
TONIX PHARMACEUTICALS HOLDING CORP. 8-K
EXHIBIT 99.02

© 2024 Tonix Pharmaceuticals Holding Corp. Presented by Seth Lederman, MD at American Society of Clinical Psychopharmacology Annual Meeting, Miami, FL May 29, 2024 Effects of Bedtime TNX - 102 SL (Sublingual Cyclobenzaprine HCl) on Mood and Anxiety Symptoms in Fibromyalgia: Results of the Phase 3 RESILIENT Trial

© 2024 Tonix Pharmaceuticals Holding Corp. 2 Disclosures • Authors: Gregory Sullivan 1 , David Hsu 1 , Mary Kelley 1 , Ben Vaughn 2 , Jean Engels 1 and Seth Lederman 1 • 1 Employees of Tonix Pharmaceuticals Holding Corp. and own stock and stock options in the company • 2 Employee of Rho, Inc. • TNX - 102 SL is an investigational new drug and is not approved for any indication
© 2024 Tonix Pharmaceuticals Holding Corp. 3 Characteristics of Fibromyalgia • Symptoms include chronic widespread pain , nonrestorative sleep , fatigue , and cognitive dysfunction • Afflicts an estimated 6 - 12 million US adults, majority are women • Those with FM struggle with daily activities, have impaired quality of life (QoL), and are frequently disabled 1 • Main correlates for QoL in FM, explaining 56% variance, do not include pain intensity or frequency; rather, greatest correlates are depression (standardized β = - 0.26), pain - related interference with everyday life ( β = - 0.19), general activity ( β =0.13), cognitive difficulties ( β = - 0.12) 2 • Conclusion: mood symptoms have big impact on QoL in FM • Physicians and patients report common dissatisfaction with currently marketed products 1 Robinson RL, et al. Burden of illness and treatment patterns for patients with fibromyalgia. Pain Medicine 2012; 13(10):1366 - 76 2 Offenbaecher M, et al. Pain is not the major determinant of quality of life in fibromyalgia. Rheumatology International 2021; 41:1995 – 2006
© 2024 Tonix Pharmaceuticals Holding Corp. 4 2016 Fibromyalgia (FM) Criteria* • How is FM currently diagnosed? By assessing pain distribution and other core symptoms of FM: • Widespread pain index (WPI): number of areas (out of 19) with pain ( WPI range 0 - 19 ) • Symptom severity scale (SSS) score calculated by: • Over past week , for following 3 symptoms rate severity 0 - 3 each (SSS subscore 0 - 9) • Fatigue (0 - 3) • Waking unrefreshed (0 - 3) • Cognitive symptoms (0 - 3) • Over previous 6 months , bothered by the following 3 symptoms (SSS subscore 0 - 3) • Headaches (0 - 1) • Pain or cramps in lower abdomen (0 - 1) • Depression (0 - 1) • Add two subscores for total ( SSS score range 0 - 12 ) • To make the diagnosis, need: • WPI ≥ 7 and SSS score ≥ 5 OR WPI of 4 - 6 and SSS score ≥ 9 • Generalized pain, defined as pain in 4 of 5 body regions^ • Symptoms generally present for at least 3 months ^pain in body areas of jaw, chest, abdomen do not contribute to meeting generalized pain definition *Wolfe F, et al. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin Arthritis Rheum. 2016; 46(3):319 - 329.

© 2024 Tonix Pharmaceuticals Holding Corp. 5 Fibromyalgia: Nonrestorative Sleep and Cyclobenzaprine • Non - restorative sleep 1,2 • Harvey Moldofsky – recognition of unrefreshing/non - restorative sleep: • Symptom • P otential causative or potentiating factor • Cyclobenzaprine 3,9 • Potentially the earliest drug studied in fibromyalgia as an oral swallowed agent • Studies showed equivocal effects and tolerability issues at “muscle spasm” doses • Bedtime, low - dose cyclobenzaprine targeting non - restorative sleep 10 - 11 • Recognition of unrefreshing sleep as a target of therapy • Primitive oral, swallowed formulation – “flat” pharmacokinetics • Bedtime, sublingual transmucosal cyclobenzaprine targeting non - restorative sleep 12 • Dynamic pharmacokinetic profile, rapid absorption, decrease in major metabolite, norcyclobenzaprine • Two studies (Phase 2 and Phase 3) at 2.8 mg; three Phase 3 studies at 5.6 mg. 1 Moldofsky H et al. Psychosom Med. 1975. 37:341 - 51. 2 Moldofsky H and Scarisbrick P. Psychosom Med. 1976. 38:35 - 44. 3 Bennett RM, et al. Arthritis Rheum 1988 . 31 : 1535 – 42 . 4 Quimby LG, et al . J Rheumatol Suppl, 1989 Nov ; 19 : 140 – 3 . 5 Reynolds WJ, et al. J Rheumatol . 1991 . 18 : 452 – 4 . 6 Santandrea S, et al . J Int Med Res. 1993 . 21 : 74 – 80 . 7 Cantini F, et al . Minerva Med. 1994 . 85 : 97 – 100 . 8 Carette S, et al. Arthritis Rheum. 1994 . 37 : 32 – 40 . 9 Tofferi JK, et al . Arthritis Rheum. 2004 . 51 : 9 – 13 . 1 10 Iglehart IW. 2003; US Patent 6,541,523. 11 Moldofsky et al. J Rheumatol . 2011. 38:2653 - 2663 12 Lederman S et al. Arthritis Care Res . 2023. 75:2359 - 2368 .
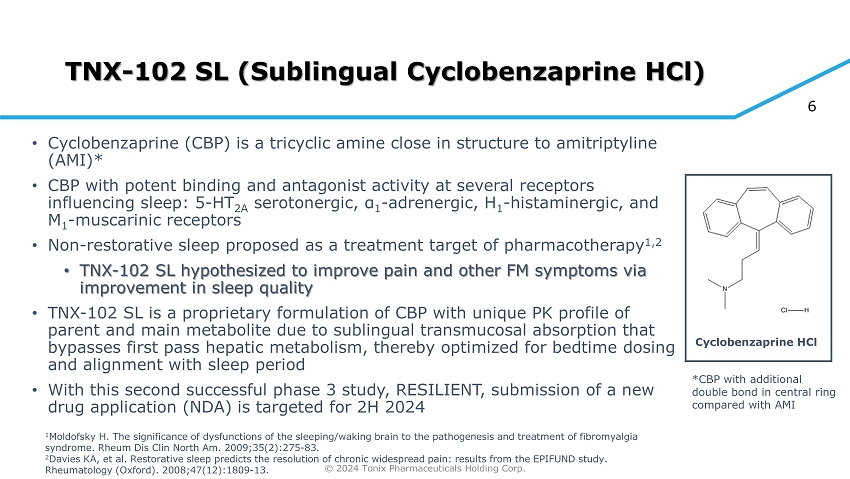
© 2024 Tonix Pharmaceuticals Holding Corp. 6 TNX - 102 SL (Sublingual Cyclobenzaprine HCl) • Cyclobenzaprine (CBP) is a tricyclic amine close in structure to amitriptyline (AMI)* • CBP with potent binding and antagonist activity at several receptors influencing sleep: 5 - HT 2A serotonergic, α 1 - adrenergic, H 1 - histaminergic, and M 1 - muscarinic receptors • Non - restorative sleep proposed as a treatment target of pharmacotherapy 1,2 • TNX - 102 SL hypothesized to improve pain and other FM symptoms via improvement in sleep quality • TNX - 102 SL is a proprietary formulation of CBP with unique PK profile of parent and main metabolite due to sublingual transmucosal absorption that bypasses first pass hepatic metabolism, thereby optimized for bedtime dosing and alignment with sleep period • With this second successful phase 3 study, RESILIENT, submission of a new drug application (NDA) is targeted for 2H 2024 Cyclobenzaprine HCl *CBP with additional double bond in central ring compared with AMI 1 Moldofsky H. The significance of dysfunctions of the sleeping/waking brain to the pathogenesis and treatment of fibromyalgia syndrome. Rheum Dis Clin North Am. 2009;35(2):275 - 83. 2 Davies KA, et al. Restorative sleep predicts the resolution of chronic widespread pain: results from the EPIFUND study. Rheumatology (Oxford). 2008;47(12):1809 - 13.
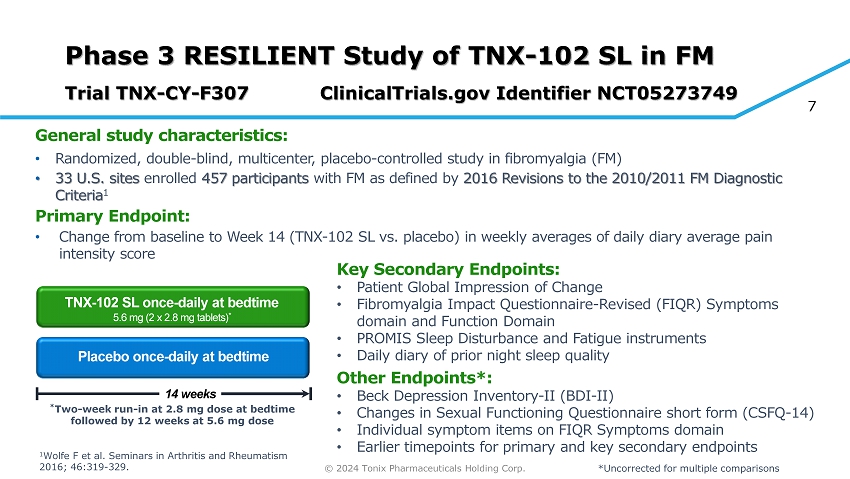
© 2024 Tonix Pharmaceuticals Holding Corp. 7 Phase 3 RESILIENT Study of TNX - 102 SL in FM Trial TNX - CY - F307 ClinicalTrials.gov Identifier NCT05273749 General s tudy c haracteristics: • Randomized, double - blind, multicenter, placebo - controlle d study in fibromyalgia (FM) • 33 U.S. sites enrolled 457 participants with FM as defined by 2016 Revisions to the 2010/2011 FM Diagnostic C riteria 1 Primary Endpoint: • Change from baseline to Week 14 (TNX - 102 SL vs. placebo) in weekly averages of daily diary average pain intensity score Placebo once - daily at bedtime TNX - 102 SL once - daily at bedtime 5.6 mg (2 x 2.8 mg tablets) * * Two - week run - in at 2.8 mg dose at bedtime followed by 12 weeks at 5.6 mg dose Key Secondary Endpoints: • Patient Global Impression of Change • Fibromyalgia Impact Questionnaire - Revised (FIQR) Symptoms domain and Function Domain • PROMIS Sleep Disturbance and Fatigue instruments • Daily diary of prior night sleep quality Other Endpoints*: • Beck Depression Inventory - II (BDI - II) • Changes in Sexual Functioning Questionnaire short form (CSFQ - 14) • Individual symptom items on FIQR Symptoms domain • Earlier timepoints for primary and key secondary endpoints 14 weeks *Uncorrected for multiple comparisons 1 Wolfe F et al. Seminars in Arthritis and Rheumatism 2016; 46:319 - 329.
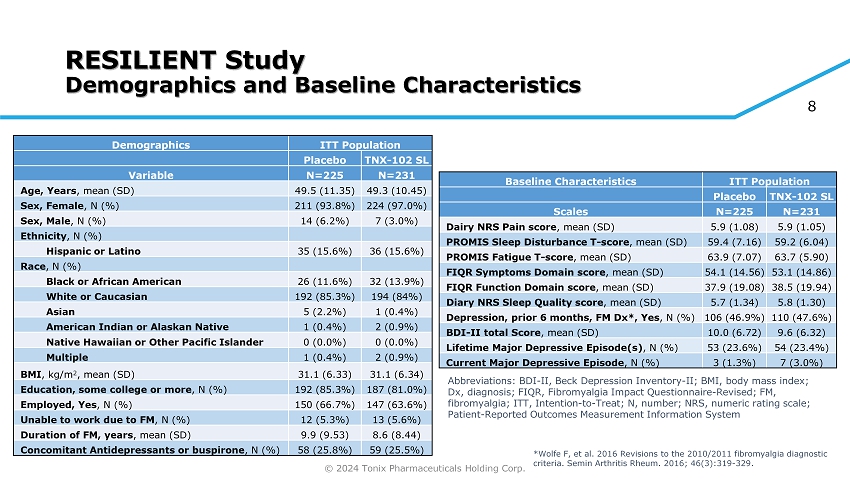
© 2024 Tonix Pharmaceuticals Holding Corp. 8 RESILIENT Study Demographics and Baseline Characteristics ITT Population Demographics TNX - 102 SL Placebo N=231 N=225 Variable 49.3 (10.45) 49.5 (11.35) Age, Years , mean (SD) 224 (97.0%) 211 (93.8%) Sex, Female , N (%) 7 (3.0%) 14 (6.2%) Sex, Male , N (%) Ethnicity , N (%) 36 (15.6%) 35 (15.6%) Hispanic or Latino Race , N (%) 32 (13.9%) 26 (11.6%) Black or African American 194 (84%) 192 (85.3%) White or Caucasian 1 (0.4%) 5 (2.2%) Asian 2 (0.9%) 1 (0.4%) American Indian or Alaskan Native 0 (0.0%) 0 (0.0%) Native Hawaiian or Other Pacific Islander 2 (0.9%) 1 (0.4%) Multiple 31.1 (6.34) 31.1 (6.33) BMI , kg/m 2 , mean (SD) 187 (81.0%) 192 (85.3%) Education, some college or more , N (%) 147 (63.6%) 150 (66.7%) Employed, Yes , N (%) 13 (5.6%) 12 (5.3%) Unable to work due to FM , N (%) 8.6 (8.44) 9.9 (9.53) Duration of FM, years , mean (SD) 59 (25.5%) 58 (25.8%) Concomitant Antidepressants or buspirone , N (%) ITT Population Baseline Characteristics TNX - 102 SL Placebo N=231 N=225 Scales 5.9 (1.05) 5.9 (1.08) Dairy NRS Pain score , mean (SD) 59.2 (6.04) 59.4 (7.16) PROMIS Sleep Disturbance T - score , mean (SD) 63.7 (5.90) 63.9 (7.07) PROMIS Fatigue T - score , mean (SD) 53.1 (14.86) 54.1 (14.56) FIQR Symptoms Domain score , mean (SD) 38.5 (19.94) 37.9 (19.08) FIQR Function Domain score , mean (SD) 5.8 (1.30) 5.7 (1.34) Diary NRS Sleep Quality score , mean (SD) 110 (47.6%) 106 (46.9%) Depression, prior 6 months, FM Dx*, Yes , N (%) 9.6 (6.32) 10.0 (6.72) BDI - II total Score , mean (SD) 54 (23.4%) 53 (23.6%) Lifetime Major Depressive Episode(s) , N (%) 7 (3.0%) 3 (1.3%) Current Major Depressive Episode , N (%) Abbreviations: BDI - II, Beck Depression Inventory - II; BMI, body mass index; Dx, diagnosis; FIQR, Fibromyalgia Impact Questionnaire - Revised; FM, fibromyalgia; ITT, Intention - to - Treat; N, number; NRS, numeric rating scale; Patient - Reported Outcomes Measurement Information System *Wolfe F, et al. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin Arthritis Rheum. 2016; 46(3):319 - 329.
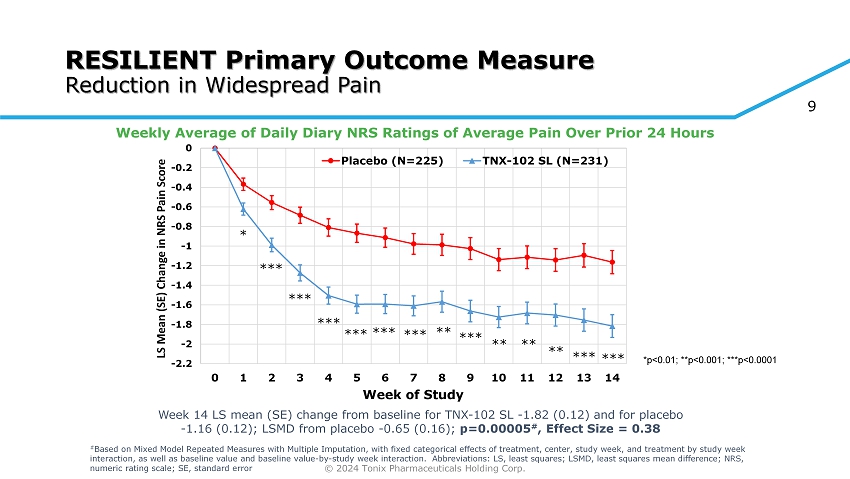
© 2024 Tonix Pharmaceuticals Holding Corp. 9 RESILIENT Primary Outcome Measure Reduction in Widespread Pain Weekly Average of Daily Diary NRS Ratings of Average Pain Over Prior 24 Hours -2.2 -2 -1.8 -1.6 -1.4 -1.2 -1 -0.8 -0.6 -0.4 -0.2 0 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 LS Mean (SE) Change in NRS Pain Score Week of Study Placebo (N=225) TNX-102 SL (N=231) * *** *** ** ** ** *** *** *** *** *** *** *** ** *p<0.01; **p<0.001; ***p<0.0001 Week 14 LS mean (SE) change from baseline for TNX - 102 SL - 1.82 (0.12) and for placebo - 1.16 (0.12); LSMD from placebo - 0.65 (0.16); p=0.00005 # , Effect Size = 0.38 # Based on Mixed Model Repeated Measures with Multiple Imputation, with fixed categorical effects of treatment, center, study w eek , and treatment by study week interaction, as well as baseline value and baseline value - by - study week interaction. Abbreviations: LS, least squares; LSMD, le ast squares mean difference; NRS, numeric rating scale; SE, standard error
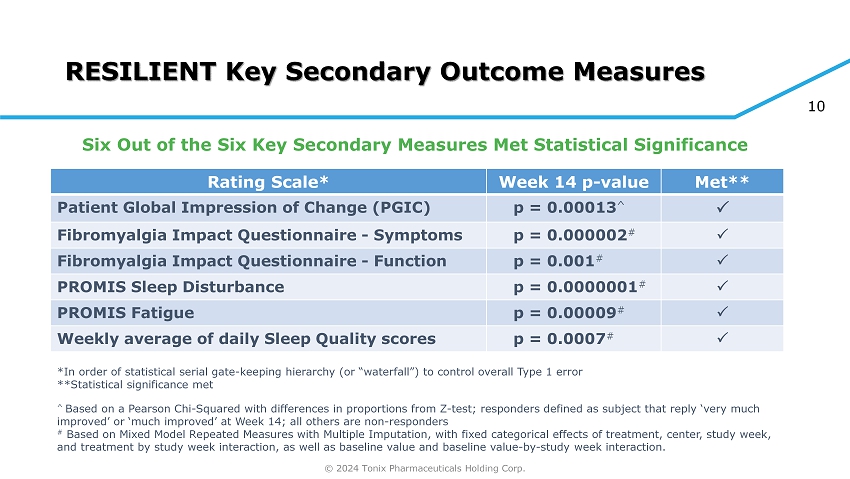
© 2024 Tonix Pharmaceuticals Holding Corp. 10 RESILIENT Key Secondary Outcome Measures Met** Week 14 p - value Rating Scale* p = 0.00013 ^ Patient Global Impression of Change (PGIC) p = 0.000002 # Fibromyalgia Impact Questionnaire - Symptoms p = 0.001 # Fibromyalgia Impact Questionnaire - Function p = 0.0000001 # PROMIS Sleep Disturbance p = 0.00009 # PROMIS Fatigue p = 0.0007 # Weekly average of daily Sleep Quality scores *In order of statistical serial gate - keeping hierarchy (or “waterfall”) to control overall Type 1 error **Statistical significance met ^ Based on a Pearson Chi - Squared with differences in proportions from Z - test; responders defined as subject that reply ‘very much improved’ or ‘much improved’ at Week 14; all others are non - responders # Based on Mixed Model Repeated Measures with Multiple Imputation, with fixed categorical effects of treatment, center, study w ee k, and treatment by study week interaction, as well as baseline value and baseline value - by - study week interaction. Six Out of the Six Key Secondary Measures Met Statistical Significance
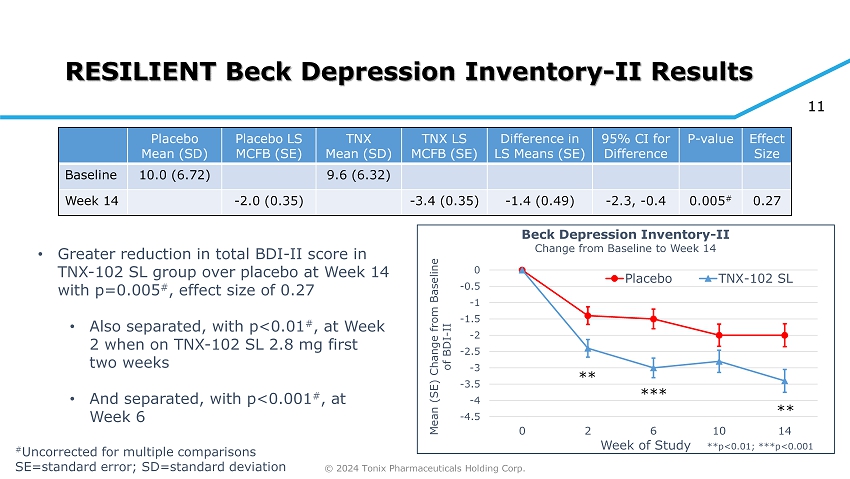
© 2024 Tonix Pharmaceuticals Holding Corp. 11 RESILIENT Beck Depression Inventory - II Results Effect Size P - value 95% CI for Difference Difference in LS Means (SE) TNX LS MCFB (SE) TNX Mean (SD) Placebo LS MCFB (SE) Placebo Mean (SD) 9.6 (6.32) 10.0 (6.72) Baseline 0.27 0.005 # - 2.3, - 0.4 - 1.4 (0.49) - 3.4 (0.35) - 2.0 (0.35) Week 14 -4.5 -4 -3.5 -3 -2.5 -2 -1.5 -1 -0.5 0 0 2 6 10 14 Mean (SE) Change from Baseline of BDI - II Week of Study Beck Depression Inventory - II Change from Baseline to Week 14 Placebo TNX-102 SL **p<0.01; ***p<0.001 ** *** ** • Greater reduction in total BDI - II score in TNX - 102 SL group over placebo at Week 14 with p=0.005 # , effect size of 0.27 • Also separated, with p<0.01 # , at Week 2 when on TNX - 102 SL 2.8 mg first two weeks • And separated, with p<0.001 # , at Week 6 # Uncorrected for multiple comparisons SE=standard error; SD=standard deviation
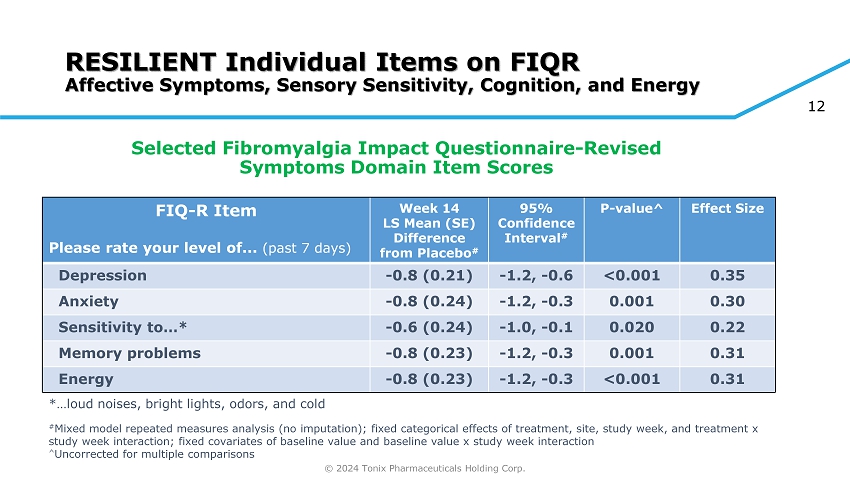
© 2024 Tonix Pharmaceuticals Holding Corp. 12 RESILIENT Individual Items on FIQR Affective Symptoms, Sensory Sensitivity, Cognition, and Energy Selected Fibromyalgia Impact Questionnaire - Revised Symptoms Domain Item Scores Effect Size P - value^ 95% Confidence Interval # Week 14 LS Mean (SE) Difference from Placebo # FIQ - R Item Please rate your level of... (past 7 days) 0.35 <0.001 - 1.2, - 0.6 - 0.8 (0.21) Depression 0.30 0.001 - 1.2, - 0.3 - 0.8 (0.24) Anxiety 0.22 0.020 - 1.0, - 0.1 - 0.6 (0.24) Sensitivity to…* 0.31 0.001 - 1.2, - 0.3 - 0.8 (0.23) Memory problems 0.31 <0.001 - 1.2, - 0.3 - 0.8 (0.23) Energy *…loud noises, bright lights, odors, and cold # Mixed model repeated measures analysis (no imputation); fixed categorical effects of treatment, site, study week, and treatme nt x study week interaction; fixed covariates of baseline value and baseline value x study week interaction ^ Uncorrected for multiple comparisons
© 2024 Tonix Pharmaceuticals Holding Corp. 13 Summary of Baseline Depression BDI - II and FIQR Item Data • While the rate of current MDE diagnosis was ~2% of the ITT, ~25% ITT had experienced a lifetime MDE, and ~47% reported past 6 month depression on FM Dx* • Also, about 25% of the ITT enrolled on concomitant antidepressant or buspirone • By end of treatment (Week 14), there was a greater reduction in depression severity by total BDI - II score in TNX - 102 SL group compared with placebo (p=0.005) • And greater reduction in FIQR items for depression (p<0.001), anxiety (p=0.001), and sensory sensitivity (p=0.020) in the TNX - 102 SL group compared with placebo • The FIQR memory item, a measure of cognitive impairment in FM, was more improved in the TNX - 102 SL group than placebo (p=0.001) • The FIQR energy item, another indicator of less fatigue in FM, was also more improved in the TNX - 102 SL group than placebo (p<0.001) • Cohen’s d effect sizes were between 0.27 and 0.35 for all Week 14 outcomes above except sensory sensitivity *Wolfe F, et al. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin Arthritis Rheum. 2016; 46(3):319 - 329. Abbreviations: BDI - II, Beck Depression Inventory - II; Dx, diagnosis; FIQR, Fibromyalgia Impact Questionnaire - Revised; FM, fibromyalgia; ITT, Intention - to - Treat
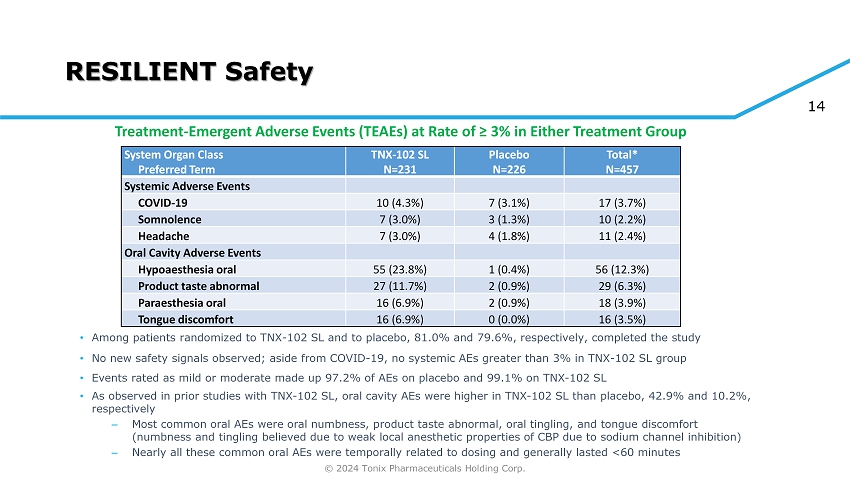
© 2024 Tonix Pharmaceuticals Holding Corp. 14 RESILIENT Safety Total* N=457 Placebo N=226 TNX - 102 SL N=231 System Organ Class Preferred Term Systemic Adverse Events 17 (3.7%) 7 (3.1%) 10 (4.3%) COVID - 19 10 (2.2%) 3 (1.3%) 7 (3.0%) Somnolence 11 (2.4%) 4 (1.8%) 7 (3.0%) Headache Oral Cavity Adverse Events 56 (12.3%) 1 (0.4%) 55 (23.8%) Hypoaesthesia oral 29 (6.3%) 2 (0.9%) 27 (11.7%) Product taste abnormal 18 (3.9%) 2 (0.9%) 16 (6.9%) Paraesthesia oral 16 (3.5%) 0 (0.0%) 16 (6.9%) Tongue discomfort • Among patients randomized to TNX - 102 SL and to placebo, 81.0% and 79.6%, respectively, completed the study • No new safety signals observed; aside from COVID - 19, no systemic AEs greater than 3% in TNX - 102 SL group • Events rated as mild or moderate made up 97.2% of AEs on placebo and 99.1% on TNX - 102 SL • As observed in prior studies with TNX - 102 SL, oral cavity AEs were higher in TNX - 102 SL than placebo, 42.9% and 10.2%, respectively ‒ Most common oral AEs were oral numbness, product taste abnormal, oral tingling, and tongue discomfort (numbness and tingling believed due to weak local anesthetic properties of CBP due to sodium channel inhibition) ‒ Nearly all these common oral AEs were temporally related to dosing and generally lasted <60 minutes Treatment - Emergent Adverse Events (TEAEs) at Rate of ≥ 3% in Either Treatment Group
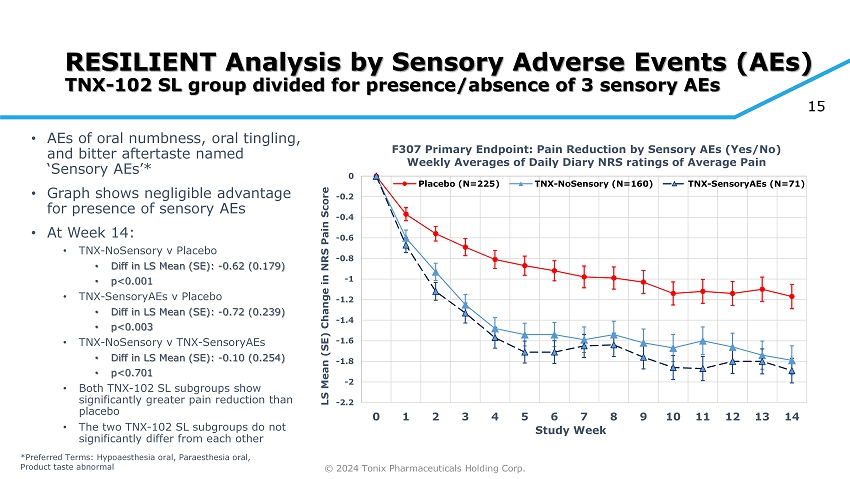
© 2024 Tonix Pharmaceuticals Holding Corp. 15 RESILIENT Analysis by Sensory Adverse Events (AEs) TNX - 102 SL group divided for presence/absence of 3 sensory AEs • AEs of oral numbness, oral tingling, and bitter aftertaste named ‘Sensory AEs’* • Graph shows negligible advantage for presence of sensory AEs • At Week 14: • TNX - NoSensory v Placebo • Diff in LS Mean (SE): - 0.62 (0.179) • p<0.001 • TNX - SensoryAEs v Placebo • Diff in LS Mean (SE): - 0.72 (0.239) • p<0.003 • TNX - NoSensory v TNX - SensoryAEs • Diff in LS Mean (SE): - 0.10 (0.254) • p<0.701 • Both TNX - 102 SL subgroups show significantly greater pain reduction than placebo • The two TNX - 102 SL subgroups do not significantly differ from each other -2.2 -2 -1.8 -1.6 -1.4 -1.2 -1 -0.8 -0.6 -0.4 -0.2 0 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 LS Mean (SE) Change in NRS Pain Score Study Week F307 Primary Endpoint: Pain Reduction by Sensory AEs (Yes/No) Weekly Averages of Daily Diary NRS ratings of Average Pain Placebo (N=225) TNX-NoSensory (N=160) TNX-SensoryAEs (N=71) *Preferred Terms: Hypoaesthesia oral, Paraesthesia oral, Product taste abnormal
© 2024 Tonix Pharmaceuticals Holding Corp. 16 RESILIENT Safety, Continued No clinically meaningful difference in mean systolic blood pressure between groups Week 14 mean (SD) change from baseline: TNX - 102 SL = 0.7 (12.38) mmHg Placebo = 0.5 (10.42) mmHg No clinically meaningful difference in mean diastolic blood pressure between groups Week 14 mean (SD) change from baseline: TNX - 102 SL = 1.1 (8.60) mmHg Placebo = 0.2 (8.22) mmHg No clinically meaningful difference in mean weight between treatment groups Week 14 mean (SD) change from baseline: TNX - 102 SL = 0.02 (2.940) kg Placebo = 0.20 (2.932) kg No Signals for Clinically Meaningful Changes in Systolic or Diastolic Blood Pressure or in Weight
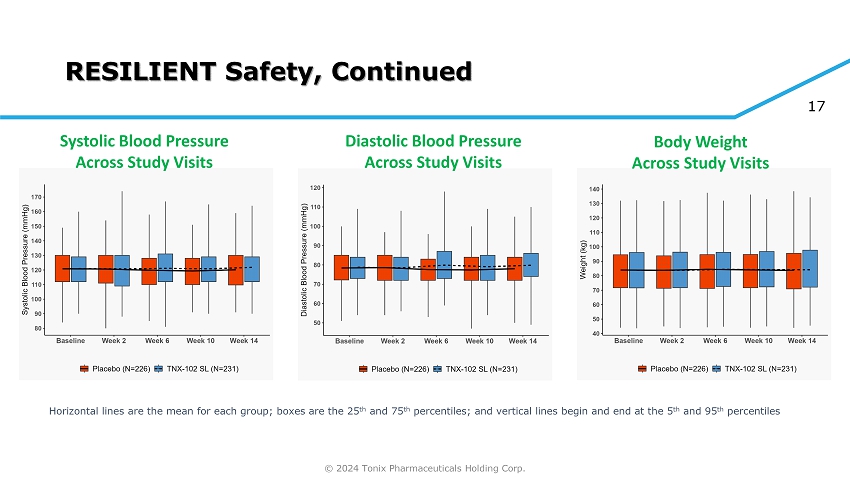
© 2024 Tonix Pharmaceuticals Holding Corp. 17 RESILIENT Safety, Continued Horizontal lines are the mean for each group; boxes are the 25 th and 75 th percentiles; and vertical lines begin and end at the 5 th and 95 th percentiles Systolic Blood Pressure Across Study Visits Diastolic Blood Pressure Across Study Visits Body Weight Across Study Visits
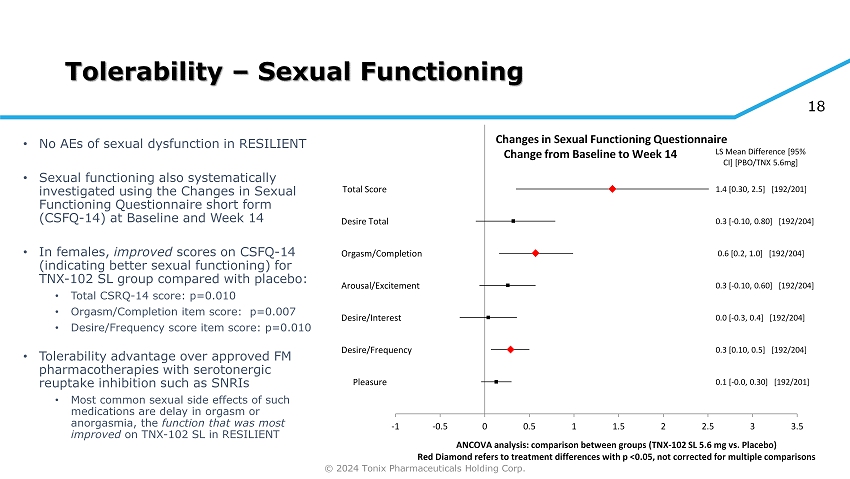
© 2024 Tonix Pharmaceuticals Holding Corp. 18 Tolerability – Sexual Functioning • No AEs of sexual dysfunction in RESILIENT • Sexual functioning also systematically investigated using the Changes in Sexual Functioning Questionnaire short form (CSFQ - 14) at Baseline and Week 14 • In females, improved scores on CSFQ - 14 (indicating better sexual functioning) for TNX - 102 SL group compared with placebo: • Total CSRQ - 14 score: p=0.010 • Orgasm/Completion item score: p=0.007 • Desire/Frequency score item score: p=0.010 • Tolerability advantage over approved FM pharmacotherapies with serotonergic reuptake inhibition such as SNRIs • Most co mmon sexual side effects of such medications are delay in orgasm or anorgasmia, the function that was most improved on TNX - 102 SL in RESILIENT Pleasure Desire/Frequency Desire/Interest Arousal/Excitement Orgasm/Completion Desire Total Total Score 0.1 [ - 0.0, 0.30] [192/201] 0.3 [0.10, 0.5] [192/204] 0.0 [ - 0.3, 0.4] [192/204] 0.3 [ - 0.10, 0.60] [192/204] 0.6 [0.2, 1.0] [192/204] 0.3 [ - 0.10, 0.80] [192/204] 1.4 [0.30, 2.5] [192/201] LS Mean Difference [95% CI] [PBO/TNX 5.6mg] -1 -0.5 0 0.5 1 1.5 2 2.5 3 3.5 ANCOVA analysis: comparison between groups (TNX - 102 SL 5.6 mg vs. Placebo) Red Diamond refers to treatment differences with p <0.05, not corrected for multiple comparisons Changes in Sexual Functioning Questionnaire Change from Baseline to Week 14
© 2024 Tonix Pharmaceuticals Holding Corp. 19 Conclusions • Fibromyalgia diagnostic criteria include depression (past 6 months) as part of the symptom burden, adding a point to symptom severity scale (SSS) score • Depressive severity reported to be prominent factor for quality of life in FM • Phase 3 RESILIENT trial demonstrated highly statistically significant effects of TNX - 102 SL on pain, patient global, fibromyalgia scale symptom and function domains, sleep/sleep quality, and fatigue • Beck Depression Inventory - II total score at Week 14 for TNX - 102 SL reduced over placebo (p=0.005; effect size [ES]=0.27) • FIQR items for depression (ES=0.35) and anxiety (ES=0.30) also reduced over placebo • Cognitive function is the 4 th core FM symptom, and FIQ - R memory item notably improved (ES=0.31), suggesting syndromal benefit of TNX - 102 SL • No new safety signals; low rates of systemic AEs, and high tolerability of therapy relative to currently approved FM therapies
© 2024 Tonix Pharmaceuticals Holding Corp. Thank you ! Questions?