
TONIX PHARMACEUTICALS HOLDING CORP. 8-K
Exhibit 99.02

© 2024 Tonix Pharmaceuticals Holding Corp. Broad - spectrum Host - directed Therapeutics: CD45 Inhibitor as Antiviral July 2024 NASDAQ: TNXP Version P0576 July 15 , 2024 (Doc 1476)

2 © 2024 Tonix Pharmaceuticals Holding Corp. Cautionary Note on Forward - Looking Statements Certain statements in this presentation regarding strategic plans, expectations and objectives for future operations or results are “forward - looking statements” as defined by the Private Securities Litigation Reform Act of 1995. These statements may be identified by the use of forward - looking words such as “anticipate,” “believe,” “forecast,” “estimate” and “intend,” among others. These forward - looking statements are based on Tonix’s current expectations and actual results could differ materially. There are a number of factors that could cause actual events to differ materially from those indicated by such forward - looking statements. These factors include, but are not limited to, the risks related to failure to obtain FDA clearances or approvals and noncompliance with FDA regulations; risks related to the failure to successfully market any of our products; risks related to the timing and progress of clinical development of our product candidates; our need for additional financing; uncertainties of patent protection and litigation; uncertainties of government or third party payor reimbursement; limited research and development efforts and dependence upon third parties; and substantial competition. As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products. The forward - looking statements in this presentation are made as of the date of this presentation, even if subsequently made available by Tonix on its website or otherwise. Tonix does not undertake an obligation to update or revise any forward - looking statement, except as required by law. Investors should read the risk factors set forth in the Annual Report on Form 10 - K for the year ended December 31, 2023, as filed with the Securities and Exchange Commission (the “SEC”) on April 1, 2024, and periodic reports and current reports filed with the SEC on or after the date thereof. All of Tonix's forward - looking statements are expressly qualified by all such risk factors and other cautionary statements.
3 © 2024 Tonix Pharmaceuticals Holding Corp. INFECTIOUS DISEASE PORTFOLIO Tonix Awarded $34M Contract from DTRA/DoD DTRA contract is expected to advance development of Tonix’s broad - spectrum oral antiviral program ( TNX - 4200 ) for medical countermeasures • Other Transaction Agreement (OTA) with a potential for up to $34 million over five years Objective : Develop an orally available small molecule that reduces CD45 enzymatic activity, with broad - spectrum protection against a range of viral families through the completion of Phase 1 clinical evaluation

4 © 2024 Tonix Pharmaceuticals Holding Corp. INFECTIOUS DISEASE PORTFOLIO DoD Moves Beyond “One Drug, One Bug” Approach 1,2 1 Vergun, D. DOD News. January 10, 2023. DoD aims to shield warfighters from novel biological agents. https://www.defense.gov/News/News - Stories/Article/Article/3261095/dod - aims - to - shield - warfighters - from - novel - biological - agents 2 US Department of Defense, Chemical and Biological Defense Program, “Approach for Research, Development and Acquisition of Med ica l Countermeasure and Test Products, Dec. 2022. https://media.defense.gov/2023/Jan/10/2003142624/ - 1/ - 1/0/APPROACH - RDA - MCM - TEST - PRODUCTS.PDF • The US Department of Defense (DoD) is moving beyond the traditional “one drug, one bug” approach to antivirals, and embracing the “one drug, multiple bugs” approach to protect against viral illness in warfighters • The Defense Threat Reduction Agency (DTRA) is a division of the DoD that supports research to protect the warfighter • DoD/DTRA collaborate with commercial partners to translate fundamental discoveries into products
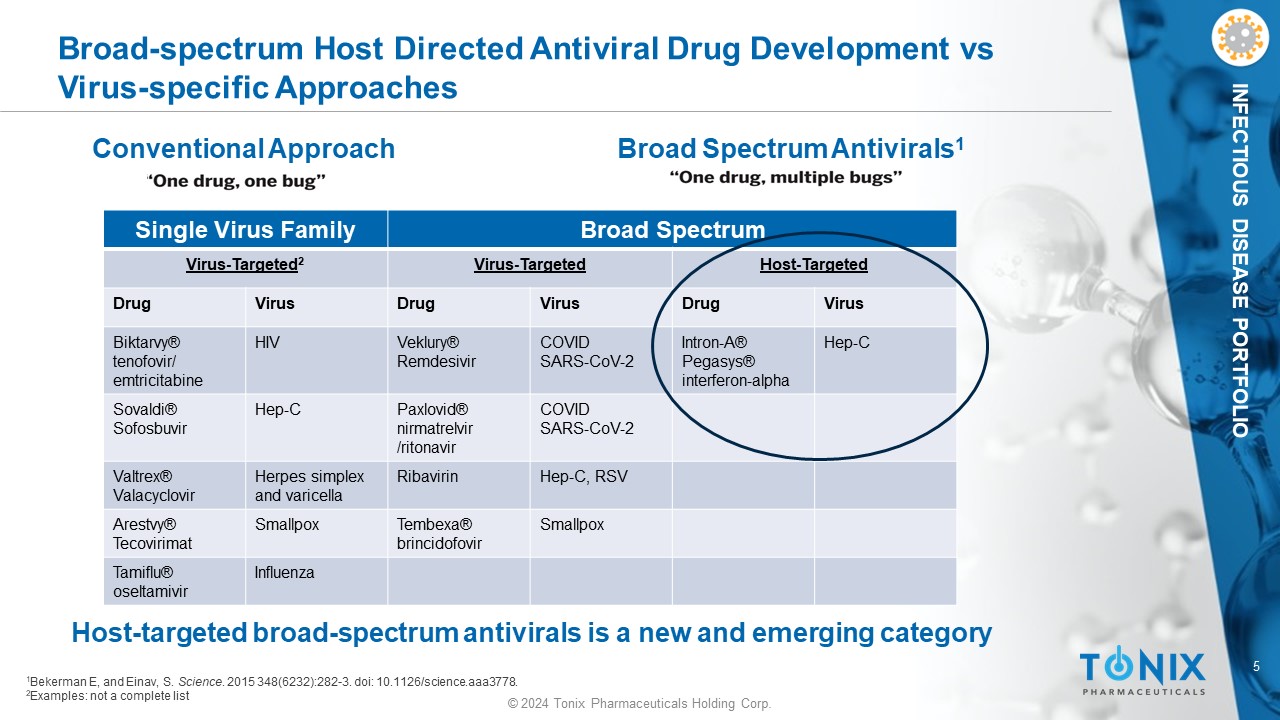
5 © 2024 Tonix Pharmaceuticals Holding Corp. INFECTIOUS DISEASE PORTFOLIO Broad Spectrum Antivirals 1 Conventional Approach Broad - spectrum Host Directed Antiviral Drug Development vs Virus - specific Approaches 1 Bekerman E, and Einav , S. Science . 2015 348(6232):282 - 3. doi : 10.1126/science.aaa3778. 2 Examples: not a complete list Broad Spectrum Single Virus Family Host - Targeted Virus - Targeted Virus - Targeted 2 Virus Drug Virus Drug Virus Drug Hep - C Intron - A® Pegasys ® interferon - alpha COVID SARS - CoV - 2 Veklury ® Remdesivir HIV Biktarvy ® tenofovir/ emtricitabine COVID SARS - CoV - 2 Paxlovid® nirmatrelvir /ritonavir Hep - C Sovaldi® Sofosbuvir Hep - C, RSV Ribavirin Herpes simplex and varicella Valtrex® Valacyclovir Smallpox Tembexa ® brincidofovir Smallpox Arestvy ® Tecovirimat Influenza Tamiflu® oseltamivir Host - targeted broad - spectrum antivirals is a new and emerging category
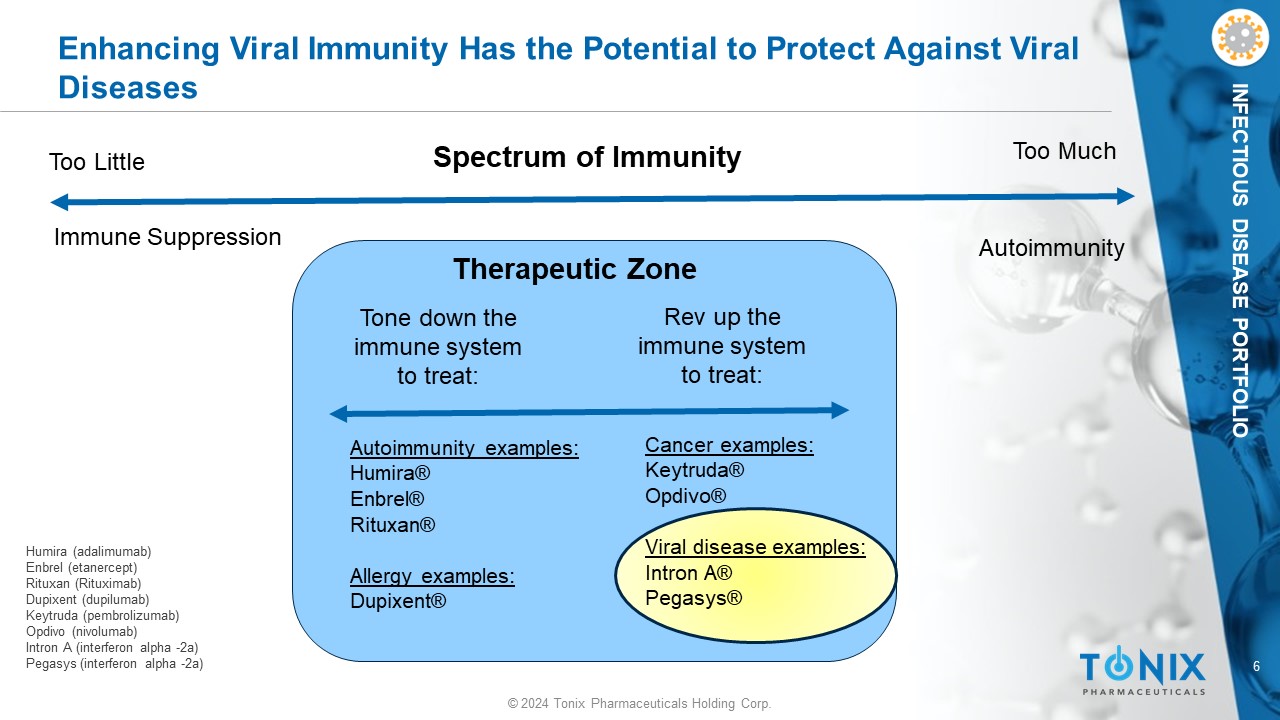
6 © 2024 Tonix Pharmaceuticals Holding Corp. INFECTIOUS DISEASE PORTFOLIO Enhancing Viral Immunity Has the Potential to Protect Against Viral Diseases Immune Suppression Autoimmunity Too Little Too Much Cancer examples: Keytruda® Opdivo ® Viral disease examples: Intron A® Pegasys ® Autoimmunity examples: Humira® Enbrel® Rituxan® Allergy examples: Dupixent® Spectrum of Immunity Tone down the immune system to treat: Rev up the immune system to treat: Therapeutic Zone Humira (adalimumab) Enbrel (etanercept) Rituxan (Rituximab) Dupixent (dupilumab) Keytruda (pembrolizumab) Opdivo (nivolumab) Intron A (interferon alpha - 2a) Pegasys (interferon alpha - 2a)
7 © 2024 Tonix Pharmaceuticals Holding Corp. INFECTIOUS DISEASE PORTFOLIO Enhancing Viral Immunity by Inhibiting CD45 Phosphatase • CD45 is a transmembrane protein tyrosine phosphatase ( PTPase ) expressed on most hematopoietic cells, including T lymphocytes • CD45 regulates receptor signaling pathways, particularly T cell activation • It dephosphorylates the negative regulatory tyrosine kinases (e.g., lck and src ) • Decreased levels of CD45 enhance antiviral 1 and antibacterial immunity in animals 2 • Oral small - molecule inhibitors of CD45 have the potential to enhance antiviral immunity 3 - 5 Goal : Develop an orally available small molecule that reduces CD45 enzymatic activity CD45 modulating host - directed antivirals would be an entirely new class of medicines 1 Panchal RG, et al., Cell Host Microbe . 2009 6(2):162 - 73. doi : 10.1016/j.chom.2009.07.003. PMID: 19683682. 2 Panchal RG, et al. J Biol Chem. 2009 284(19):12874 - 85. doi : 10.1074/jbc.M809633200 3 Miski M, et al. Bioorg Med Chem Lett . 1995 5(14):1519 - 22. doi : 10.1016/0960 - 894X(95)00250 - W. 4 Hamaguchi T, et al. Bioorg Med Chem Lett . 2000 10(23):2657 - 60. doi : 10.1016/s0960 - 894x(00)00539 - 4. PMID: 11128645. 5 Carr G, et al. Methods . 2014. 65(2):229 - 38, doi : 10.1016/j.ymeth.2013.09.007.
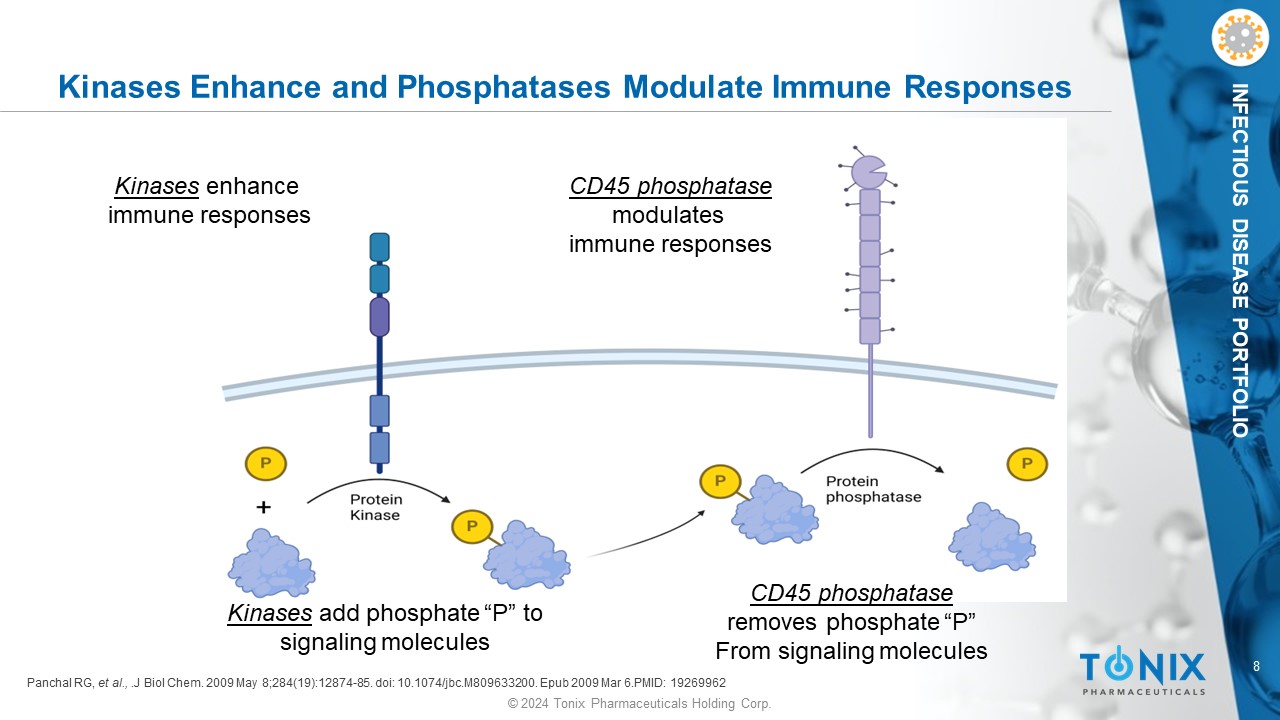
8 © 2024 Tonix Pharmaceuticals Holding Corp. INFECTIOUS DISEASE PORTFOLIO Kinases Enhance and Phosphatases Modulate Immune Responses Panchal RG, et al., .J Biol Chem. 2009 May 8;284(19):12874 - 85. doi : 10.1074/jbc.M809633200. Epub 2009 Mar 6.PMID: 19269962 Kinases enhance immune responses CD45 phosphatase modulates immune responses Kinases add phosphate “P” to signaling molecules CD45 phosphatase removes phosphate “P” From signaling molecules
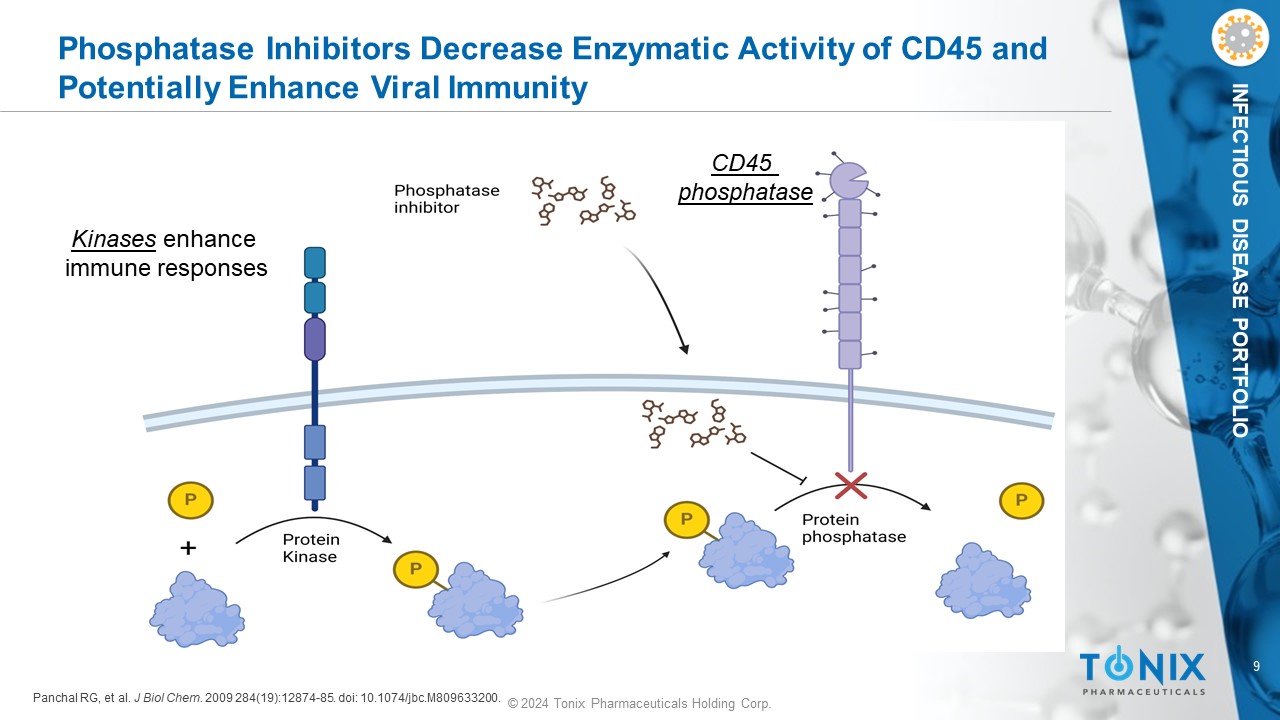
9 © 2024 Tonix Pharmaceuticals Holding Corp. INFECTIOUS DISEASE PORTFOLIO Phosphatase Inhibitors Decrease Enzymatic Activity of CD45 and Potentially Enhance Viral Immunity Panchal RG, et al. J Biol Chem. 2009 284(19):12874 - 85. doi : 10.1074/jbc.M809633200. CD45 phosphatase Kinases enhance immune responses
10 © 2024 Tonix Pharmaceuticals Holding Corp. INFECTIOUS DISEASE PORTFOLIO TNX - 4200: Orally Available CD45 Antagonists • Tonix is exploiting regenerative AI and computational biology to identify modulators of CD45 expression and inhibitors of CD45 function to develop candidate broad - spectrum antiviral drugs 1 • Drugs that strengthen the body’s immunity and protect against viral illness are called “host - directed” antivirals 2,3 Goal: broad spectrum antivirals CD45 modulating host - directed antivirals would be an entirely new class of medicines 1 Panchal RG, et al. J Biol Chem. 2009 284(19):12874 - 85. doi : 10.1074/jbc.M809633200 2 Radoshitzky SR, et al., PLoS Pathog . 2016 Mar 31;12(3):e1005466. doi : 10.1371/journal.ppat.1005466. eCollection 2016 PMID: 27031835 3 Loureiro ME, et al., PLoS Pathog . 2018 Jul 12;14(7):e1007125. doi : 10.1371/journal.ppat.1007125. eCollection 2018 Jul.PMID : 30001425

11 © 2024 Tonix Pharmaceuticals Holding Corp. INFECTIOUS DISEASE PORTFOLIO Tonix Research and Development Center (RDC) • Located in Frederick, MD (close to Fort Detrick/ USAMRIID) • 48,000 square foot facility; main building is BSL - 2 with some areas designated BSL - 3 • Supports expanding infectious disease pipeline by accelerating internal discovery and development of vaccines and antiviral drugs • At full capacity, the RDC can employ 80 - 100 scientists and technical support staff

12 © 2024 Tonix Pharmaceuticals Holding Corp. INFECTIOUS DISEASE PORTFOLIO Sina Bavari, PhD – Director of Infectious Disease Reserach • Sina Bavari, PhD, Tonix’s Executive Director of Infectious Disease R&D ‒ Formerly served as head of science at the United States Army Medical Research Institute of Infectious Diseases (USAMRIID) • At DoD/USAMRIID, his laboratory found that a small decrease in the expression or function of CD45 protects animals from multiple pathogens such as anthrax and Ebola virus 1,2 ‒ CD45 is a lymphocyte transmembrane receptor phosphatase • Dr. Bavari’s lab also discovered Remdesivir GS - 5734 in collaboration with Gilead and CDC ‒ That work was published in in Nature 2016 and showed showing that GS - 5734 (Remdesivir) works against SARS - CoV and MERS, which led to the testing of Remdesivir/GS - 5734 in SARS - CoV - 2 3 1 Panchal RG, et al., Cell Host Microbe . 2009 6(2):162 - 73. doi : 10.1016/j.chom.2009.07.003. PMID: 19683682. 2 Panchal RG, et al. J Biol Chem. 2009 284(19):12874 - 85. doi : 10.1074/jbc.M809633200 3 Warren TK, et al. Nature . 2016 531(7594):381 - 5. doi : 10.1038/nature17180.

13 © 2024 Tonix Pharmaceuticals Holding Corp. INFECTIOUS DISEASE PORTFOLIO Other Programs at Tonix – Non - dilutive Funding • Tonix has other broad spectrum antiviral programs ongoing ‒ Cathepsin inhibitors ‒ Protein - engineered lectins • Other Government Support ‒ Tonix’s TNX - 1800 vaccine was selected by the National Institutes of Health (NIH), the National Institute of Allergic and Infectious Diseases (NIAID) for Project NextGen, an in - kind award in which NIAID will study Tonix’s COVID - 19 vaccine platform in a Phase 1 study ‒ The DoD CDMRP* has also provided $3 M of funding to support a University of North Carolina (UNC) study of Tonix’s TNX - 102 SL drug to treat Acute Stress Disorder (ASD) and prevent PTSD after motor vehicle collisions ‒ The National Institute of Drug Abuse (NIDA) has awarded $5.2 M in a grant to support Tonix’s Phase 2 development of TNX - 1300, a cocaine antidote *CDMRP = Congressionally directed medical research program

© 2024 Tonix Pharmaceuticals Holding Corp. THANK YOU