
TONIX PHARMACEUTICALS HOLDING CORP. 8-K
Exhibit 99.03

Extended Survival of 9 - and 10 - Gene Edited Pig Heart Xenografts with Ischemia Minimization and CD154 Costimulation Blockade – Based Immunosuppression June 4, 2024 Ɣ Amir Sanatkar MD. 1 Center for Transplantation Sciences, Massachusetts General Hospital and Harvard Medical School, Boston, MA

Relevant Disclosures • R. Chaban was supported by the Benjamin Research Fellowship from the German Research Foundation (DFG) • I. Ileka is supported by T32 5T32 AI007529 - 24 • Gene edited pigs were provided by Revivicor and NSRRC (NIH grant U42OD011140) • Tonix Pharmaceuticals provided TNX - 1500*, a humanized, Fc - modified, dimeric anti - CD154 mAb • This work was supported by NIH grants UO1 AI153612 (Pierson), U19 AI090959 (Cooper), and sponsored research agreements with Tonix Pharmaceuticals * TNX - 1500 is an investigational new biologics and is not approved for any indication
Background • Gene - edited (GE) pigs for Xenotransplantation. – Removal of CHO antigen targets of preformed Ab • TKO (Gal - 1,3 - Gal, Neu5Gc, 4Gal) – Addition of human regulatory molecules • Complement: CD46, CD55, CD59 • Coagulation: TBM, EPCR, TFPI – Addition of human anti - inflammatory ‘transgenes’ • CD47, HLA - E/ 2 g, HO - 1, A20, CD39

Methods • Current study: – 3 - , 9 - , or 10 - GE pig hearts – novel costimulation - based immunosuppressive (TONIX - 1500) – cold - perfused storage technique designed to minimize graft ischemia . • Eight baboon recipients received heterotopic heart transplants » 3 Reference pig hearts (National Swine Resource & Research Ctr: NSRRC) • 3 - GE pigs (n=3): GalKO . β4 GALNT2KO.Hcd55 » 5 Multi - GE pig hearts ( Revivicor ) • 9 - GE pigs (n=3): GalKO . β4 GalNT2KO. GHRKO. hCD46.hCD55 . hTBM.hEPCR . hCD47 .hHO - 1 • 10 - GE pigs (n=2): GalKO . β4 GalNT2KO. CMAHKO. GHRKO. hCD46.CD55 . hTBM.hEPCR . hCD47.hHO - 1 .
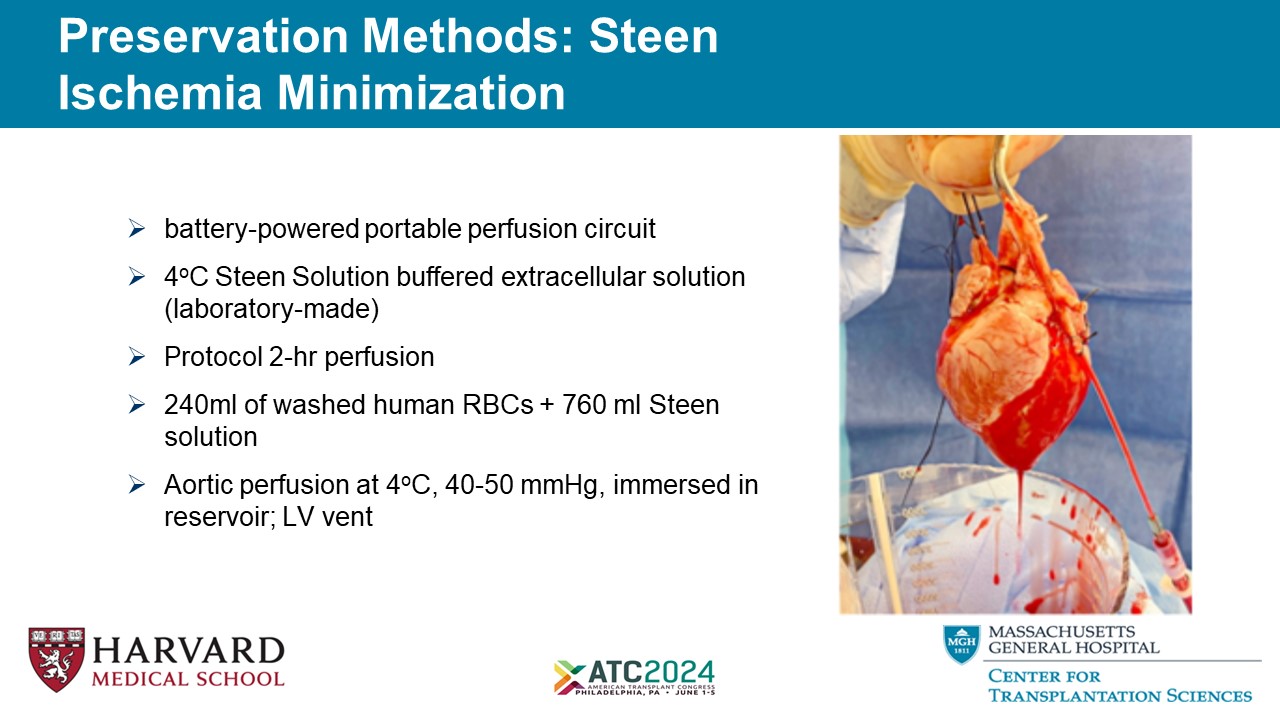
Preservation Methods: Steen Ischemia Minimization 5 » battery - powered portable perfusion circuit » 4 o C Steen Solution buffered extracellular solution (laboratory - made) » Protocol 2 - hr perfusion » 240ml of washed human RBCs + 760 ml Steen solution » Aortic perfusion at 4 o C, 40 - 50 mmHg, immersed in reservoir; LV vent
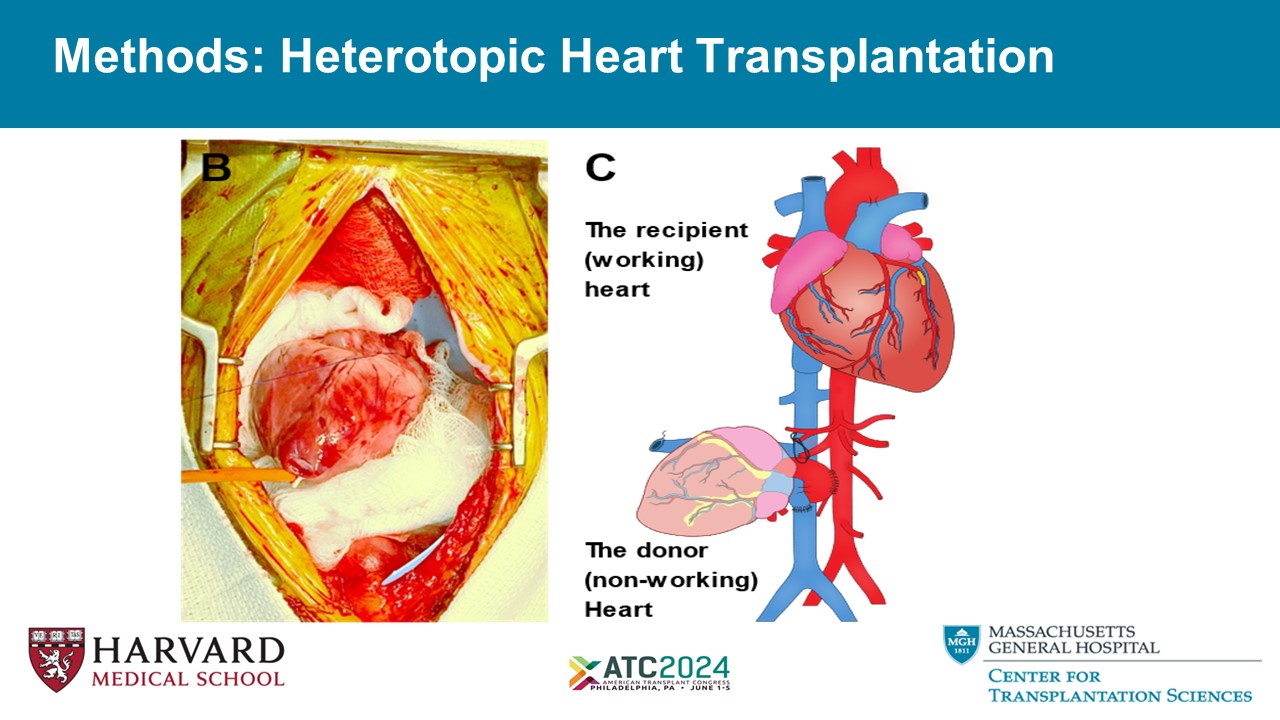
Methods: Heterotopic Heart Transplantation
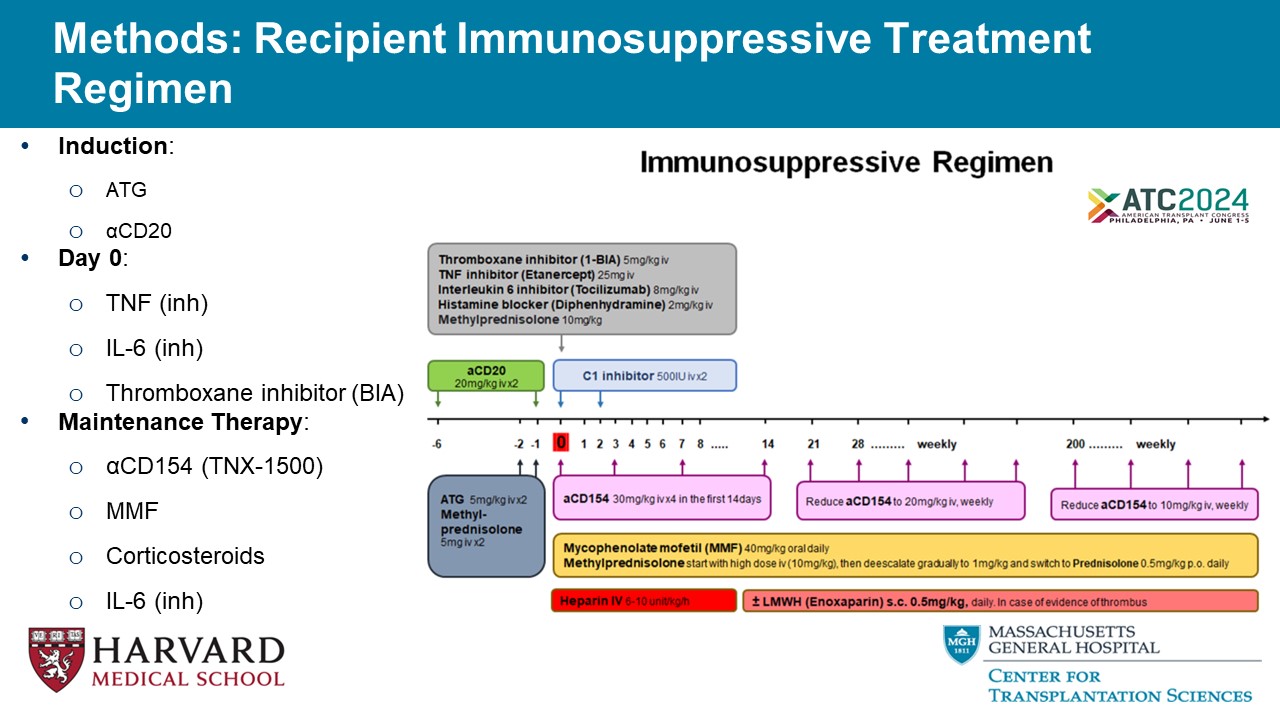
Methods: Recipient Immunosuppressive Treatment Regimen • Induction : o ATG o α CD20 • Day 0 : o TNF ( inh ) o IL - 6 ( inh ) o Thromboxane inhibitor (BIA) • Maintenance Therapy : o α CD154 (TNX - 1500) o MMF o Corticosteroids o IL - 6 ( inh )
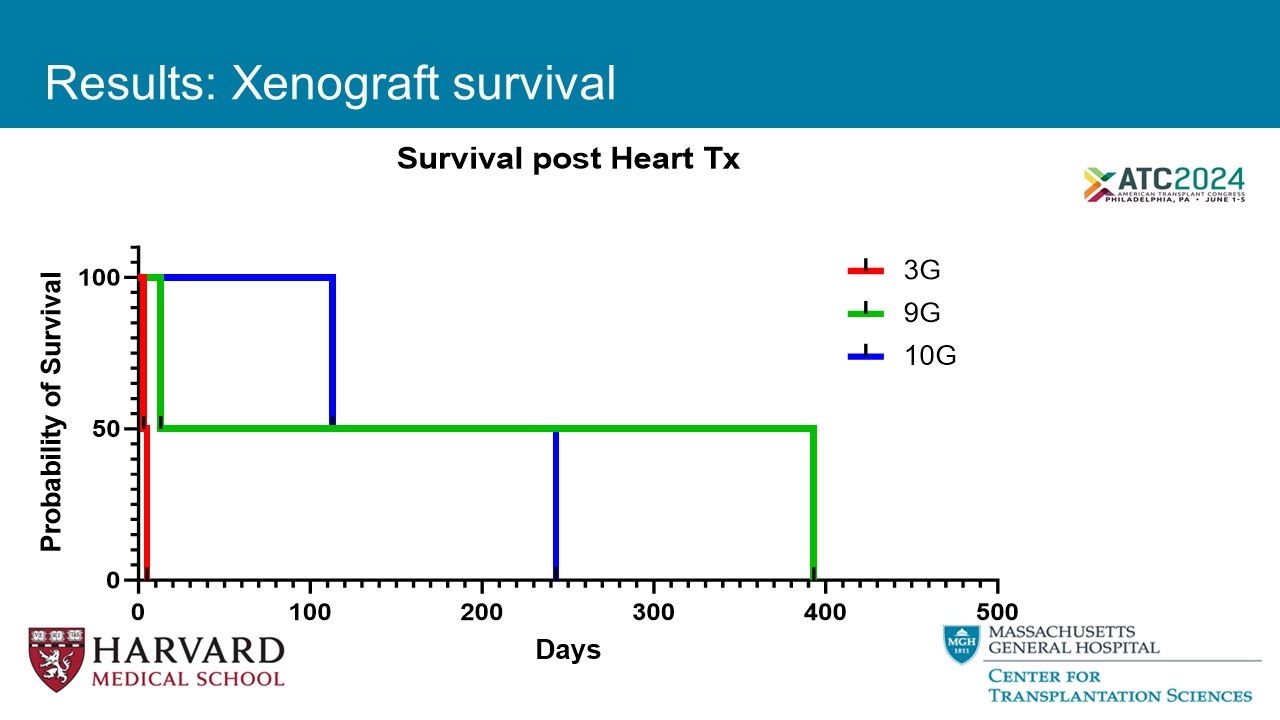
0 100 200 300 400 500 0 50 100 Survival post Heart Tx Days P r o b a b i l i t y o f S u r v i v a l 3G 9G 10G Results: Xenograft survival
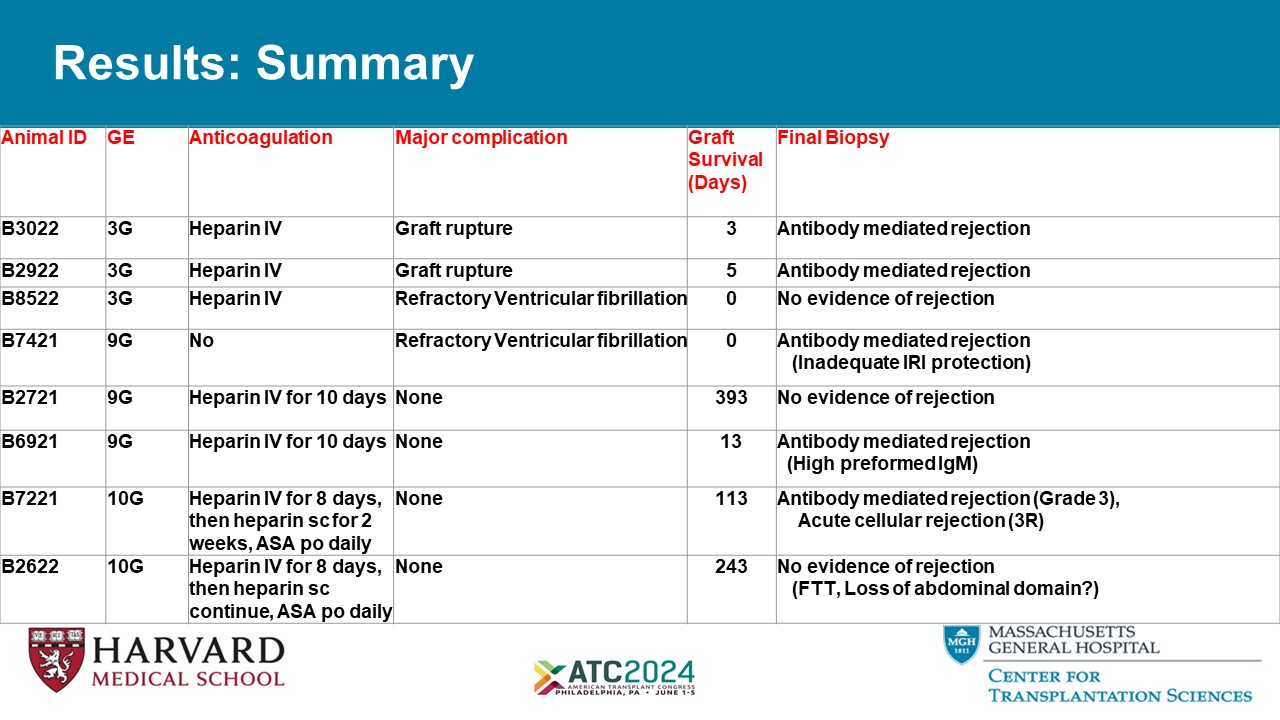
Results: Summary Final Biopsy Graft Survival (Days) Major complication Anticoagulation GE Animal ID Antibody mediated rejection 3 Graft rupture Heparin IV 3G B3022 Antibody mediated rejection 5 Graft rupture Heparin IV 3G B2922 No evidence of rejection 0 Refractory Ventricular fibrillation Heparin IV 3G B8522 Antibody mediated rejection (Inadequate IRI protection) 0 Refractory Ventricular fibrillation No 9G B7421 No evidence of rejection 393 None Heparin IV for 10 days 9G B2721 Antibody mediated rejection (High preformed IgM) 13 None Heparin IV for 10 days 9G B6921 Antibody mediated rejection (Grade 3), Acute cellular rejection (3R) 113 None Heparin IV for 8 days, then heparin sc for 2 weeks, ASA po daily 10G B7221 No evidence of rejection (FTT, Loss of abdominal domain?) 243 None Heparin IV for 8 days, then heparin sc continue, ASA po daily 10G B2622
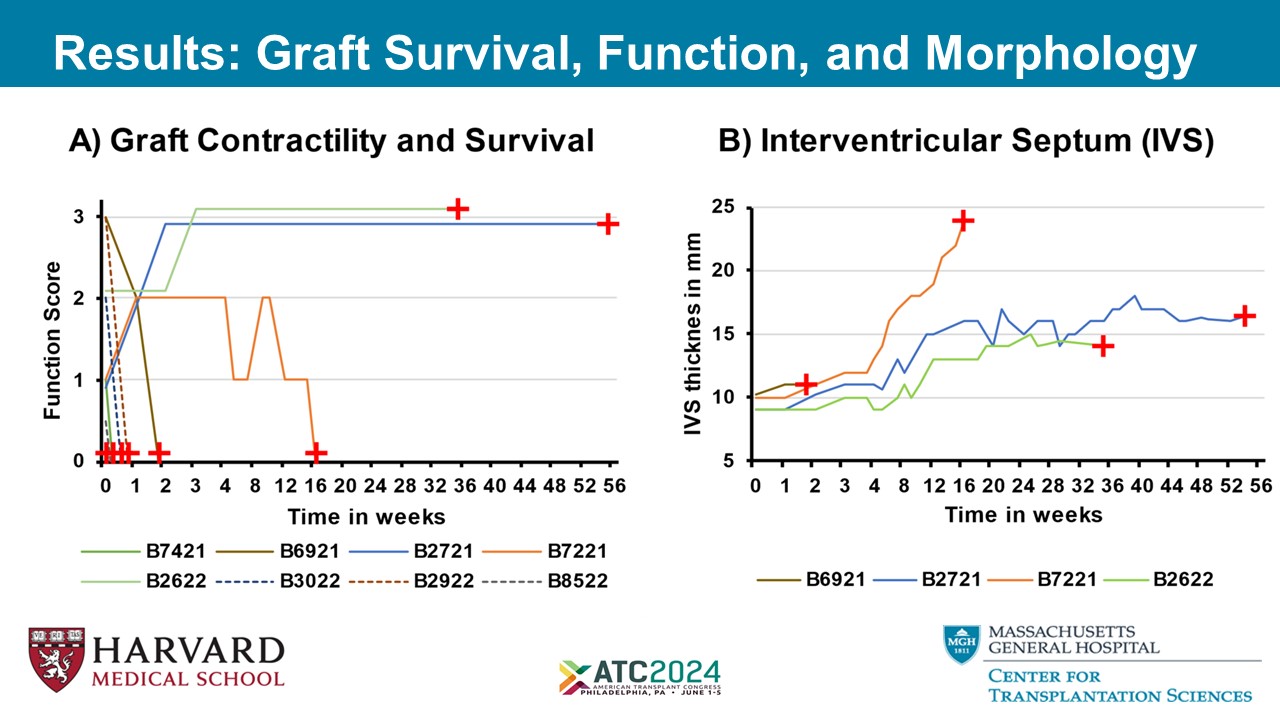
Results: Graft Survival, Function, and Morphology
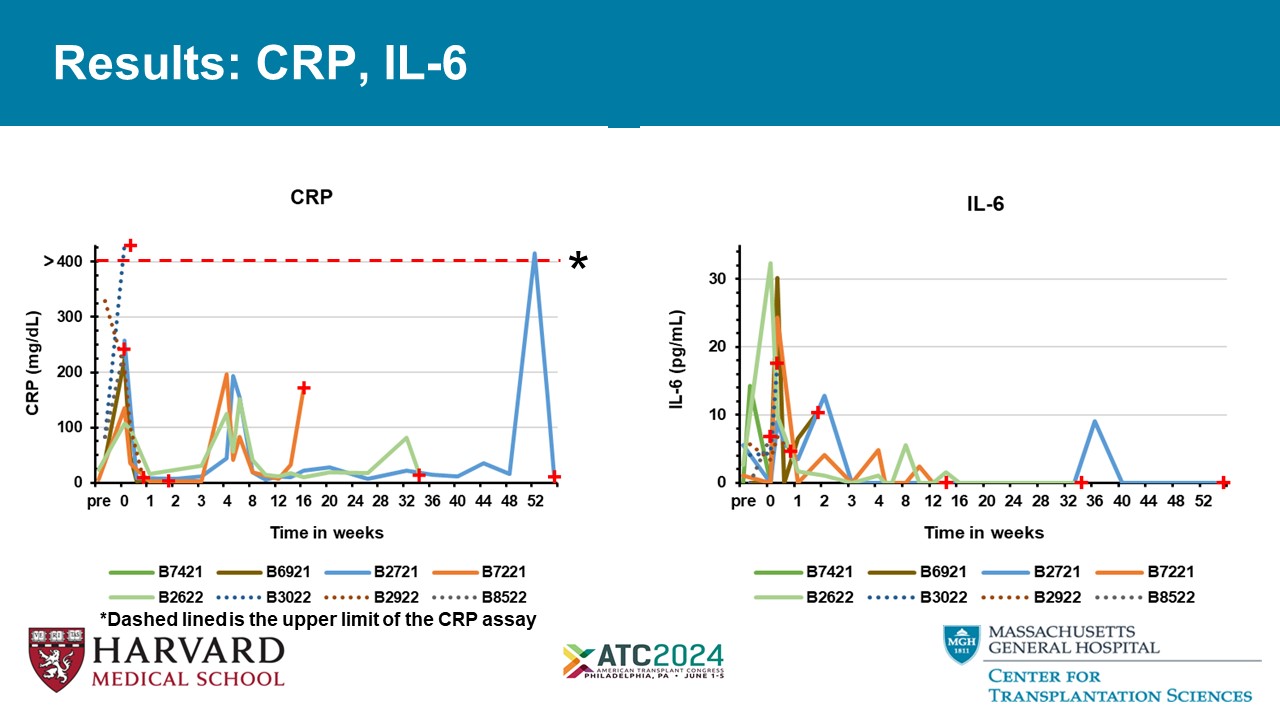
Results: CRP, IL - 6 *Dashed lined is the upper limit of the CRP assay *
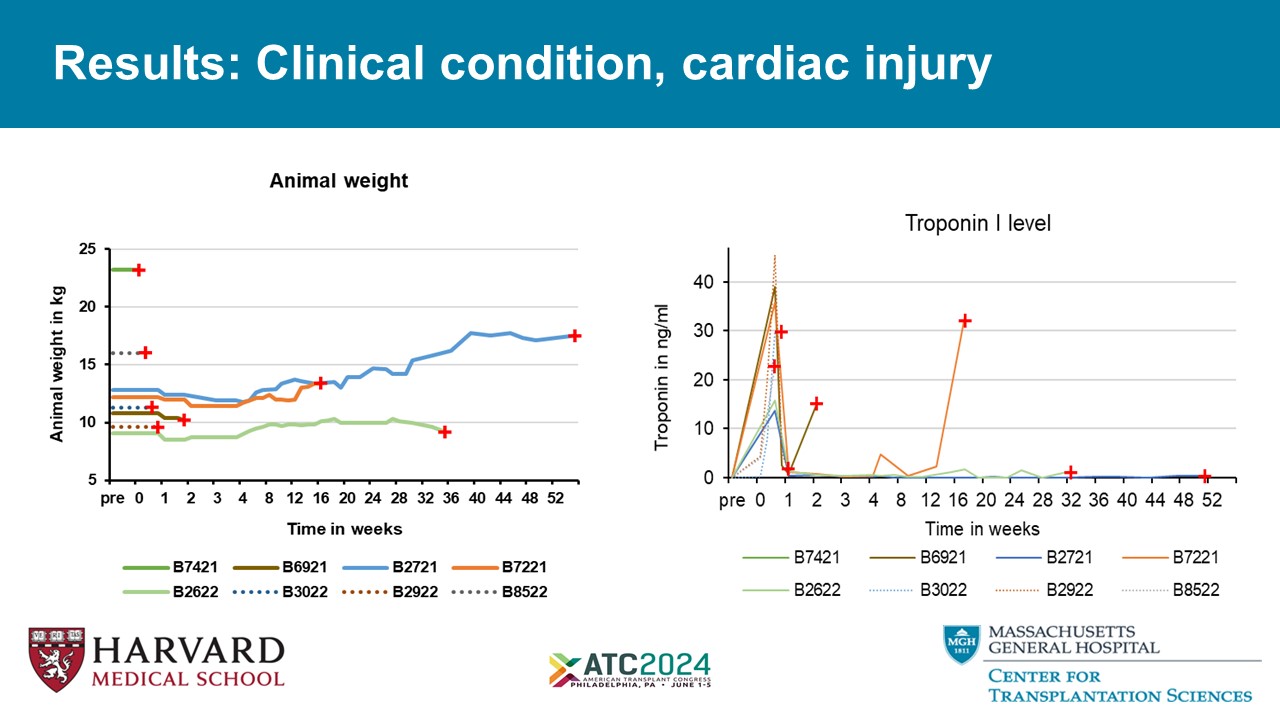
Results: Clinical condition, cardiac injury
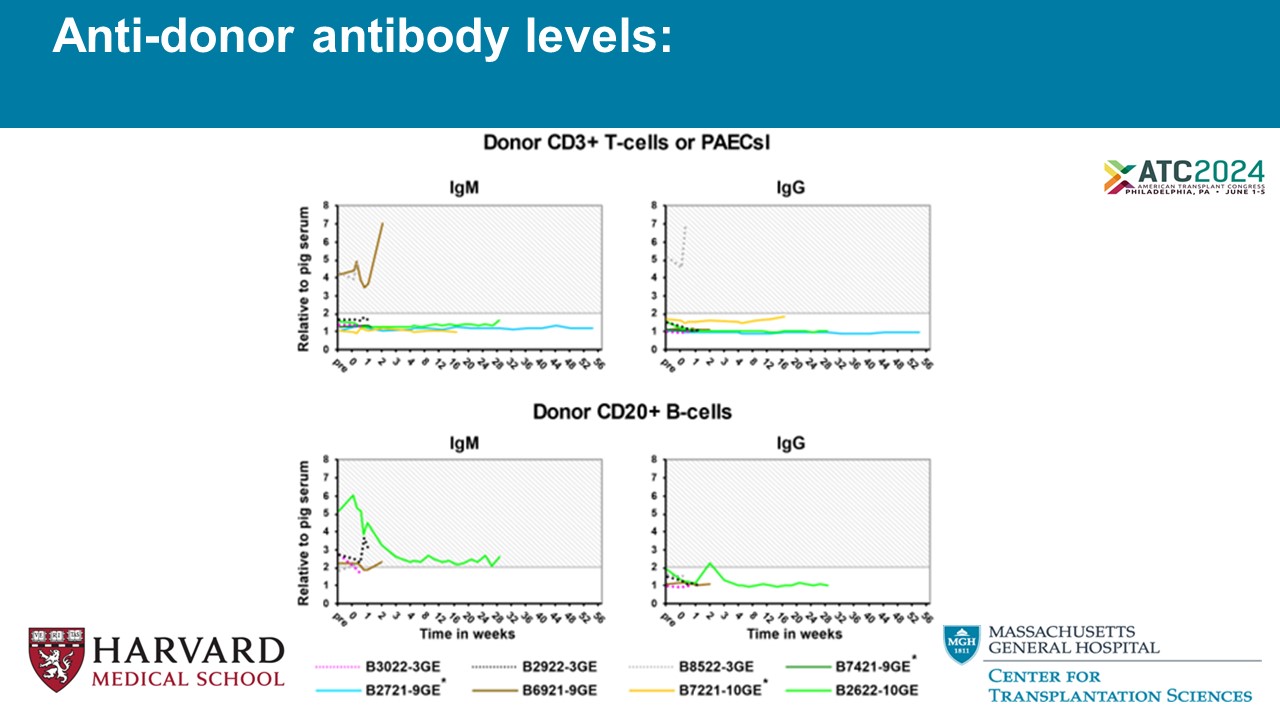
Anti - donor antibody levels:
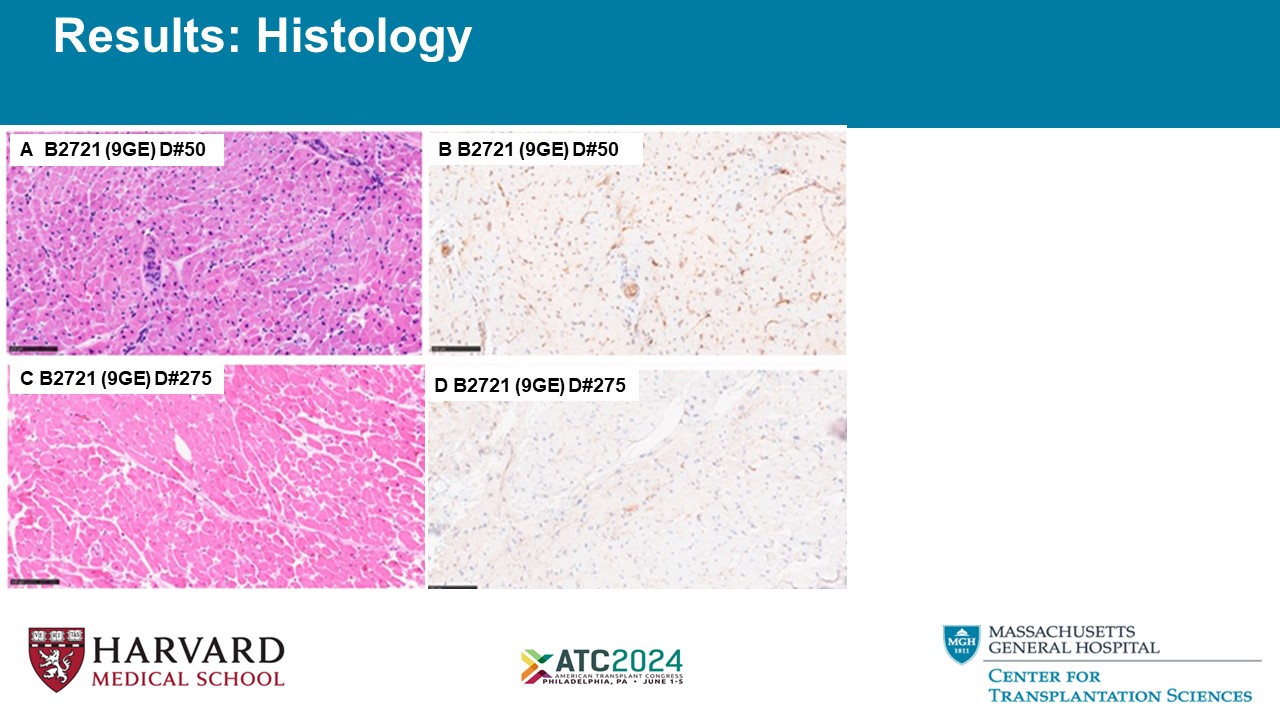
A B2721 (9GE) D#50 B B2721 (9GE) D#50 C B2721 (9GE) D#275 D B2721 (9GE) D#275 Results: Histology
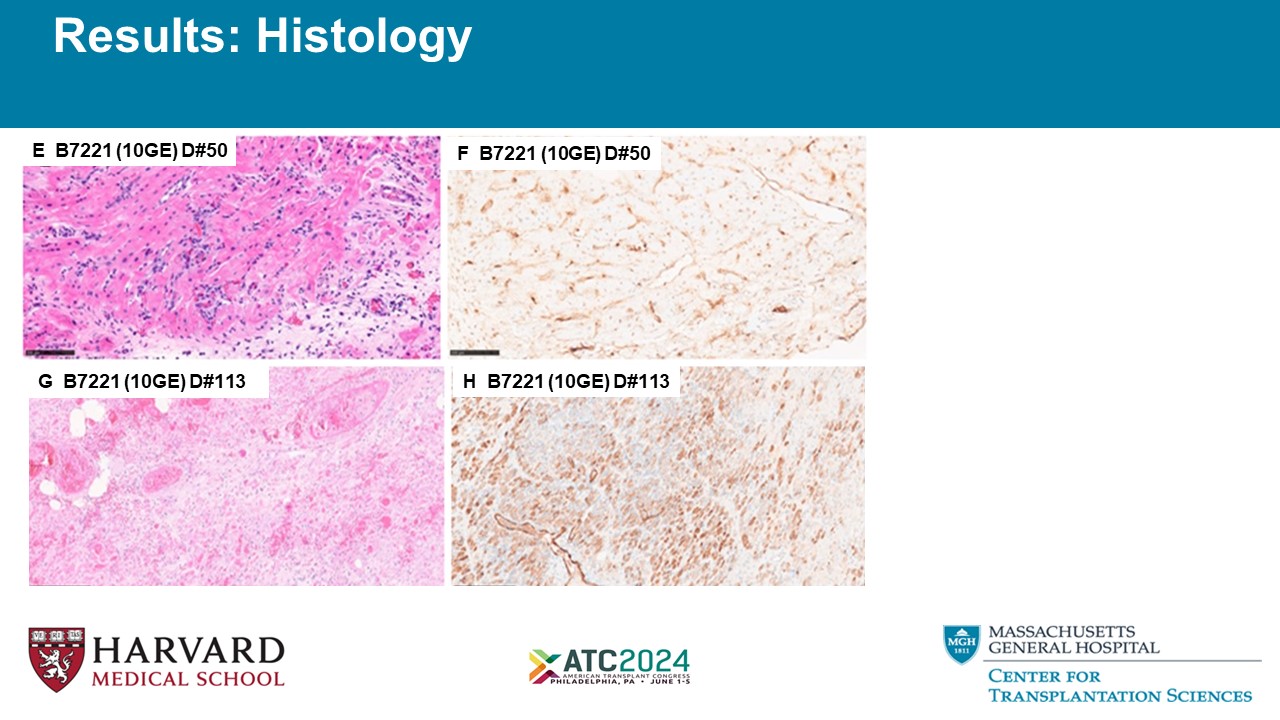
E B7221 (10GE) D#50 F B7221 (10GE) D#50 G B7221 (10GE) D#113 H B7221 (10GE) D#113 Results: Histology
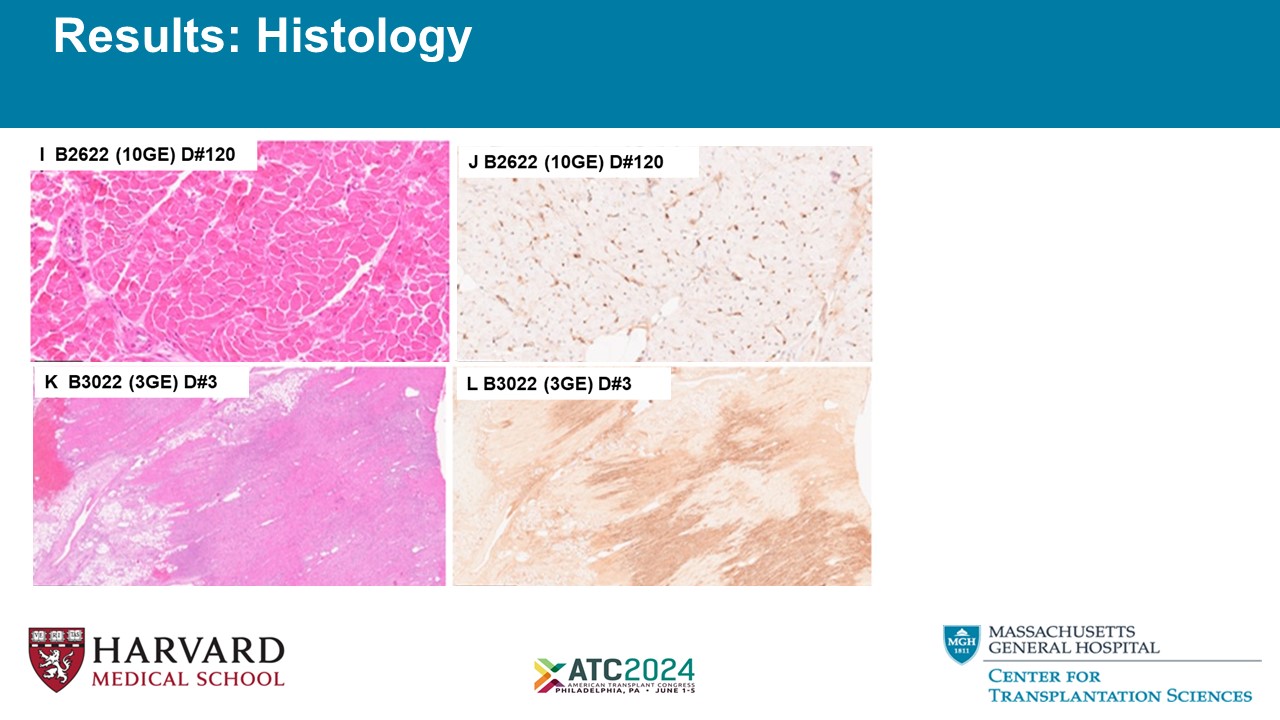
Results: Histology
Conclusions • 9 - or 10 - GE pig hearts exhibit promising performance.(Compared to reference basic genetics) in a clinically applicable regimen • Peri - transplant myocardial injury and systemic inflammation occur consistently, However; recovery and survival can be achieved using Gene modifications and Ischemia minimization. • Further evaluation in an orthotopic heart model.

Acknowledgments