - concept study, supporting the decision to advance the program b ased on a proposed primary endpoint using the PROMIS Fatigue scale • TNX - 102 SL showed robust effect size in improving fatigue and consistent activity across secondary measures of sleep quality, cognitive function, disability and Patient Global Impression of Change (PGIC) • Was g enerally well tolerated with an adverse event (AE) profile comparable to prior studies with TNX - 102 SL: ‒ AE - related discontinuations were similar in drug and placebo arms ‒ No new safety signals were observed Fatigue is the signature symptom of Long COVID and has been identified as the dominant symptom contributing to disability 2 • W e observed numerical improvement in the PROMIS fatigue score (in RELIEF p= 0.007 MMRM and in RALLY p= 0.007 MMRM) in both prior Phase 3 studies of TNX - 102 SL in fibromyalgia, • W e believe the results of PREVAIL, toge ther with extensive data from studies in other chronic conditions 3 - 5 , makes PROMIS Fatigue a solid candidate for the primary endpoint of future Long COVID registrational studies 1 Tonix Press Release, September 5, 2023 - https://bit.ly/3Z6FQHQ 2 Walker S, et al . BMJ Open 2023;13:e069217. doi:10.1136/ bmjopen - 2022 - 069217 3 Cook, K.F., et al. 2016. Journal of Clinical Epidemiology , 73, 89 - 102 4 Cella, D., et al. 2016. Journal of Clinical Epidemiology , 73, 128 – 134 5 Lai, J.S., et al. 2011. Archives of Physical Medicine and Rehabilitation , 92(10 Supplement), S20 - S27.

29 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO CNS PORTFOLIO Acute Stress Reaction (ASR) / Acute Stress Disorder (ASD) ASR/ASD are acute stress conditions resulting from trauma which c an affect both civilian and military populations. Large unmet need: • According to National Center for PTSD, about 60% of men and 50% of women in the US are exposed least one traumatic experience in their lives 1 • In the US alone, one - third of emergency department visits (40 - 50 million patients per year) are for evaluation after trauma exposures 2 Current standard of care: • No medications are currently available at or near the point of care to treat patients suffering from acute traumatic events and support long - term health 1 National Center for PTSD. How Common is PTSD in Adults? https://www.ptsd.va.gov/understand/common/common_adults.asp 2 Wisco et al. J Clin Psychiatry . 2014.75(12):1338 - 46
30 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 102 SL for ASR/ASD: Program Status Status: Expect to start Phase 2 in 1Q 2024 ; received IND clearance from FDA Phase 2 Trial Funded by DoD grant to University of North Carolina (UNC) • UNC Institute for Trauma Recovery awarded a $3M grant from the Department of Defense (DoD) • OASIS trial will build upon infrastructure developed through the UNC - led, $40M AURORA initiative ‒ AURORA study is a major national research initiative to improve the understanding, prevention, and recovery of individuals who have experienced a traumatic event ‒ Supported in part by funding from the National Institutes of Health (NIH) and the health care arm of Google’s parent company Alphabet • Opportunity to investigate the correlation between motor vehicle collisions and the emergence of ASD and PTSD • Supported by multiple clinical trials: • Phase 2 trial in military - related PTSD ( AtEase or NCT02277704) • Phase 3 trial in military - related PTSD (HONOR or NCT03062540) • Phase 3 trial in primarily civilian PTSD (RECOVERY or NCT03841773) • In each of these studies, early and sustained improvements in sleep were associated with TNX - 102 SL treatment by the PROMIS sleep disturbance (SD) scale and the Clinician Administered PTSD Scale (CAPS - 5) “sleep disturbance” item. Together these studies provide preliminary evidence that TNX - 102 SL is well - tolerated and may promote recovery from PTSD via a pharmacodynamic facilitation of sleep - dependent emotional memory processing

31 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 102 SL for ASR/ASD: Phase 2 OASIS Study Design General s tudy c haracteristics: • Randomized, double - blind, placebo - controlle d study in Acute Stress Reaction (ASR) / Acute Stress Disorder (ASD) • The proposed O ptimizing A cute S tress reaction I nterventions with TNX - 102 S L (OASIS) trial will examine the safety and efficacy of TNX - 102 SL to reduce adverse posttraumatic neuropsychiatric sequelae among patients presenting to the emergency department after a motor ve hicle collision (MVC) • The trial will enroll approximately 180 individuals who acutely experienced trauma at study sites across the US • Participants will be randomized in the emergency department to receive a two - week course of either TNX - 102 SL or placebo • Investigator - initiated IND Objective: • Investigate the potential of Tonix’s TNX - 102 SL (cyclobenzaprine HCl sublingual tablets) to reduce the frequency and severity of the adverse effects of traumatic exposure, including acute stress reaction (ASR), acute stress disorder (ASD), and posttraumatic stress d iso rder (PTSD). • ASR refers to the body’s immediate response to trauma, whereas ASD is the short - term effects of trauma (within 1 month), and PTS D is the long - term effects of trauma (beyond 1 month) * First dose of TNX - 102 SL 5.6 mg versus placebo taken in the emergency department, and then daily at bedtime to finish 2 weeks of treatment A Phase 2 Study to Evaluate the Efficacy and Safety of TNX - 102 SL Taken Daily in Patients With ASR/ ASD (OASIS) • Primary outcome measure: Acute Stress Disorder Scale (ASDS) assessed at 7 and 21 days post MVC • Posttraumatic stress symptom severity assessed at 6 and 12 weeks post MVC using the PTSD Checklist for DSM - 5 (PCL - 5) • Standardized survey instruments of sleep disturbances, anxiety and depression symptoms, general physical and mental health, and clinical global improvement also employed • Detailed and brief neurocognitive assessments are performed from baseline to 12 weeks after MVC at specific timepoints throughout study participation period Placebo once - daily at bedtime 2 weeks TNX - 102 SL once - daily at bedtime 5.6 mg (2 x 2.8 mg tablets) *
32 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Tonmya Œ (TNX - 102 SL): Patents and Patent Applications • U.S. Composition:* • A 75:25 cyclobenzaprine HCl - mannitol eutectic (dependent claims add a basifying agent). • 5 US Patents (Expire November 2034) • 1 Pending US Application (Would expire November 2034) • A composition of a cyclobenzaprine HCl and a basifying agent suitable for sublingual absorption. • 1 Pending US Application (Would e xpire June 2033) • U.S. Methods of Use* (Specific Indications): • Fibromyalgia • Pain, Sleep Disturbance, Fatigue • 1 Pending US Application (Would e xpire December 2041) • Early Onset Response • 1 Pending US Provisional Application (Would e xpire December 2044) • Depressive Symptoms • 1 Pending US Application (Would e xpire March 2032) • Sexual Dysfunction • 1 Pending US Application (Would expire October 2041) • PASC • 1 Pending US Application (Would e xpire June 2043) • PTSD • 1 US Patent ( Expires November 2030) • Agitation (Dementia) • 1 US Patent ( Expires December 2038) • 1 Pending US Application (Would expire December 2038) • Alcohol Use Disorder • 1 Pending US Application (Would e xpire November 2041) • Foreign Filings • Corresponding foreign patents have been filed and some have issued: • Composition (25 patents, 3 allowed applications, 16 pending applications) • Methods of Use (9 patents, 54 pending applications) *US Patents: Issued: US Patent Nos. 9,636,408; 9,956,188; 10,117,936; 10,864,175; 11,839,594; 9,918,948; 11,826,321. Pending: US Patent Application Nos. 13/918,692; 18/385,468; 13/412,571; 18/265,525; 63/612,352; 18/382,262; 18/037,815; 17/226,058; 18/212,500.

© 2024 Tonix Pharmaceuticals Holding Corp. TONIX MEDICINES: MARKETED PRODUCTS

34 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Tonix Medicines: Commercial - Stage Specialty Pharma Subsidiary • Tonix Medicines is a w holly - owned subsidiary of Tonix ( NASDAQ: TNXP) • Currently marketing two products indicated for the treatment of acute migraine: Zembrace ® SymTouch ® and Tosymra ® • ~16 M in net sales 1 • Nascent commercial organization • Tonix Medicines is led by James (Jim) Hunter • Veteran pharma executive with a track record for growing early businesses • Hunter previously founded Validus with Tonix CEO, Dr. Lederman • Tonix Medicines is preparing to launch Tonmya Œ (TNX - 102 SL) for fibromyalgia • Fibromyalgia care is relatively concentrated to specialized providers • We believe prescribing physicians can be targeted effectively by a specialty sales force • Evolving landscape in commercial markets favors distribution channels such as specialty pharmacies 1 Tonix 10 - Q for 3Q23
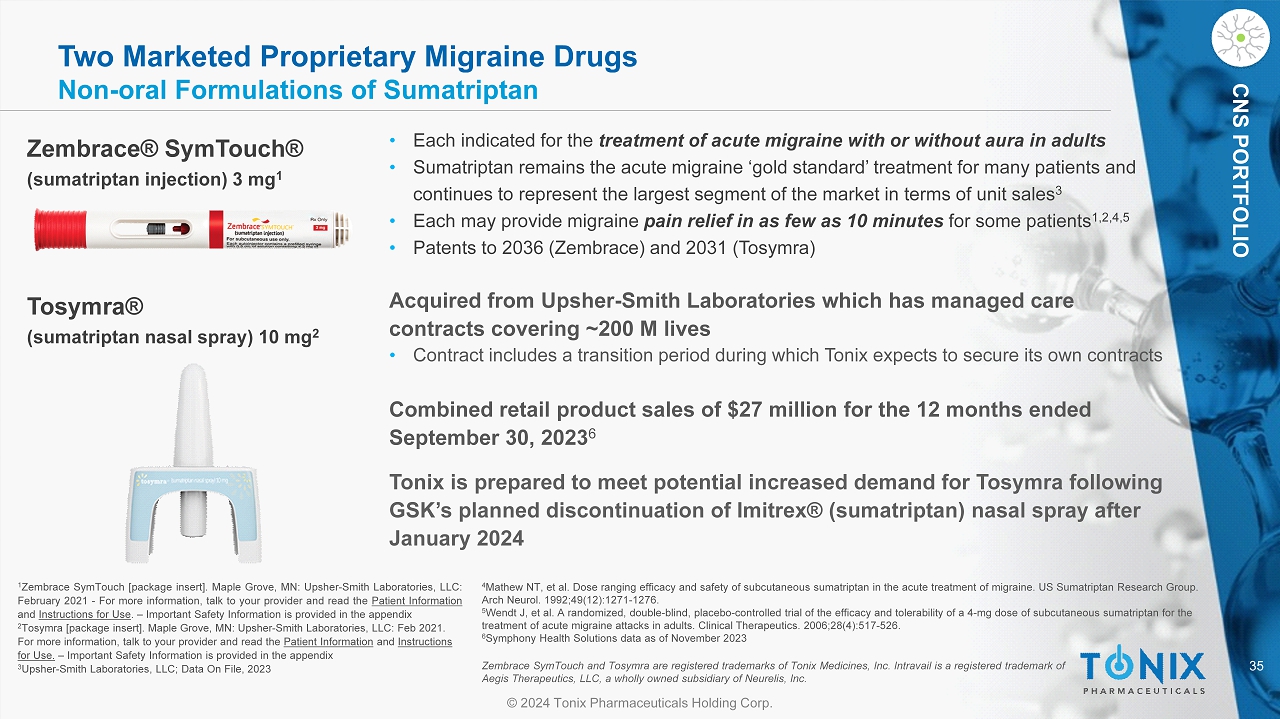
35 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Two Marketed Proprietary Migraine Drugs Non - oral Formulations of Sumatriptan • Each indicated for the tr eatment of acute migraine with or without aura in adults • Sumatriptan remains the acute migraine ‘gold standard’ treatment for many patients and continues to represent the largest segment of the market in terms of unit sales 3 • Each may provide migraine pain relief in as few as 10 minutes for some patients 1,2,4,5 • Patents to 2036 ( Zembrace ) and 2031 ( Tosymra ) 1 Zembrace SymTouch [package insert] . Maple Grove, MN : Upsher - Smith Laboratories, LLC : February 2021 - For more information, talk to your provider and read the Patient Information and Instructions for Use . – Important Safety Information is provided in the appendix 2 Tosymra [package insert]. Maple Grove, MN: Upsher - Smith Laboratories, LLC: Feb 2021. For more information, talk to your provider and read the Patient Information and Instructions for Use. – Important Safety Information is provided in the appendix 3 Upsher - Smith Laboratories, LLC; Data On File, 2023 Zembrace® SymTouch ® (sumatriptan injection) 3 mg 1 Tosymra® (sumatriptan nasal spray) 10 mg 2 Acquired from Upsher - Smith Laboratories which has managed care contracts covering ~200 M lives • Contract includes a transition period during which Tonix expects to secure its own contracts Combined retail product sales of $27 million for the 12 months ended September 30, 2023 6 Tonix is prepared to meet potential increased demand for Tosymra following GSK’s planned discontinuation of Imitrex® (sumatriptan) nasal spray after January 2024 4 Mathew NT, et al. Dose ranging efficacy and safety of subcutaneous sumatriptan in the acute treatment of migraine. US Sumatri pta n Research Group. Arch Neurol. 1992;49(12):1271 - 1276. 5 Wendt J, et al. A randomized, double - blind, placebo - controlled trial of the efficacy and tolerability of a 4 - mg dose of subcutan eous sumatriptan for the treatment of acute migraine attacks in adults. Clinical Therapeutics. 2006;28(4):517 - 526. 6 Symphony Health Solutions data as of November 2023 Zembrace SymTouch and Tosymra are registered trademarks of Tonix Medicines, Inc. Intravail is a registered trademark of Aegis Therapeutics, LLC, a wholly owned subsidiary of Neurelis , Inc.
36 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Zembrace and Tosymra Bypass the GI Tract Bypassing gastrointestinal (GI) tract is potential advantage for treating acute migraine • GI absorption may be inconsistent in migraineurs due to gastric stasis (also called “gastroparesis”) 1 - 4 • Nausea and vomiting are symptoms of migraine 5 which can complicate oral treatment Existing intranasal products • Imitrex® nasal spray (sumatriptan) • Migranal ® (dihydroergotamine) nasal spray – developed by Novartis, sold by Bausch Health New intranasal products bringing attention to non - oral route • Pfizer’s Zavzpret ® ( zavegepant ), FDA approved in March, 2023 1 is the first intranasal gepant • Impel NeuroPharma’s Trudhesa ® (dihydroergotamine) FDA approved 2021 2 1 Pfizer Press Release March 10, 2023. – https://www.pfizer.com/news/press - release/press - release - detail/pfizers - zavzprettm - zavegepant - migraine - nasal - spray 2 Impel Press Release September 3, 2021 - https://impelpharma.com/2021/09/03/impel - neuropharma - announces - u - s - fda - approval - of - trudhesa - dihydroergotamine - mesylate - nasal - spr ay - for - the - acute - treatment - of - migraine/

37 © 2024 Tonix Pharmaceuticals Holding Corp. Pipeline Programs and Strategy for Partnerships

38 © 2024 Tonix Pharmaceuticals Holding Corp. Pipeline Development Strategy Focusing on government and academic collaborations • Validates Tonix’s scientific expertise and technology • Reduces internal spend • Increases number of trials • Potentially speeds tim e to market • Grants, contracts, cost - sharing or “in - kind” arrangements

39 © 2024 Tonix Pharmaceuticals Holding Corp. External Partnerships Government partners providing direct funding, cost sharing or in - kind support include: • National Institutes of Health (NIH) • National Institute of Allergy and Infectious Disease (NIAID) ▪ TNX - 1800 selected for Project NextGen • National Institute on Drug Abuse (NIDA) ▪ TNX - 1300 for cocaine intoxication; Phase 2 study funding • Department of Defense (DoD) ▪ TNX - 102 SL for ASD; Phase 2 study funding Academic partners sponsoring clinical trials of Tonix’s investigational drug products include: • Massachusetts General Hospital (MGH) • University of Washington • University of North Carolina

40 © 2024 Tonix Pharmaceuticals Holding Corp. TNX - 1300: COCAINE INTOXICATION Key Partnerships TNX - 1500: ALLOGRAFT REJECTION TNX - 1800 : COVID - 19 VACCINE TNX - 2900: PRADER - WILLI SYNDROME TNX - 102 SL: ACUTE STRESS DISORDER

41 © 2024 Tonix Pharmaceuticals Holding Corp. TNX - 2900 Intranasal Potentiated Oxytocin with Magnesium A n ovel, non - CGRP antagonist approach to treatment
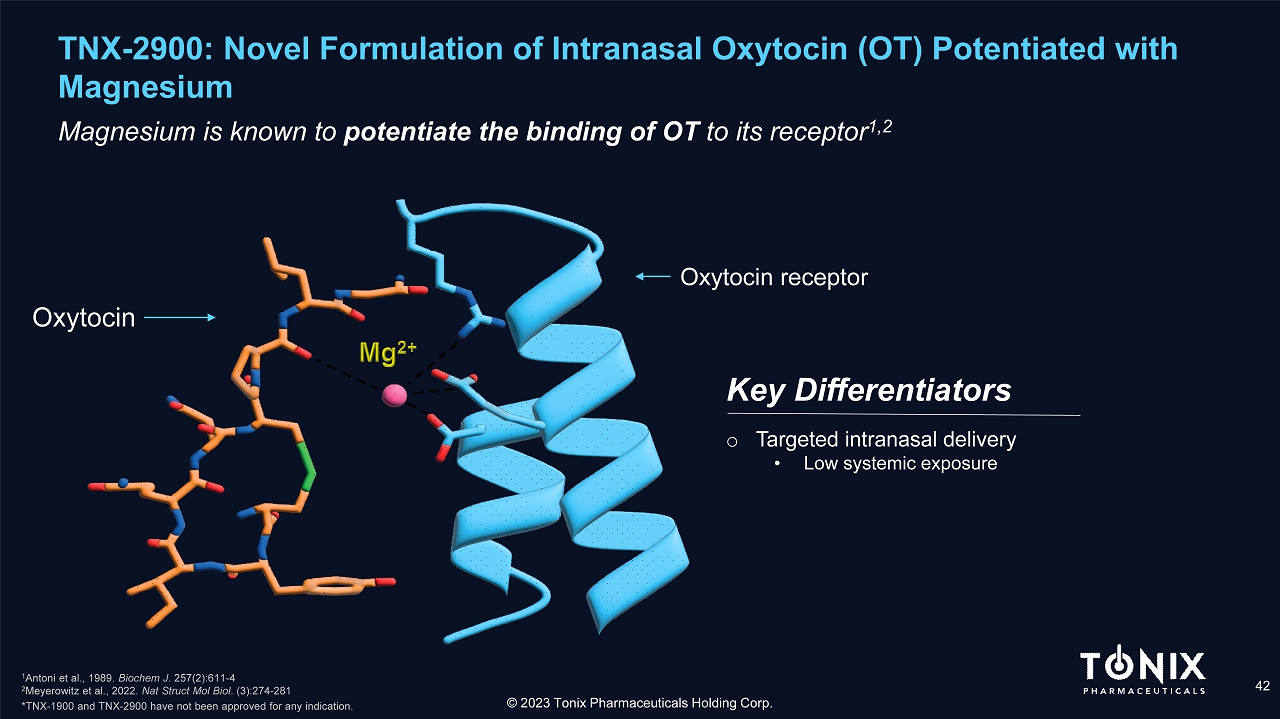
TNX - 2900: Novel Formulation of Intranasal Oxytocin (OT) Potentiated with Magnesium 42 Magnesium is known to potentiate the binding of OT to its receptor 1 ,2 Key Differentiators © 2023 Tonix Pharmaceuticals Holding Corp. Oxytocin receptor Oxytocin o Targeted intranasal delivery • Low systemic exposure 1 Antoni et al., 1989. Biochem J . 257(2):611 - 4 2 Meyerowitz et al., 2022. Nat Struct Mol Biol . (3):274 - 281 *TNX - 1900 and TNX - 2900 have not been approved for any indication.
43 © 2024 Tonix Pharmaceuticals Holding Corp. RARE DISEASE PORTFOLIO Rare genetic diseas e that afflicts 10 - 20 thousand individuals in the US About Prader - Willi Syndrome Prader - Willi Syndrome (PWS) is the most common genetic cause of life - threatening childhood obesity. PWS causes unhealthy behaviors around food 1 - 4 , c onsequences such as obesity, type 2 diabetes, and cardiovascular disease 1 - 5 , and creates significant caretaker burden 1 - 4 10 - 20 thousand individuals *TNX - 2900 has been granted FDA Orphan Drug Designation and received IND clearance by FDA for Phase 2 Trial 1 Miller et al., 2011. Am J Med Genet A . 1 55A(5):1040 - 1049 2 Butler et al., 2017. Genet Med. 19(6):635 - 642 3 Butler MG. NORD. Updated 2018. Accessed May 25, 2022. https://rarediseases.org/rare - diseases/prader - willi - syndrome/ 4 Prader - Willi Syndrome Association USA. Accessed May 25, 2022. https://www.pwsausa.org/what - is - prader - willi - syndrome/ 5 Muscogiuri et al., 2021. J Endocrinol Invest . 44(10):2057 - 2070 Current standard of care: • Human growth hormone treatment is FDA - approved for growth failure in PWS children Large unmet need: • Currently no cure, and no treatment for PWS - related hyperphagia • Consequences can be life threatening - obesity and cardiovascular disease are leading cause of death

44 © 2024 Tonix Pharmaceuticals Holding Corp. TNX - 1300 Cocaine Esterase Fast acting antidote for life threatening cocaine intoxication
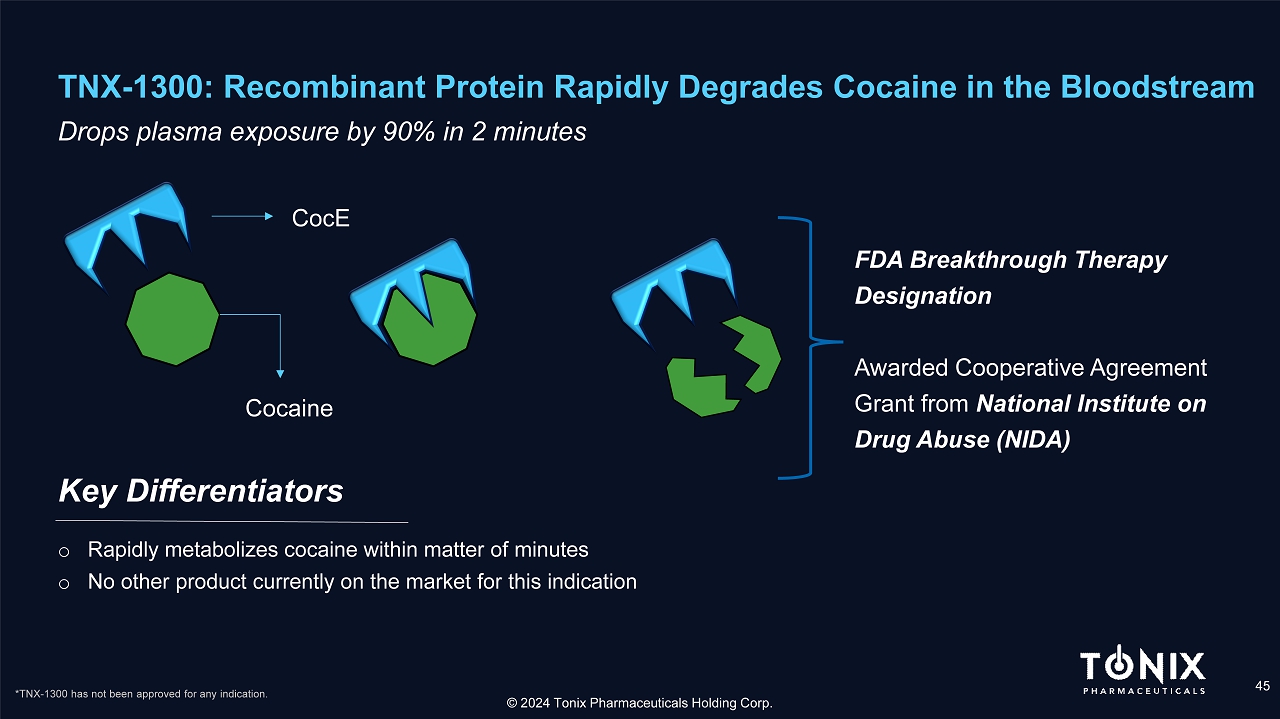
TNX - 1300: Recombinant Protein Rapidly Degrades Cocaine in the Bloodstream 45 Drops plasma exposure by 90% in 2 minutes Key Differentiators © 2024 Tonix Pharmaceuticals Holding Corp. o Rapidly metabolizes cocaine within matter of minutes o N o other product currently on the market for this indication CocE Cocaine FDA Breakthrough Therapy Designation Awarded Cooperative Agreement Grant from National Institute on Drug Abuse (NIDA) *TNX - 1300 has not been approved for any indication.
46 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO CNS PORTFOLIO Over 500,000 emergency department visits for cocaine, annually 3,4 About Cocaine Intoxication Over 5 million Americans reported current cocaine use in 2020, which is almost 2% of the population 1 . In 2021, more than 24,900 individuals in the US died from drug overdose deaths involving cocaine 2 500k 1 Substance Abuse and Mental Health Services Administration. (2021). Results from the 2020 National Survey on Drug Use and Health: Detailed Tables: Prevalence Estimates, Standard Errors, and Sample Sizes. 2 Centers for Disease Control and Prevention (CDC) - https://www.cdc.gov/nchs/nvss/vsrr/drug - overdose - data.htm 3 Substance Mental Health Services Administration, Drug Abuse Warning Network, 2011: National Estimates of Drug - Related Emergency Department Visits. HHS Publication No. (SMA) 13 - 4760, DAWN Series D - 39. Rockville, MD: Substance Abuse and Mental Health Services Administration, 2013. 4 Drug Abuse Warning Network, 2011: Selected Tables of National Estimates of Drug - Related Emergency Department Visits. Rockville, MD: Center for Behavioral Health Statistics and Quality, SAMHSA, 2013. Current standard of care: • Patients are currently managed only by supportive care for the adverse effects of cocaine intoxication on the cardiovascular and central nervous systems Large unmet need: • N o other product currently on the market for this indication • TNX - 1300 could significantly reduce the time and resources required for other detox services • Potentially r educes the risk of morbidity and mortality

© 2024 Tonix Pharmaceuticals Holding Corp. IMMUNOLOGY: KEY CANDIDATES

48 © 2024 Tonix Pharmaceuticals Holding Corp. TNX - 1500 Anti - CD40L Monoclonal Antibody Next Generation mAb preserves efficacy without risk of thrombosis
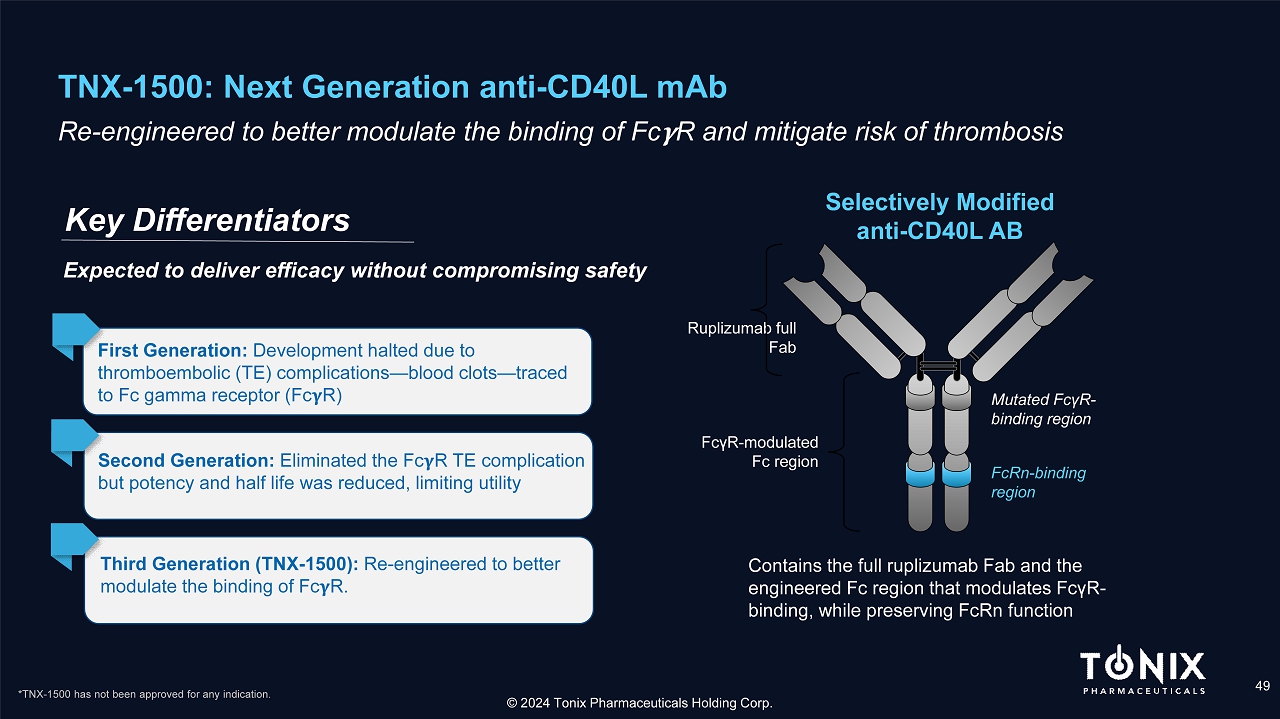
TNX - 1500: Next Generation anti - CD40L mAb 49 Re - engineered to better modulate the binding of Fc R and mitigate risk of thrombosis Key Differentiators © 2024 Tonix Pharmaceuticals Holding Corp. Selectively Modified anti - CD40L AB Ruplizumab full Fab Contains the full ruplizumab Fab and the engineered Fc region that modulates Fc γ R - binding, while preserving FcRn function Mutated Fc γ R - binding region FcRn - binding region Fc γ R - modulated Fc region First Generation: Development halted due to thromboembolic (TE) complications — blood clots — traced to Fc gamma receptor (Fc R) Second Generation: Eliminated the Fc R TE complication but potency and half life was reduced, limiting utility Third Generation (TNX - 1500): Re - engineered to better modulate the binding of Fc R. Expected to deliver efficacy without compromising safety *TNX - 1500 has not been approved for any indication.
50 © 2024 Tonix Pharmaceuticals Holding Corp. IMMUNOLOGY PORTFOLIO TNX - 1500 Strategy and Status Third Indication (and beyond): Autoimmune Diseases ( e.g., Multiple Sclerosis, Sj ö gen’s Syndrome, Systemic Lupus Erythematosus) • These indications require large studies, but represent large target markets Proposed Initial Indication: Prevention of Allograft Rejection Status: Phase 1 enrollment and dosing complete; data readout expected 3Q’24 • Collaborations ongoing with Mass General Hospital on heart and kidney transplantation in non - human primates • Collaboration with Boston Children’s on bone marrow transplantation in non - human primates Next Steps: Initiate Phase 2 study in Kidney Transplant Recipients 1 2 Second Indication: Hematopoietic Cell Transplant (Bone Marrow Transplant) • Potential to reduce GvHD 3 Current ly e xploring strategic partnerships and out - licensing opportunities

51 © 2024 Tonix Pharmaceuticals Holding Corp. IMMUNOLOGY PORTFOLIO TNX - 1500 Preclinical Data and Publications Non - human Primate Kidney Allo - Transplantation • TNX - 1500 monotherapy consistently prevents kidney transplant rejection with n o thrombosis observed • April 2023 Publication: Lassiter, G., et al. (2023). TNX - 1500, an Fc - modified Anti - CD154 Antibody, Prolongs Nonhuman Primate Renal Allograft Survival. American Journal of Transplantation . www.sciencedirect.com/science/article/pii/S1600613523003714 Non - human Primate Heart Heterotopic Allo - Transplantation • TNX - 1500 monotherapy consistently prevents heart transplant rejection. Similar activity to chimeric hu5c8 2 during treatment phase in prior studies • April 2023 Publication: Miura, S., et al. (2023) TNX - 1500, an Fc - modified Anti - CD154 Antibody, Prolongs Nonhuman Primate Cardiac Allograft Survival. American Journal of Transplantation. www.sciencedirect.com/science/article/pii/S1600613523003969 Non - Human Primate Kidney Xenograft Transplantation • TNX - 1500 therapy is part of a regiment to prevent rejection in kidney xenograft transplants ‒ Anand, R.P., Layer, J.V., Heja , D. et al. (2023). Design and testing of a humanized porcine donor for xenotransplantation. Nature. https://www.nature.com/articles/s41586 - 023 - 06594 - 4 ‒ Kozlov, M. (2023). Monkey survives two years after gene - edited pig - kidney transplant. Nature. https://www.nature.com/articles/d41586 - 023 - 03176 - 2 ‒ Mohiuddin, M. (2023). Pig - to - primate organ transplants require genetic modifications of donor. Nature. https://www.nature.com/articles/d41586 - 023 - 02817 - w

52 © 2024 Tonix Pharmaceuticals Holding Corp. TNX - 1700 Recombinant Trefoil Factor Family Member 2 (rTFF2 - HSA) Fusion Protein Targeting the toxic tumor micro - environment
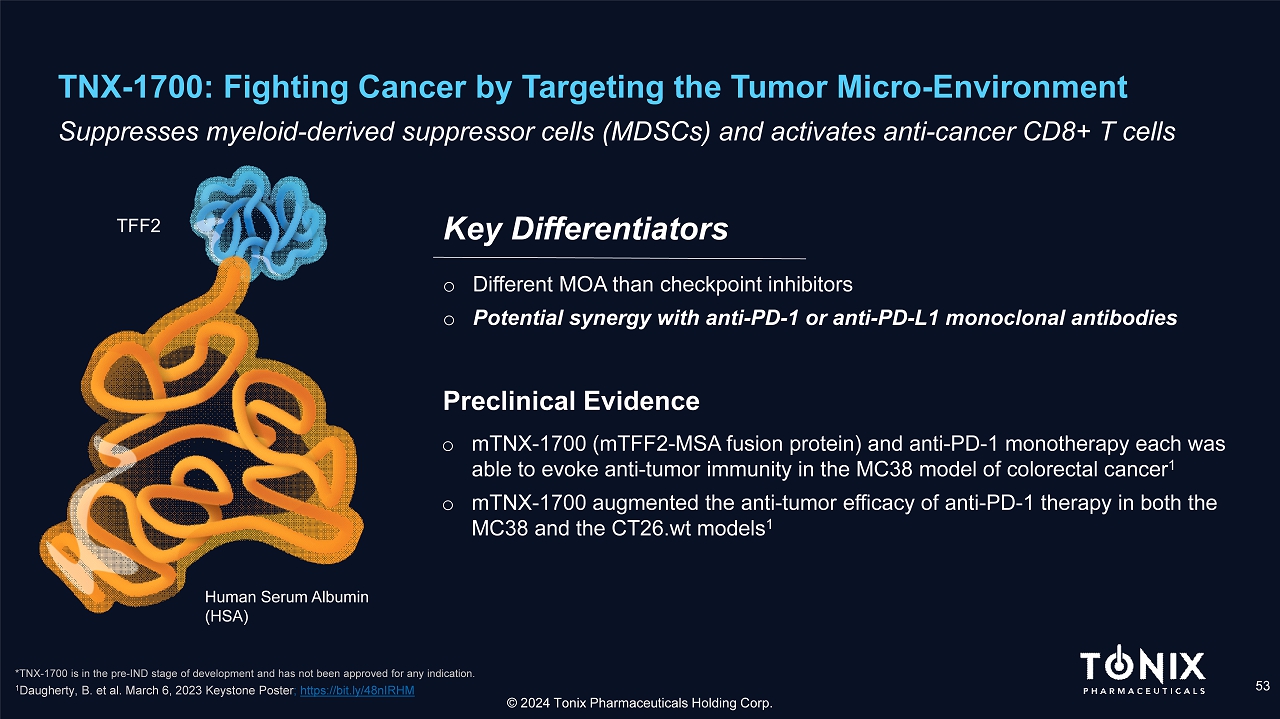
TNX - 1700: Fighting Cancer by Targeting the Tumor Micro - Environment 53 S uppresses myeloid - derived suppressor cells (MDSCs) and activates anti - cancer CD8+ T cells o Different MOA than checkpoint inhibitors o Potential synergy with anti - PD - 1 or anti - PD - L1 monoclonal antibodies Key Differentiators © 2024 Tonix Pharmaceuticals Holding Corp. *TNX - 1700 is in the pre - IND stage of development and has not been approved for any indication. Human Serum Albumin (HSA) TFF2 o mTNX - 1700 (mTFF2 - MSA fusion protein) and anti - PD - 1 monotherapy each was able to evoke anti - tumor immunity in the MC38 model of colorectal cancer 1 o mTNX - 1700 augmented the anti - tumor efficacy of anti - PD - 1 therapy in both the MC38 and the CT26.wt models 1 Prec linical Evidence 1 Daugherty, B. et al. March 6, 2023 Keystone Poster ; https://bit.ly/48nIRHM
54 © 2024 Tonix Pharmaceuticals Holding Corp. IMMUNOLOGY PORTFOLIO People living with colorectal cancer in the US 2 About Gastric and Colorectal Cancer Gastric and colorectal cancer are both leading cancers in the US. C olorectal cancer is the 3 rd leading cause of cancer - related deaths in both men and women. 1 >1.3M Current standard of care: • PD - 1 blockade − However, gastric and colorectal cancer are relatively unresponsive Large unmet need: • Gastric and colorectal cancer have a relative 5 - year survival rate of 35.7% and 65%, respectively − Despite advances in the field, patients are still in need of life saving treatment 1 American Cancer Society, accessed September 2023 - https://www.cancer.org/cancer/types/colon - rectal - cancer/about/key - statistics.html 2 NIH, accessed September 2023 - https://seer.cancer.gov/statfacts/html/colorect.html 3 NIH, accessed September 2023 - https://seer.cancer.gov/statfacts/html/stomach.html >125k People living with gastric cancer in the US 3

© 2024 Tonix Pharmaceuticals Holding Corp. INFECTIOUS DISEASE: KEY CANDIDATES

56 © 2024 Tonix Pharmaceuticals Holding Corp. INFECTIOUS DISEASE PORTFOLIO Internal Development & Manufacturing Capabilities R&D Center (RDC): Frederick, MD • Research advancing CNS and immunology drugs • Accelerated development of vaccines and antiviral drugs against infectious diseases • ~48,000 square feet, BSL - 2 with some areas designated BSL - 3 Advanced Development Center (ADC): North Dartmouth, MA • Development and clinical scale manufacturing of biologics • ~45,000 square feet, BSL - 2

57 © 2024 Tonix Pharmaceuticals Holding Corp. INFECTIOUS DISEASE PORTFOLIO Broad - Spectrum Antiviral Discovery Programs Host - directed antiviral discovery programs CD45 targeted therapeutics • Small molecule therapeutics that reduce endogenous levels of CD45, a protein tyrosine phosphatase • Reduction in CD45 protects against many viruses including the Ebola virus Cathepsin inhibitors • Small molecule therapeutics that inhibit essential cathepsins which are required by viruses such as coronaviruses and filoviruses to infect cells • Activity as monotherapy and in combination with other antivirals Virus - directed antivirals discovery program Viral glycan - targeted engineered biologics • B ind to viral densely branched high - mannose (DBH) glycans • Neutralize circulating virus and stop the entry of the progeny virus into cells • Antiviral activity against a broad range of RNA viruses • Activity as monotherapy and in combination with other antivirals

58 © 2024 Tonix Pharmaceuticals Holding Corp. TNX - 801 Live Virus Vaccine Live virus vaccine platform with multitude of potential applications
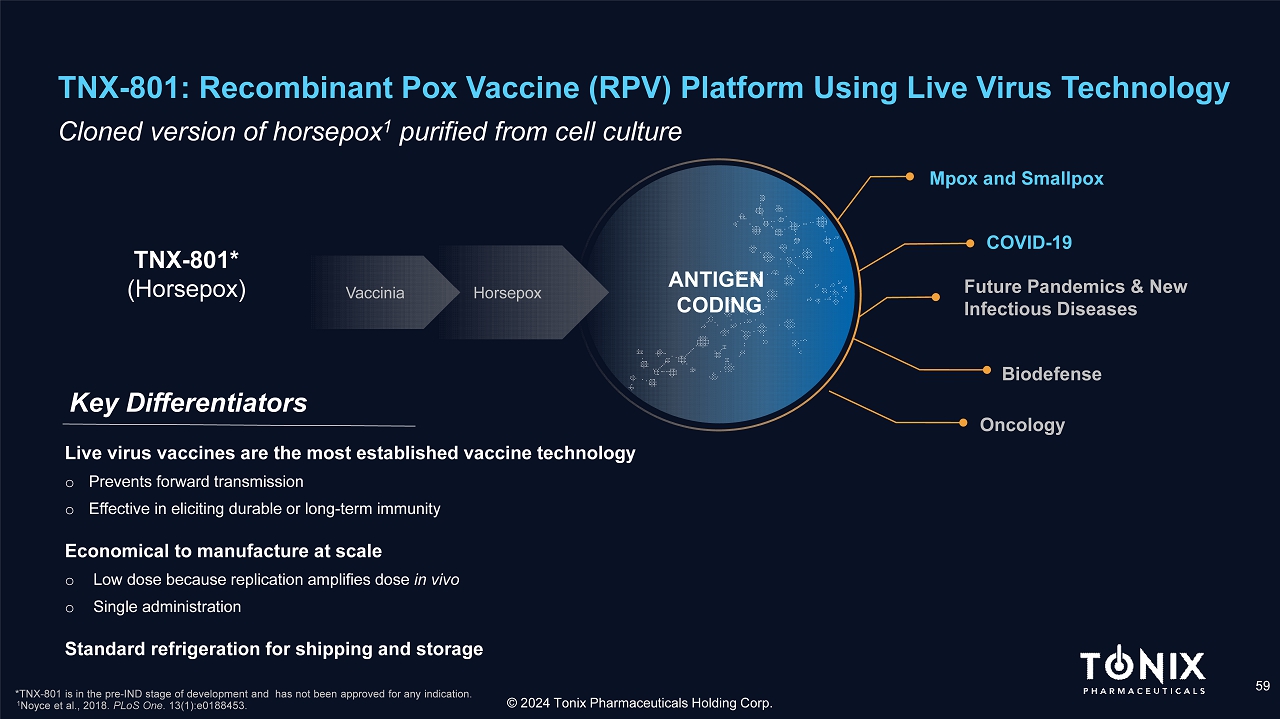
TNX - 801: Recombinant Pox Vaccine (RPV) Platform Using Live Virus Technology 59 C loned version of horsepox 1 purified from cell culture Key Differentiators © 2024 Tonix Pharmaceuticals Holding Corp. TNX - 801* (Horsepox) *TNX - 801 is in the pre - IND stage of development and has not been approved for any indication. 1 Noyce et al., 2018. PLoS One . 13(1):e0188453. Live virus vaccines are the most established vaccine technology o Prevents forward transmission o Effective in eliciting durable or long - term immunity Economical to manufacture at scale o Low dose because replication amplifies dose in vivo o Single administration Standard refrigeration for shipping and storage Mpox and Smallpox Future Pandemics & New Infectious Diseases COVID - 19 Biodefense Vaccinia Horsepox Oncology ANTIGEN CODING
60 © 2024 Tonix Pharmaceuticals Holding Corp. INFECTIOUS DISEASE PORTFOLIO TNX - 1800: Designed to Express the SARs - CoV - 2 Spike Protein TNX - 1800 (recombinant horsepox virus) is a live virus vaccine based on Tonix’s TNX - 801 that is designed to express the spike protein of the SARS - CoV - 2 virus and to elicit a predominant T cell response • I mmunogenic and well tolerated 1 • S howed promise in protecting animals from challenge with SARS - CoV - 2 delivered directly into the lungs 1 Status: National Institute of Allergy and Infectious Diseases (NIAID) will conduct a Phase 1 clinical trial with TNX - 1800 • First vaccine candidate using Tonix’s live virus recombinant pox virus (RPV) platform technology to enter clinical trials • “Project NextGen” is an initiative by the U.S. Department of Health and Human Services (HHS) to advance a pipeline of new, innovative vaccines and therapeutics for COVID - 19. NIAID will be conducting clinical trials to evaluate several early - stage vaccine candidates, including TNX - 1800 • Phase 1 study is designed to assess safety and immunogenicity in approximately 60 healthy adult volunteers • Upon completion of the trial, NIAID and Tonix will assess the results and determine the next steps for the development of TNX - 1800 1 Awasthi, M. et al. Viruses . 2023. 15(10):2131. 2 Awasthi, M. et al. BioRxiv . 2023.

© 2024 Tonix Pharmaceuticals Holding Corp. TEAM, NETWORK, & UPCOMING MILESTONES

62 © 2024 Tonix Pharmaceuticals Holding Corp. Management Team Seth Lederman, MD Co - Founder, CEO & Chairman Jessica Morris Chief Operating Officer Gregory Sullivan, MD Chief Medical Officer Bradley Saenger, CPA Chief Financial Officer

63 © 2023 Tonix Pharmaceuticals Holding Corp. Milestones: Recently Completed and Upcoming □ 4 th Quarter 2023 Positive t opline results of Phase 3 RESILIENT study for Tonmya Œ for the management of fibromyalgia x Upcoming Milestones □ 1 st Quarter 2024 Initiate Phase 2 study of TNX - 102 SL for acute stress disorder □ 1 st Quarter 2024 Initiate Phase 2 study of TNX - 1300 for the treatment of cocaine intoxication □ 1 st Half 2024 Pre - NDA meeting with FDA for Tonmya Œ for fibromyalgia □ 3 rd Quarter 2024 Results of Phase 1 study of TNX - 1500 □ 2 nd Half 2024 Submit NDA to FDA for Tonmya Œ for fibromyalgia

© 2024 Tonix Pharmaceuticals Holding Corp. THANK YOU

65 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Zembrace ® Important Safety Information (1 of 2) Zembrace SymTouch ( Zembrace ) can cause serious side effects, including heart attack and other heart problems, which may lead to death. Stop use and get emergency help if you have any signs of a heart attack: D iscomfort in the center of your chest that lasts for more than a few minutes or goes away and comes back; severe tightness, pain, pressure, or heaviness in your chest, throat, neck, or jaw; pain or discomfort in your arms, back, neck, jaw or stomach ; shortness of breath with or without chest discomfort ; breaking out in a cold sweat ; nausea or vomiting ; feeling lightheaded Zembrace is not for people with risk factors for heart disease (high blood pressure or cholesterol, smoking, overweight, diabetes, family history of heart disease) unless a heart exam shows no problem. Do not use Zembrace if you have: H istory of heart problems ; narrowing of blood vessels to your legs, arms, stomach, or kidney (peripheral vascular disease) ; uncontrolled high blood pressure ; hemiplegic or basilar migraines. If you are not sure if you have these, ask your provider. H ad a stroke, transient ischemic attacks (TIAs), or problems with blood circulation ; severe liver problems ; taken any of the following medicines in the last 24 hours: almotriptan , eletriptan , frovatriptan , naratriptan, rizatriptan, ergotamines , dihydroergotamine ; are taking certain antidepressants, known as monoamine oxidase (MAO) - A inhibitors or it has been 2 weeks or less since you stopped taking a MAO - A inhibitor. Ask your provider for a list of these medicines if you are not sure. A n allergy to sumatriptan or any of the components of Zembrace Tell your provider about all of your medical conditions and medicines you take, including vitamins and supplements. Zembrace can cause dizziness, weakness, or drowsiness. If so, do not drive a car, use machinery, or do anything where you need to be alert.

66 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Zembrace ® Important Safety Information (2 of 2) Zembrace may cause serious side effects including: • Changes in color or sensation in your fingers and toes; sudden or severe stomach pain, stomach pain after meals, weight loss, na usea or vomiting, constipation or diarrhea, bloody diarrhea, fever; cramping and pain in your legs or hips; feeling of heaviness or t igh tness in your leg muscles; burning or aching pain in your feet or toes while resting; numbness, tingling, or weakness in your legs; cold fe eli ng or color changes in one or both legs or feet; increased blood pressure including a sudden severe increase even if you have no history of high blood pressure; medication overuse headaches from using migraine medicine for 10 or more days each month. If your headaches g et worse, call your provider. • Serotonin syndrome, a rare but serious problem that can happen in people using Zembrace , especially when used with anti - depressant medicines called SSRIs or SNRIs. Call your provider right away if you have: mental changes such as seeing things that are not th ere (hallucinations), agitation, or coma; fast heartbeat; changes in blood pressure; high body temperature; tight muscles; or tro ubl e walking. • Hives (itchy bumps); swelling of your tongue, mouth, or throat • Seizures even in people who have never had seizures before The most common side effects of Zembrace include: pain and redness at injection site; tingling or numbness in your fingers or toes; dizziness; warm, hot, burning feeling to your face (flushing); discomfort or stiffness in your neck; feeling weak, drowsy, or ti red. Tell your provider if you have any side effect that bothers you or does not go away. These are not all the possible side effe cts of Zembrace . For more information, ask your provider. This is the most important information to know about Zembrace but is not comprehensive. For more information, talk to your provider and read the Patient Information and Instructions for Use . You can also visit www.upsher - smith.com or call 1 - 888 - 650 - 3789. For full Prescribing Information, visit: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=6e5b104f - 2b9e - 416e - 92fb - ef1bdaea867d You are encouraged to report adverse effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1 - 800 - FDA - 1088. Zembrace is a prescription medicine used to treat acute migraine headaches with or without aura in adults who have been diagnosed with migraine. Zembrace is not used to prevent migraines. It is not known if it is safe and effective in children under 18 years of age.

67 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Tosymra® Important Safety Information (1 of 2) Tosymra® can cause serious side effects, including heart attack and other heart problems, which may lead to death. Stop Tosymra and get emergency medical help if you have any signs of heart attack: • D iscomfort in the center of your chest that lasts for more than a few minutes or goes away and comes back ; severe tightness, pain, pressure, or heaviness in your chest, throat, neck, or jaw ; pain or discomfort in your arms, back, neck, jaw, or stomach ; shortness of breath with or without chest discomfort; breaking out in a cold sweat ; nausea or vomiting ; feeling lightheaded Tosymra is not for people with risk factors for heart disease (high blood pressure or cholesterol, smoking, overweight, diabetes, family history of heart disease) unless a heart exam is done and shows no problem. Do not use Tosymra if you have: • H istory of heart problems ; narrowing of blood vessels to your legs, arms, stomach, or kidney (peripheral vascular disease) ; uncontrolled high blood pressure ; severe liver problems ; hemiplegic or basilar migraines. If you are not sure if you have these, ask your healthcare provider. • H ad a stroke, transient ischemic attacks (TIAs), or problems with blood circulation ; taken any of the following medicines in the last 24 hours: almotriptan , eletriptan , frovatriptan , naratriptan, rizatriptan, ergotamines , or dihydroergotamine. Ask your provider if you are not sure if your medicine is listed above • are taking certain antidepressants, known as monoamine oxidase (MAO) - A inhibitors or it has been 2 weeks or less since you stopped taking a MAO - A inhibitor . A sk your provider for a list of these medicines if you are not sure • A n allergy to sumatriptan or any ingredient in Tosymra Tell your provider about all of your medical conditions and medicines you take, including vitamins and supplements. Tosymra can cause dizziness, weakness, or drowsiness. If so, do not drive a car, use machinery, or do anything where you need to be alert.

68 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Tosymra may cause serious side effects including: • Changes in color or sensation in your fingers and toes; sudden or severe stomach pain, stomach pain after meals, weight loss, nausea or vomiting, constipation or diarrhea, bloody diarrhea, fever; cramping and pain in your legs or hips, feeling of heaviness or tightness in your leg muscles, burning or aching pain in your feet or toes while resting, numbness, tingling, or weakness in your legs, cold feeling or color changes in one or both legs or feet; increased blood pressure including a sudden severe increase even if you have no history of high blood pressure; medication overuse headaches from using migraine medicine for 10 or more days each month. If your headaches get worse, call your provider . • Serotonin syndrome, a rare but serious problem that can happen in people using Tosymra, especially when used with anti - depressant medicines called SSRIs or SNRIs. Call your provider right away if you have : mental changes such as seeing things that are not there (hallucinations), agitation, or coma; fast heartbeat; changes in blood pressure; high body temperature; tight muscles; or trouble walking. • Seizures even in people who have never had seizures before The most common side effects of Tosymra include : tingling, dizziness, feeling warm or hot, burning feeling, feeling of heaviness, feeling of pressure, flushing, feeling of tightness, numbness, application site (nasal) reactions, abnormal taste, and throat irritation. Tell your provider if you have any side effect that bothers you or does not go away. These are not all the possible side effects of Tosymra. For more information, ask your provider. This is the most important information to know about Tosymra but is not comprehensive. For more information, talk to your provider and read the Patient Information and Instructions for Use . You can also visit www.upsher - smith.com or call 1 - 888 - 650 - 3789. For full Prescribing Information, visit: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=015a5cf9 - f246 - 48bc - b91e - cd730a53d8aa You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch , or call 1 - 800 - FDA - 1088. Tosymra is a prescription medicine used to treat acute migraine headaches with or without aura in adults. Tosymra is not used to treat other types of headaches such as hemiplegic or basilar migraines or cluster headaches. Tosymra is not used to prevent migraines. It is not known if Tosymra is safe and effective in children under 18 years of age. Tosymra ® Important Safety Information (2 of 2)