
TONIX PHARMACEUTICALS HOLDING CORP. - 8-K
Exhibit 99.02

Redox ©2024 Mannitol as a eutectic forming agent for improved sublingual delivery of Cyclobenzapine HCl Prof. Marino Nebuloni 11 th Global Conference on Pharmaceutics and Novel Drug Delivery Systems (PDDS), Rome September 19, 2024. Oral Presentation.

Redox ©2024 Disclosures • Prof . Nebuloni is an employee of Redox Analytical Science Srl • Research funded by Tonix Pharmaceuticals, Inc . 2

Redox ©2024 Content • Drug – excipient compatibility strategy • Eutectic solid state complex formation : benefit on dissolution profile and physical and chemical stability • Case study : Cyclobenzaprine HCl - excipient compatibility for sublingual drug product formulation • D - mannitol polymorphism influence on eutectic formation • Investigation by suitable techniques - thermal analysis, X - ray spectroscopy, Intrinsic Dissolution Rate, Scanning Electron Microscopy, C 13 NMR at solid state, etc . • Characterization and pharmaceutical influence of eutectic in pharmaceutical properties - stability, dissolution, particle morphology and machinability on sublingual tablet formulation - • Influence of basic excipient present in the drug product . • Conclusion 3

Redox ©2024 Compatibility strategy excipient impact 4 • Pharmaceutical excipients have important functions such as serving as carriers, improving drug stability, solubilization and increasing or slowing down the drug release . • The laws and regulatory agencies have made rigid regulations on the compatibility of APIs and excipients of various dosage forms . • Pharmaceutical Development Q 8 (R 2 ) – ICH . Chapter “ 2 . 1 . 1 ” points out that a compatibility test of APIs and excipients is required at the beginning of formulation development • The early prediction of drug - excipient incompatibility is vital in the pharmaceutical industry to avoid costly material wastage and time delays
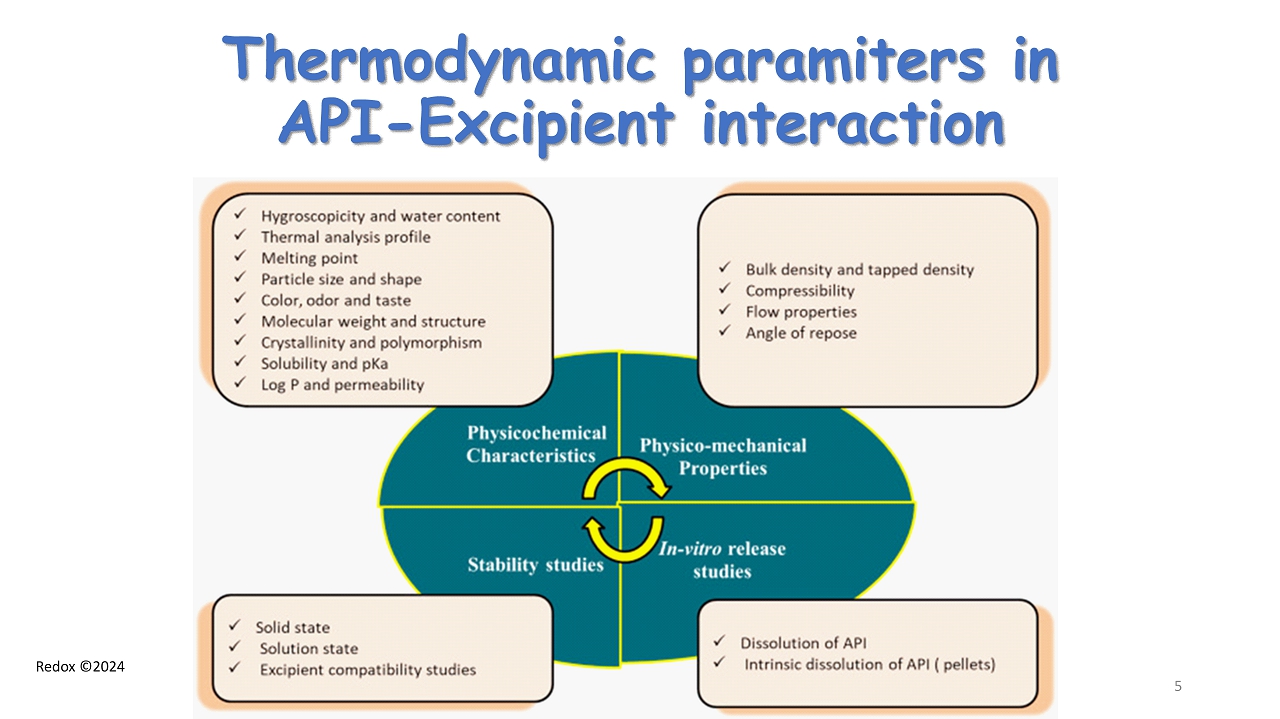
Redox ©2024 Thermodynamic paramiters in API - Excipient interaction 5
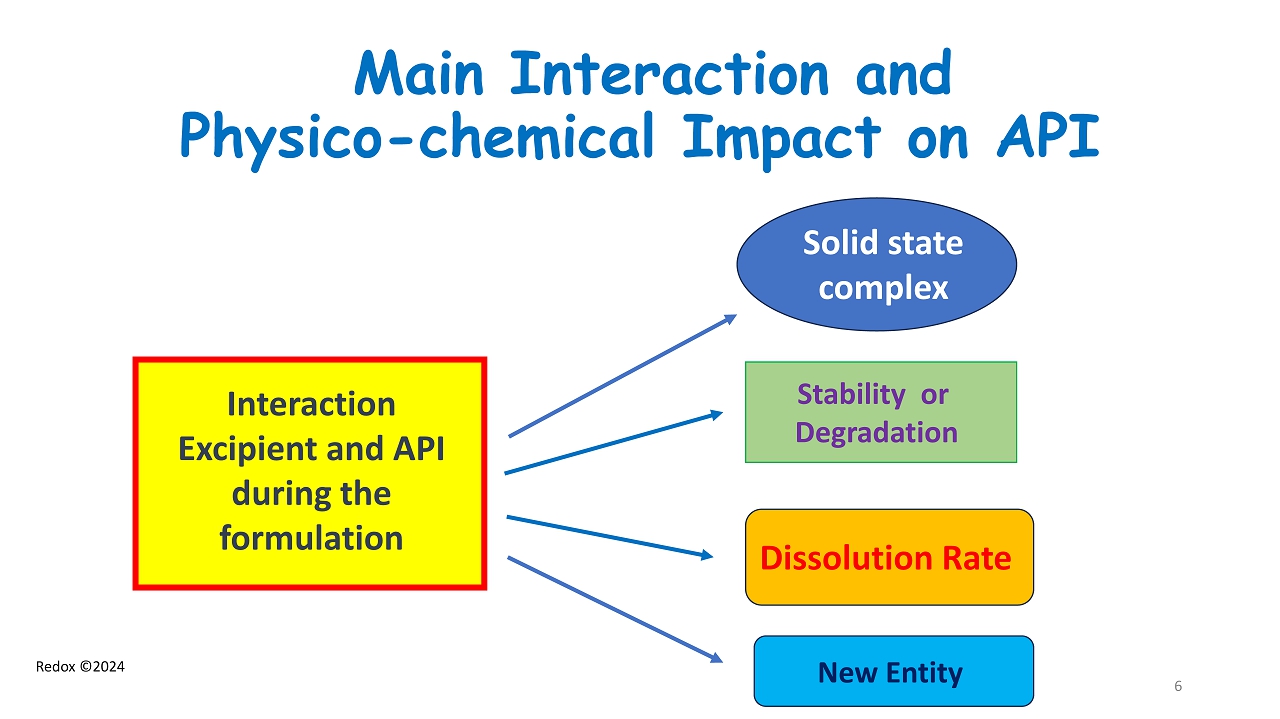
Redox ©2024 Main Interaction and Physico - chemical Impact on API Solid state complex Dissolution Rate Stability or Degradation New Entity Interaction Excipient and API during the formulation 6

Redox ©2024 Drug Excipient Compatibility - Experimental Design - • Before initiating drug product development , the formulation scientist must fully consider the chemical structure of the API and the type of delivering system • Initial selection of excipients should be based on appropriate delivery characterisation . • Potential mechanism of degradation of the drug . • Know chemical incompatibility reported in published information 7

Redox ©2024 Analytical Methods for drug - excipient compatibility assessment • Thermal Methods (DSC, TGA, Hot Stage Micros .) • FT - IR Spectroscopy • X - Ray Powder Diffraction • Scanning Electron Microscopy (SEM) • Dissolution Rate profile & Intrinsic Dissolution Rate • NMR at the solid state 8
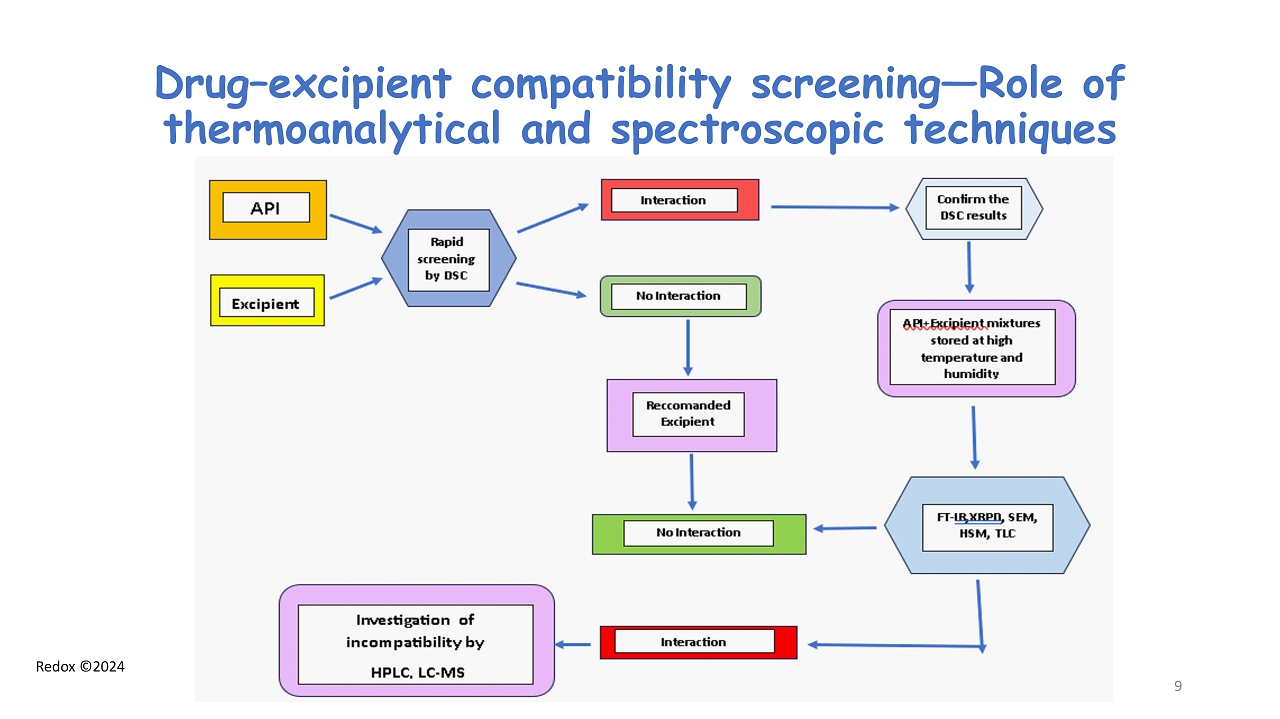
Redox ©2024 Drug – excipient compatibility screening — Role of thermoanalytical and spectroscopic techniques 9
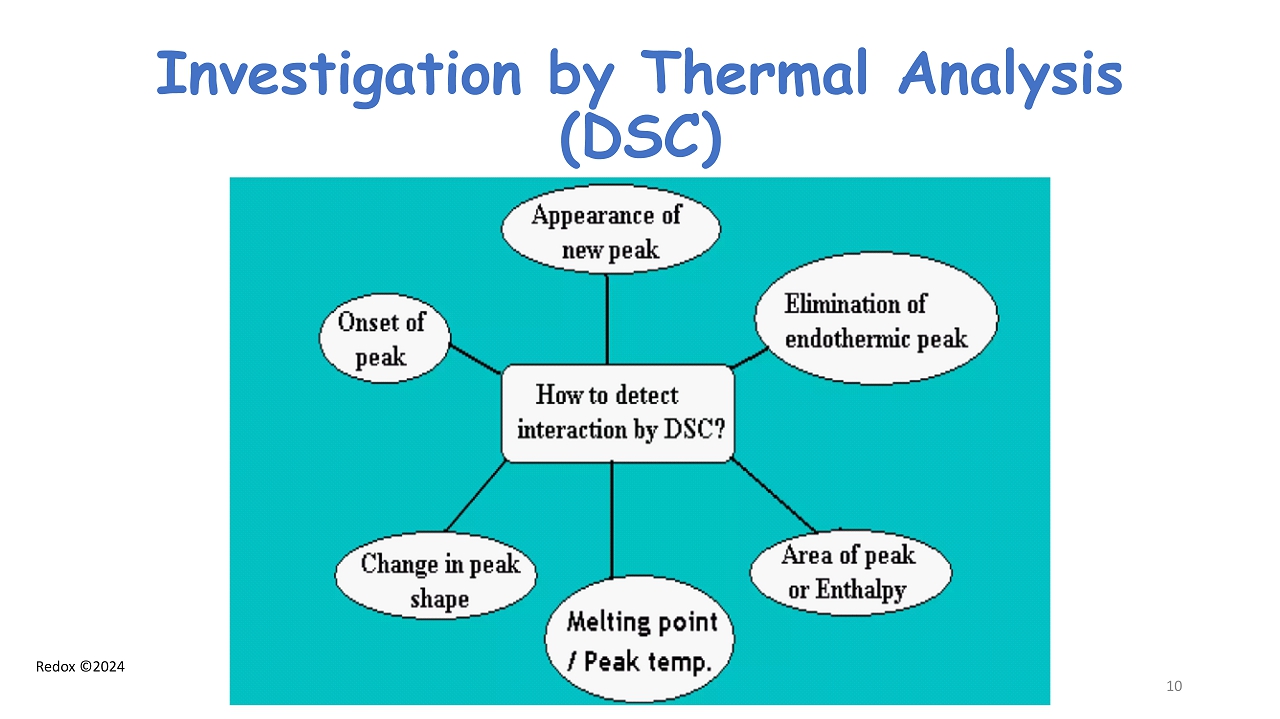
Redox ©2024 Investigation by Thermal Analysis (DSC) 10
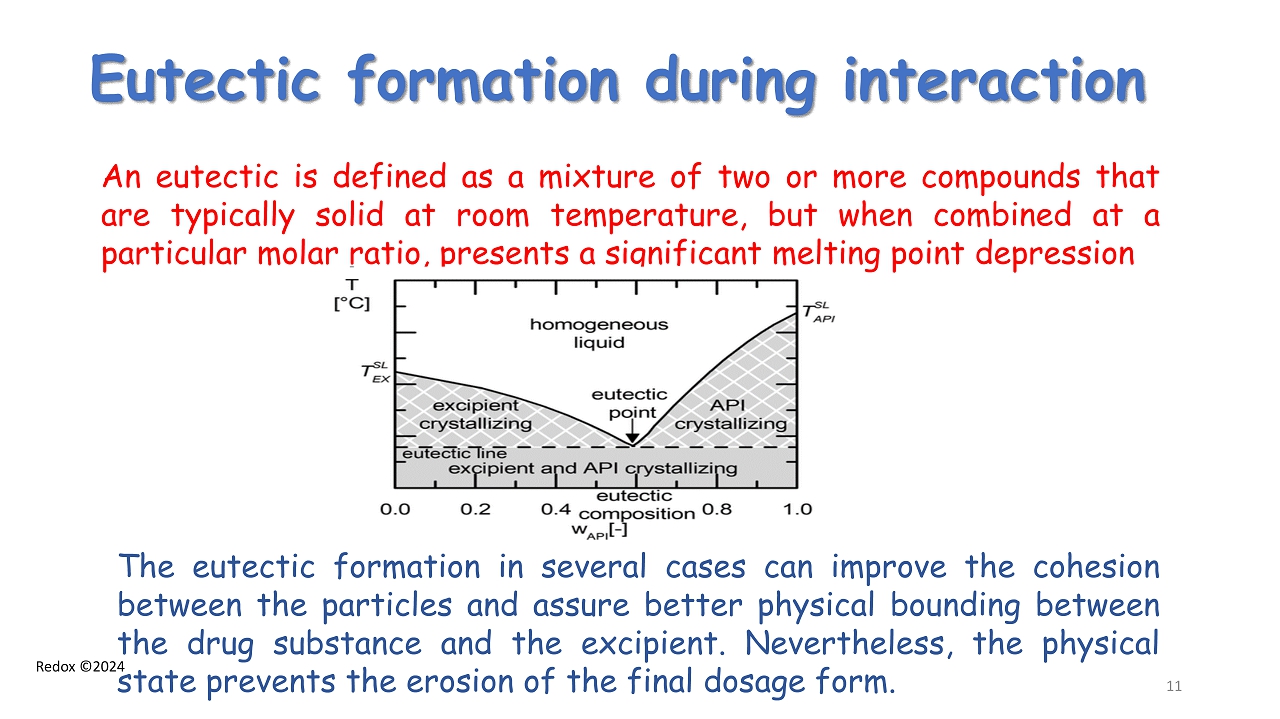
Redox ©2024 Eutectic formation during interaction The eutectic formation in several cases can improve the cohesion between the particles and assure better physical bounding between the drug substance and the excipient . Nevertheless, the physical state prevents the erosion of the final dosage form . An eutectic is defined as a mixture of two or more compounds that are typically solid at room temperature, but when combined at a particular molar ratio, presents a significant melting point depression 11

Redox ©2024 Case Study: compatibility investigation in sublingual formulation TNX - 102 SL ( Cyclobenzaprine HCl Sublingual Tablets ) 1 Tonix Pharmaceuticals Activity Fibromyalgia, Long COVID, Acute Stress Disorder…… 12 1 TNX - 102 SL is an experimental new drug and has not been approved for any indication
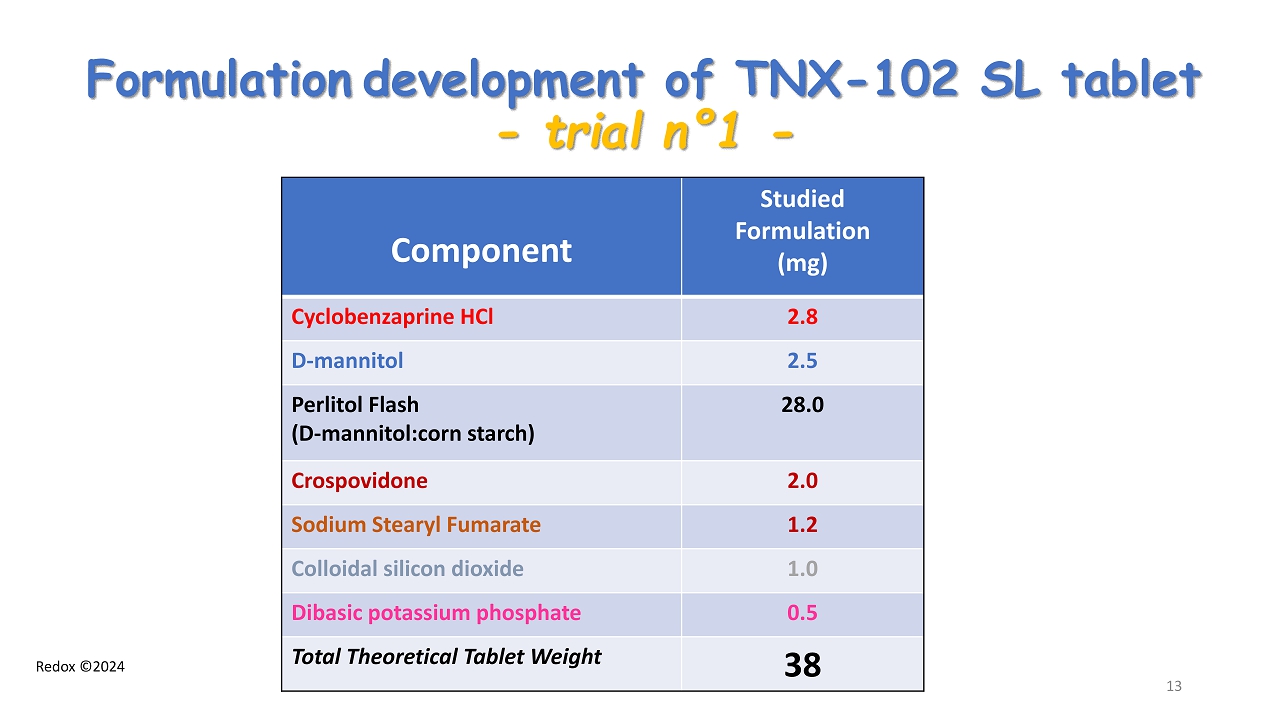
Redox ©2024 Formulation development of TNX - 102 SL tablet - trial n ° 1 - Component Studied Formulation (mg) Cyclobenzaprine HCl 2.8 D - mannitol 2.5 Perlitol Flash ( D - mannitol:corn starch ) 28.0 Crospovidone 2.0 Sodium Stearyl Fumarate 1.2 Colloidal silicon dioxide 1.0 Dibasic potassium phosphate 0.5 Total Theoretical Tablet Weight 38 13
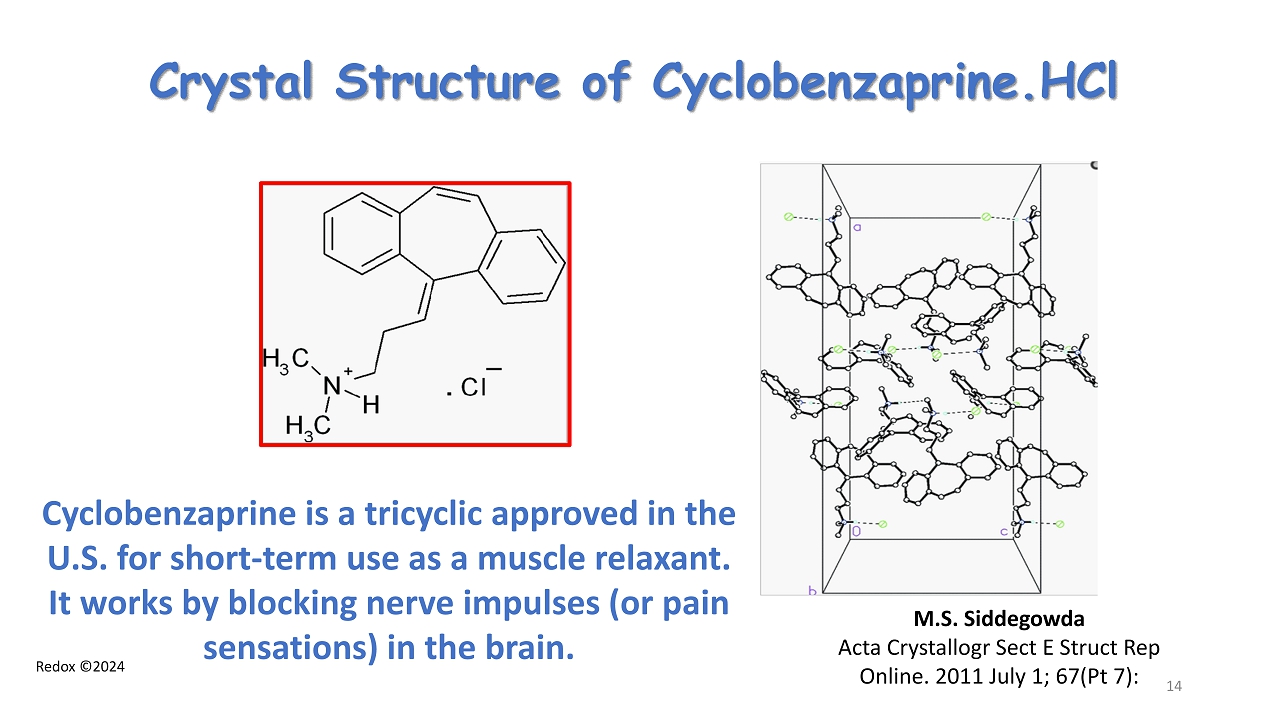
Redox ©2024 Crystal Structure of Cyclobenzaprine.HCl M.S. Siddegowda Acta Crystallogr Sect E Struct Rep Online. 2011 July 1; 67( Pt 7): 14 Cyclobenzaprine is a tricyclic approved in the U.S. for short - term use as a muscle relaxant. It works by blocking nerve impulses (or pain sensations) in the brain.
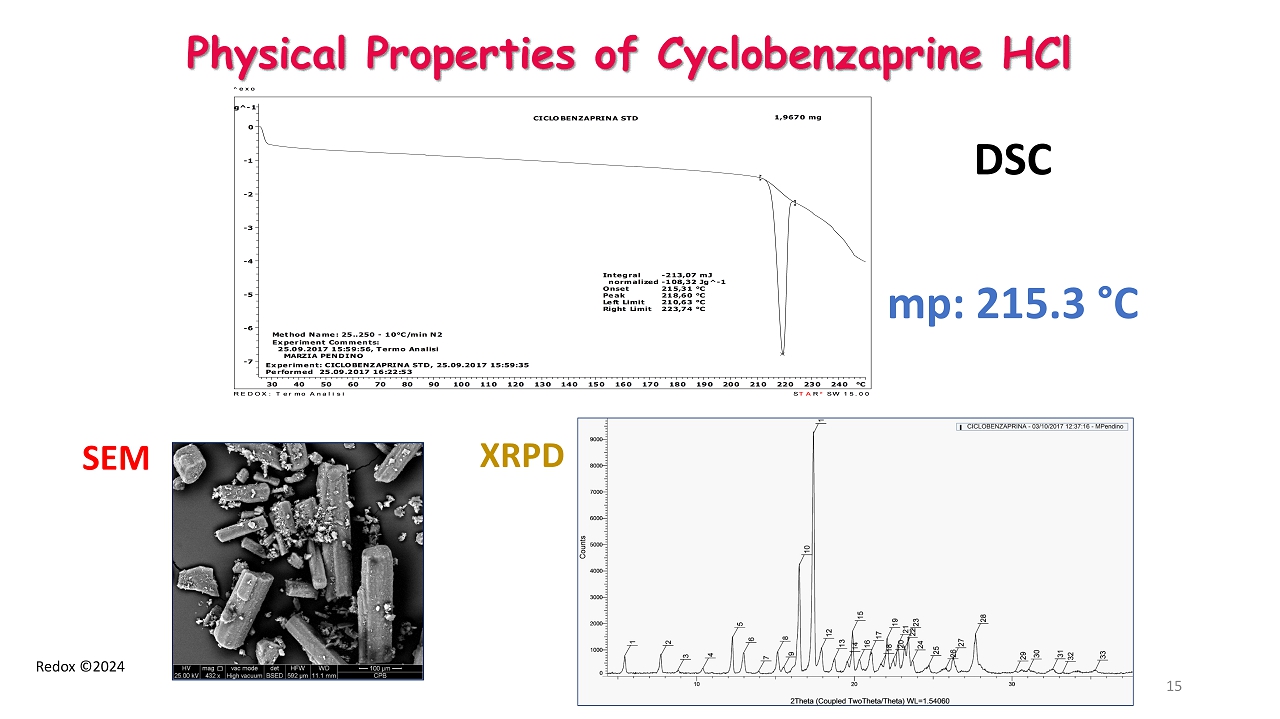
Redox ©2024 Physical Properties of Cyclobenzaprine HCl Integral -213,07 mJ normalized -108,32 Jg^-1 Onset 215,31 °C Peak 218,60 °C Left Limit 210,63 °C Right Limit 223,74 °C 1,9670 mg CICLOBENZAPRINA STD Experiment Comments: 25.09.2017 15:59:56, Termo Analisi MARZIA PENDINO Method Name: 25..250 - 10°C/min N2 Experiment: CICLOBENZAPRINA STD, 25.09.2017 15:59:35 Performed 25.09.2017 16:22:53 Wg^-1 -7 -6 -5 -4 -3 -2 -1 0 °C30 40 50 60 70 80 90 100 110 120 130 140 150 160 170 180 190 200 210 220 230 240 ^exo STARe SW 15.00 REDOX: Termo Analisi DSC mp: 215.3 ° C XRPD SEM 15
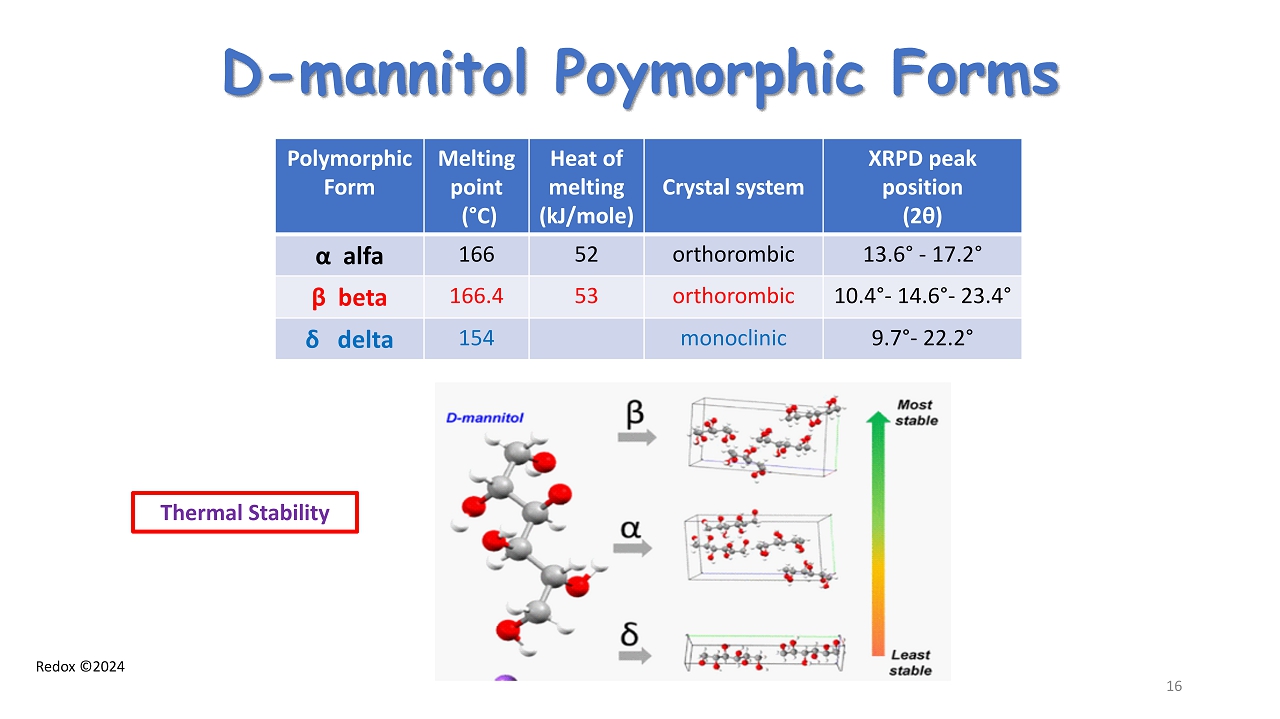
Redox ©2024 D - mannitol Poymorphic Forms Polymorphic Form Melting point ( ° C) Heat of melting (kJ/mole) Crystal system XRPD peak position (2 θ ) α alfa 166 52 orthorombic 13.6 ° - 17.2 ° β beta 166.4 53 orthorombic 10.4 ° - 14.6 ° - 23.4 ° δ delta 154 monoclinic 9.7 ° - 22.2 ° Thermal Stability 16
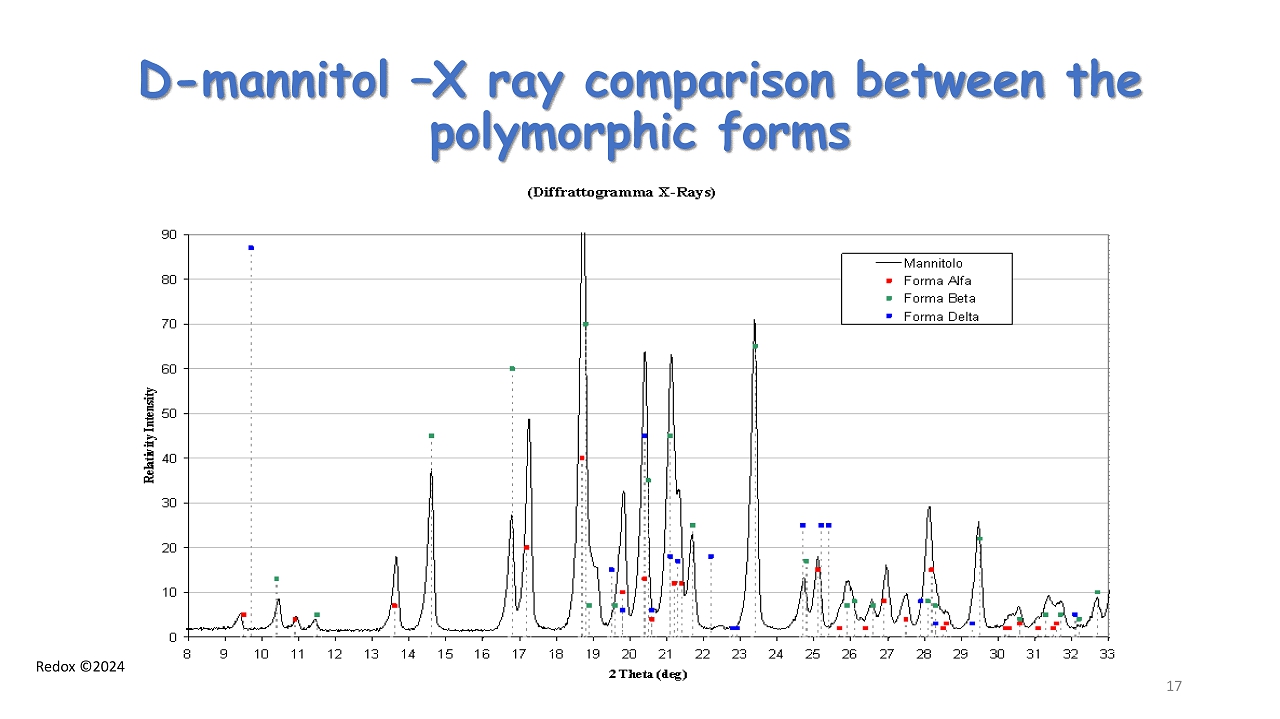
Redox ©2024 D - mannitol – X ray comparison between the polymorphic forms 17

Redox ©2024 D - mannitol crystallization and polymorphic transition Optical microscopy polarized light Metastable phase nucleation Delta Form Delta to Beta Metastable + Delta + Beta Crystalline Beta Form 18
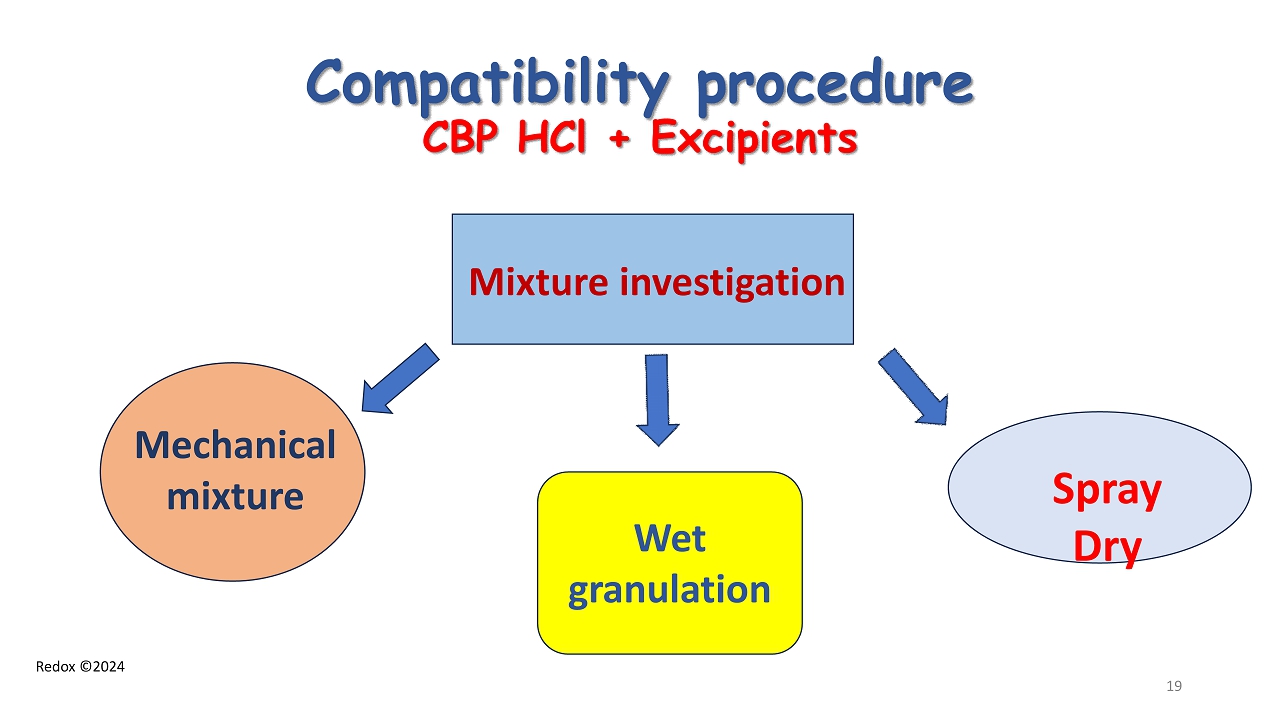
Redox ©2024 Compatibility procedure CBP HCl + Excipients Mechanical mixture Wet granulation Spray Dry Mixture investigation 19
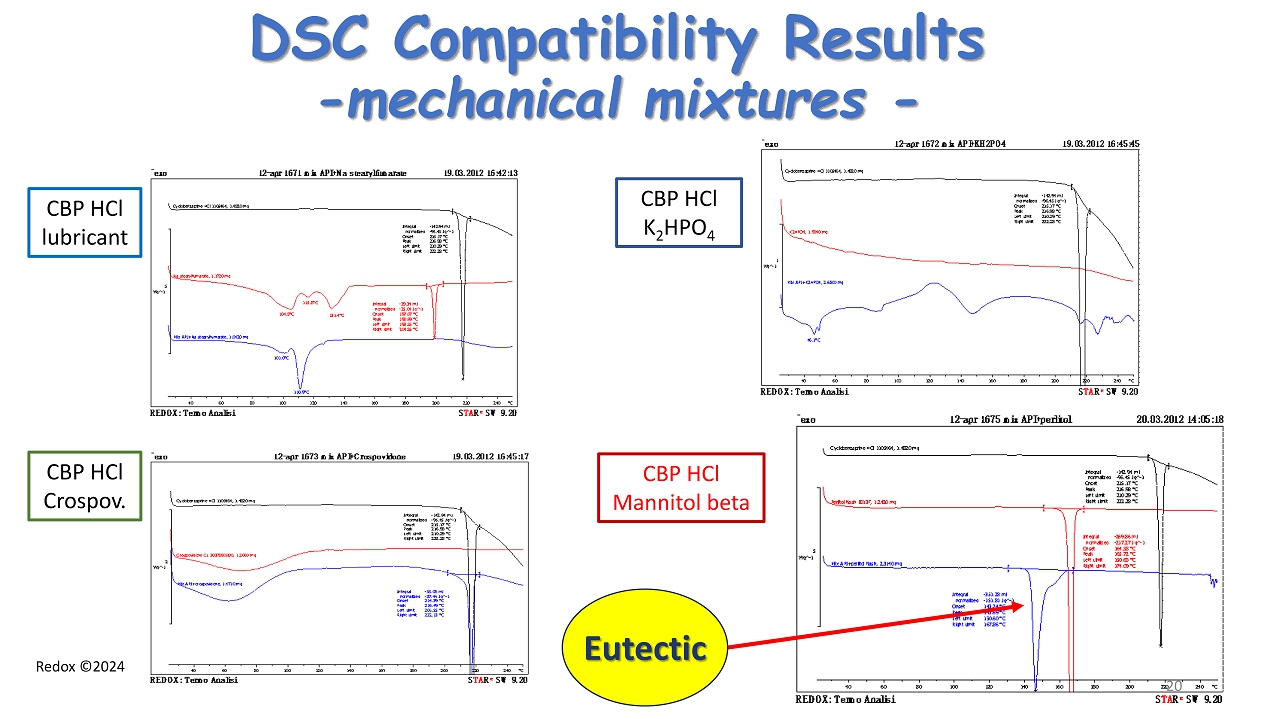
Redox ©2024 DSC Compatibility Results - mechanical mixtures - CBP HCl lubricant CBP HCl Crospov . CBP HCl K 2 HPO 4 CBP HCl Mannitol beta Eutectic 20
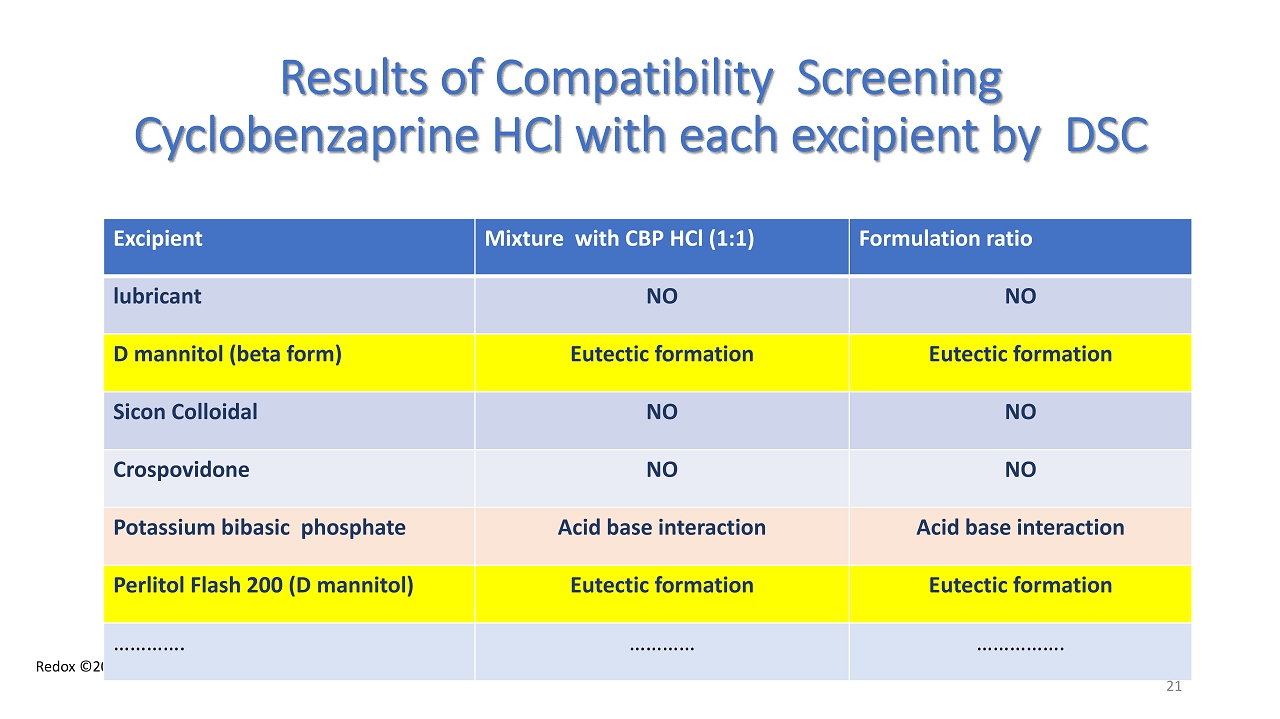
Redox ©2024 Results of Compatibility Screening Cyclobenzaprine HCl with each excipient by DSC Excipient Mixture with CBP HCl (1:1) Formulation ratio lubricant NO NO D mannitol (beta form ) Eutectic formation Eutectic formation Sicon Colloidal NO NO Crospovidone NO NO Potassium bibasic phosphate Acid base interaction Acid base interaction Perlitol Flash 200 (D mannitol ) Eutectic formation Eutectic formation …………. ………… ……………. 21
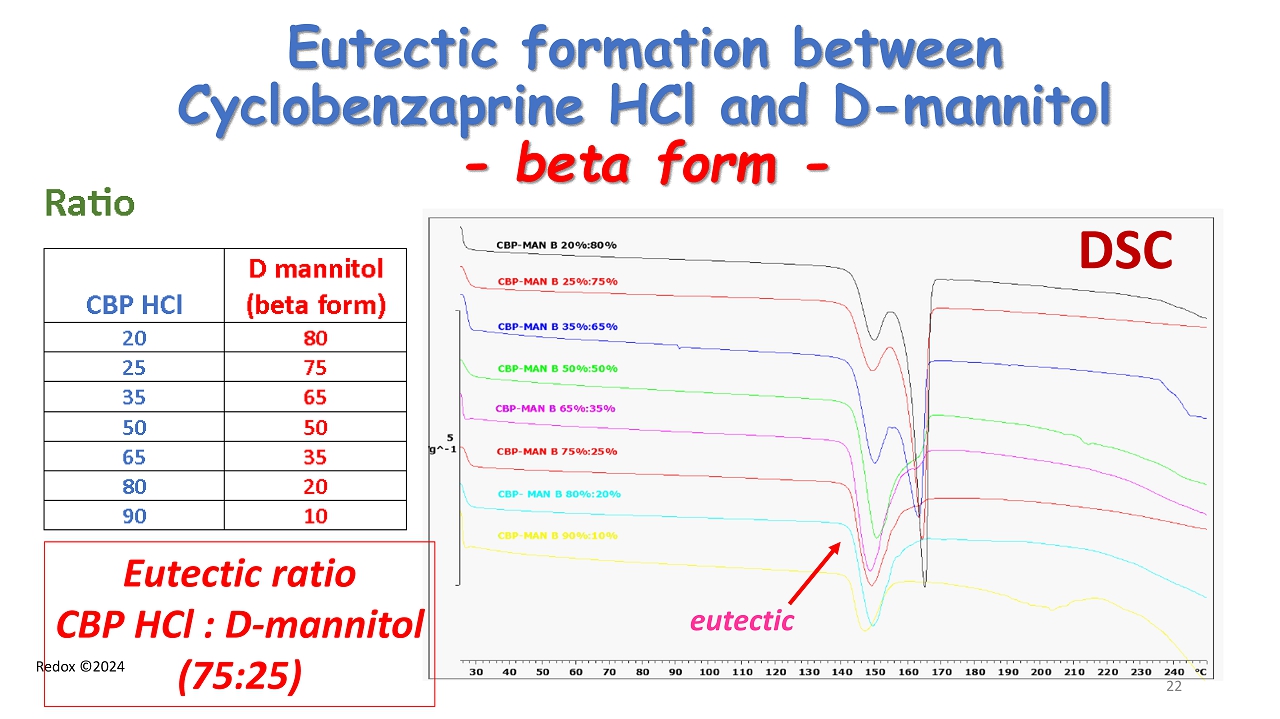
Redox ©2024 Eutectic formation between Cyclobenzaprine HCl and D - mannitol - beta form - DSC Eutectic ratio CBP HCl : D - mannitol (75:25) 22 eutectic
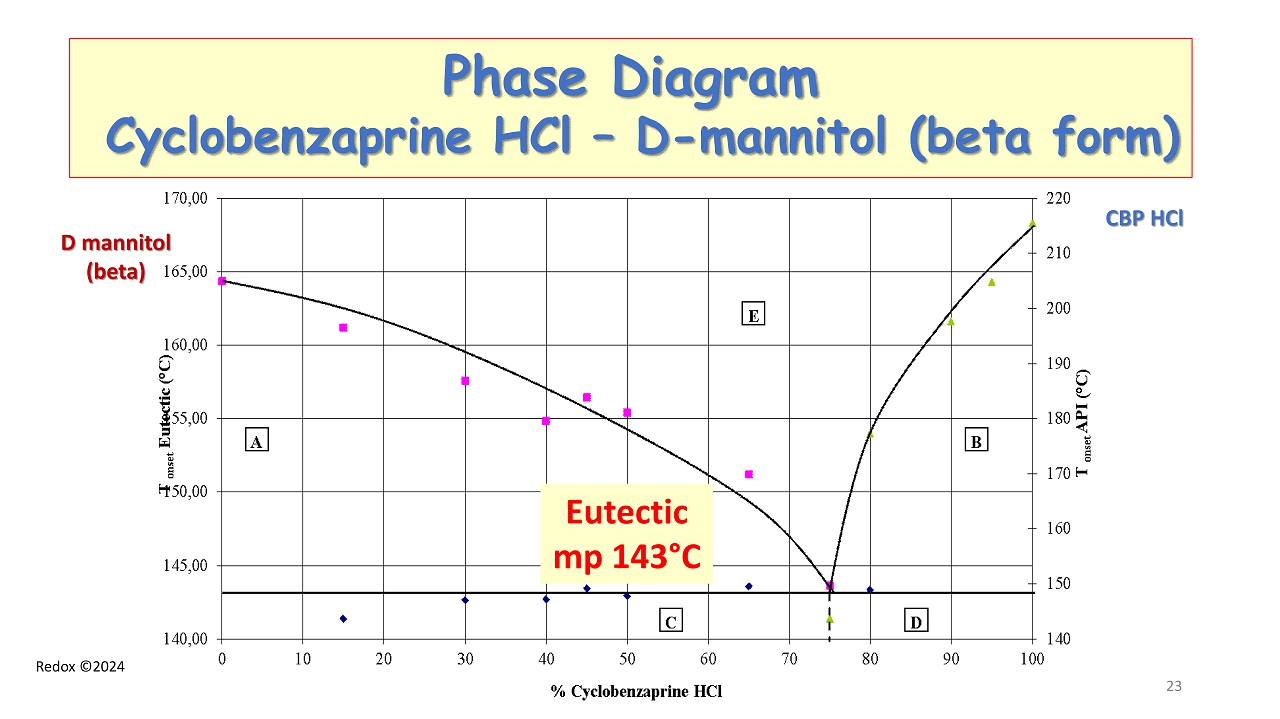
Redox ©2024 Phase Diagram Cyclobenzaprine HCl – D - mannitol (beta form ) CBP HCl D mannitol (beta) Eutectic mp 143 ° C 23
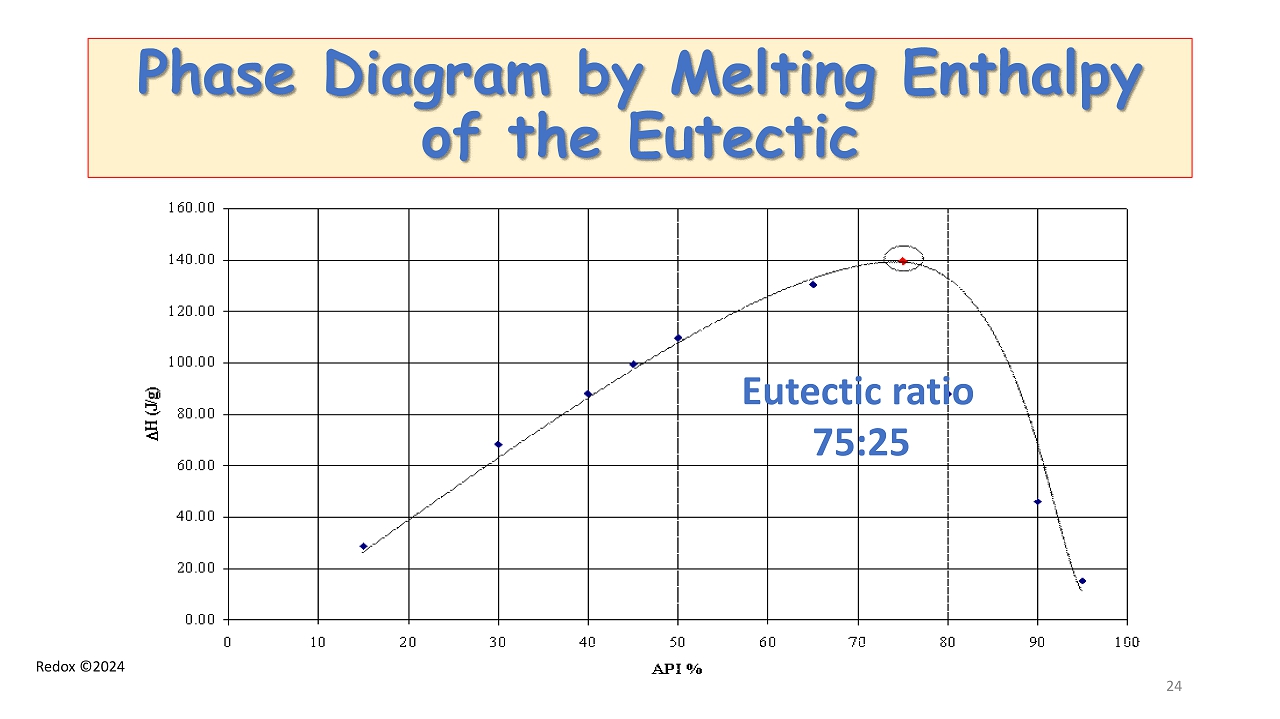
Redox ©2024 Phase Diagram by Melting Enthalpy of the Eutectic Eutectic ratio 75:25 24
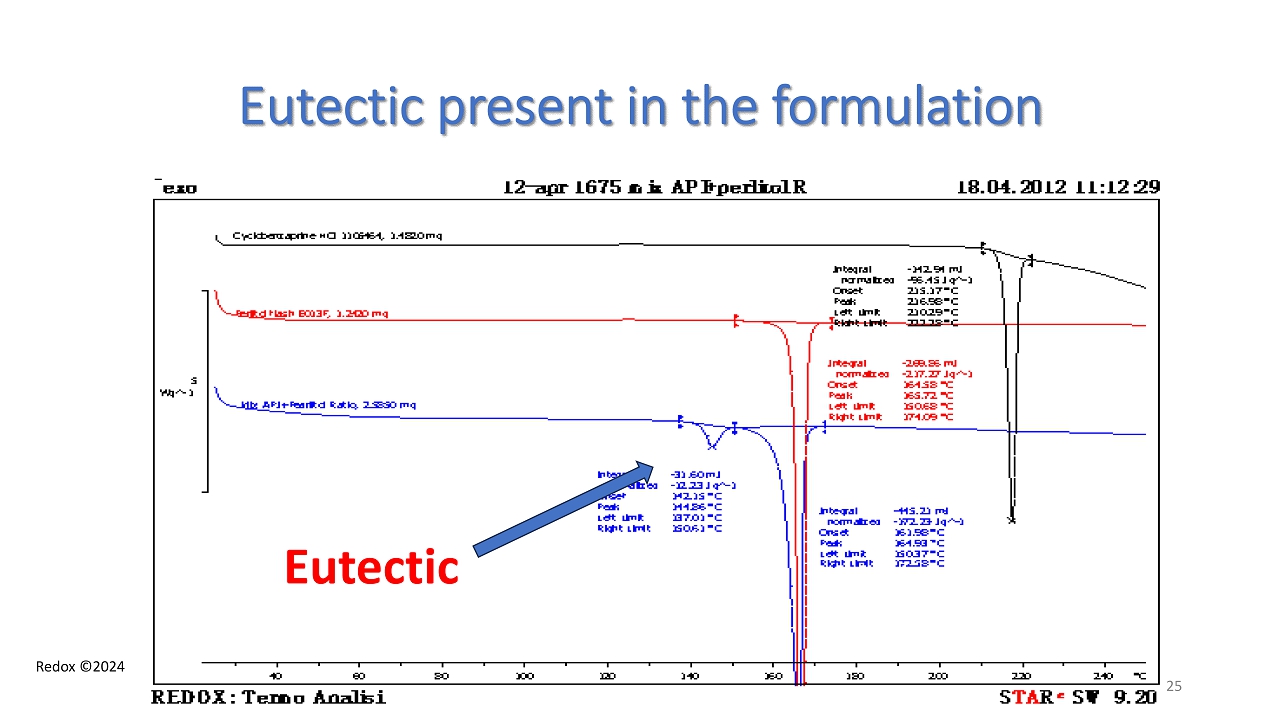
Redox ©2024 Eutectic present in the formulation Eutectic 25
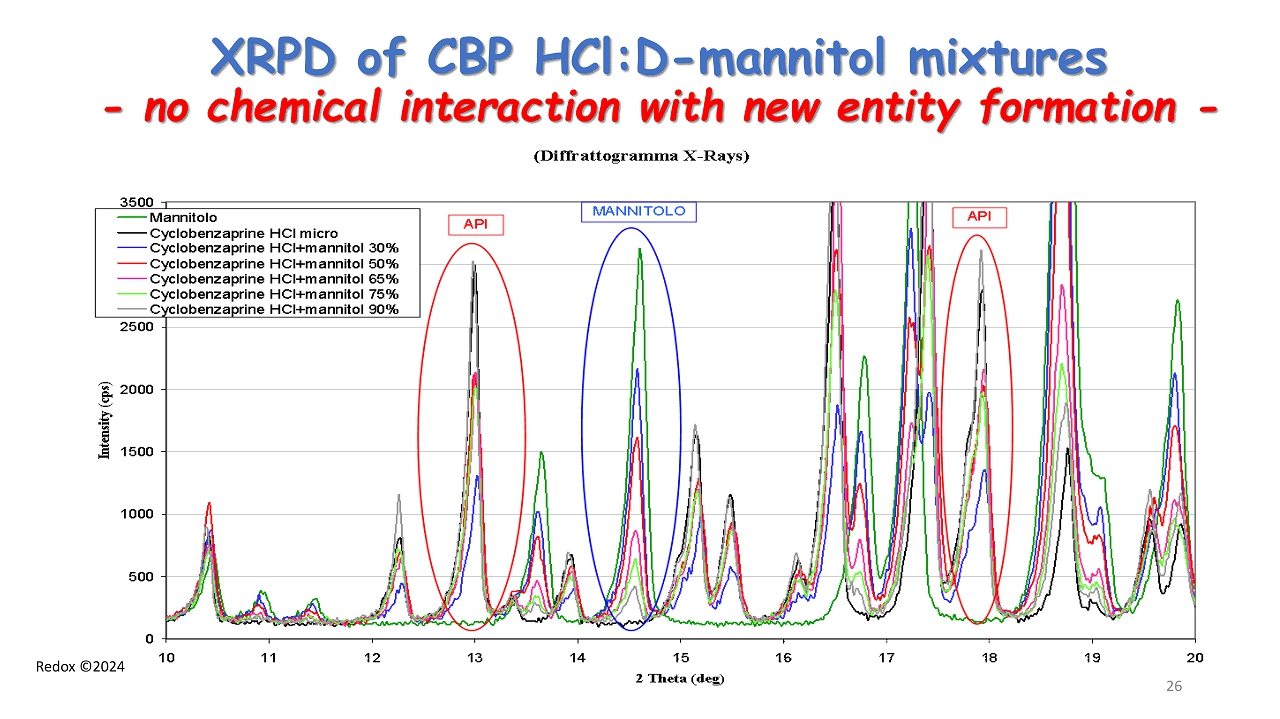
Redox ©2024 XRPD of CBP HCl:D - mannitol mixtures - no chemical interaction with new entity formation - 26
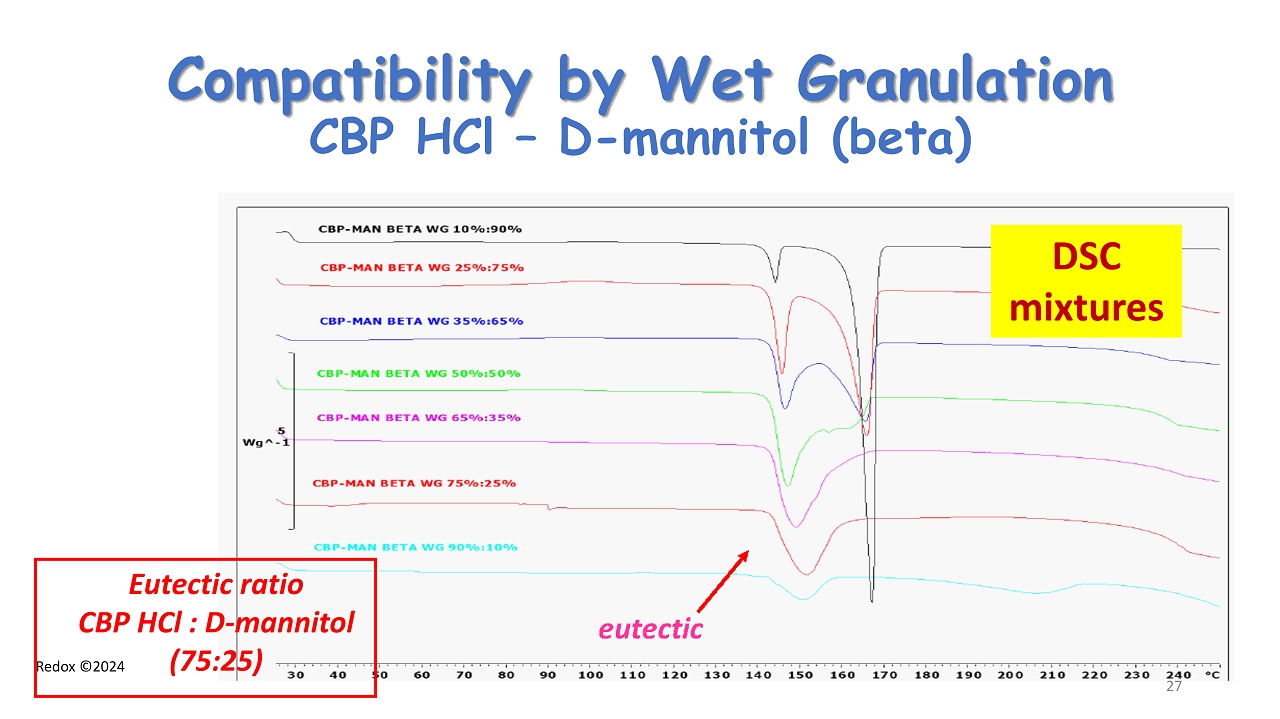
Redox ©2024 Compatibility by Wet Granulation CBP HCl – D - mannitol (beta) DSC mixtures Eutectic ratio CBP HCl : D - mannitol (75:25) 27
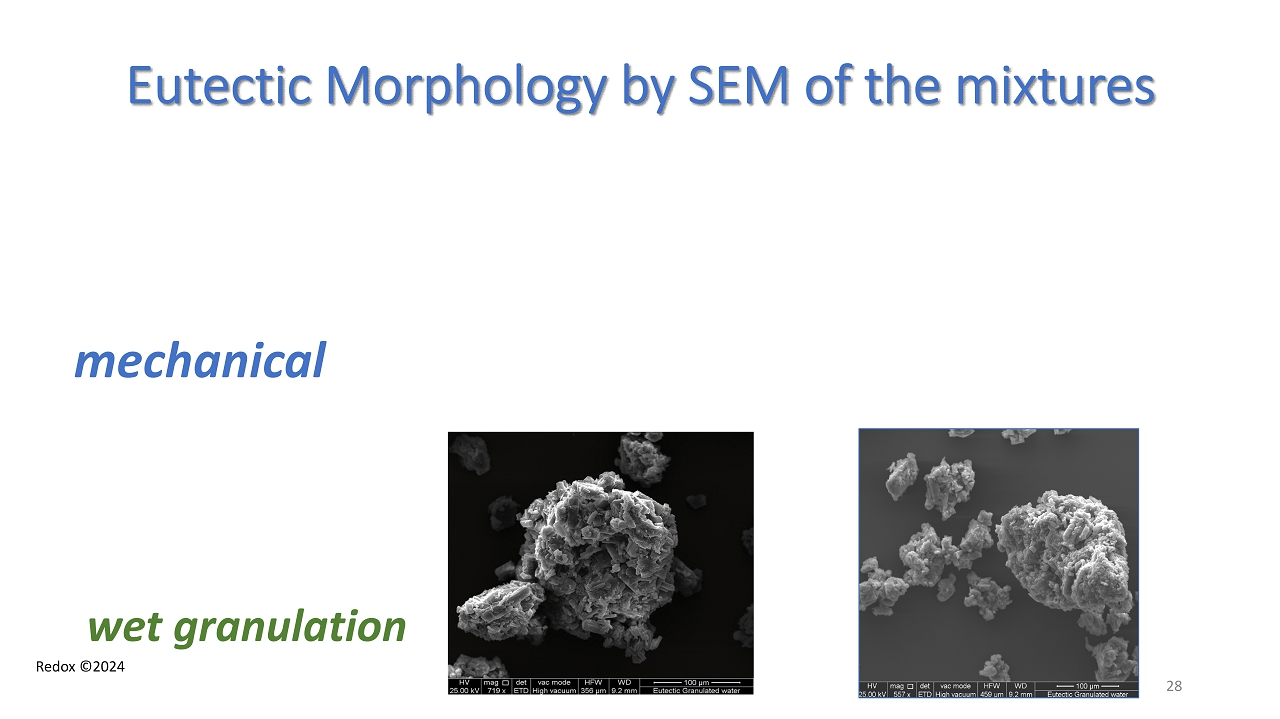
Redox ©2024 Eutectic Morphology by SEM of the mixtures mechanical wet granulation 28
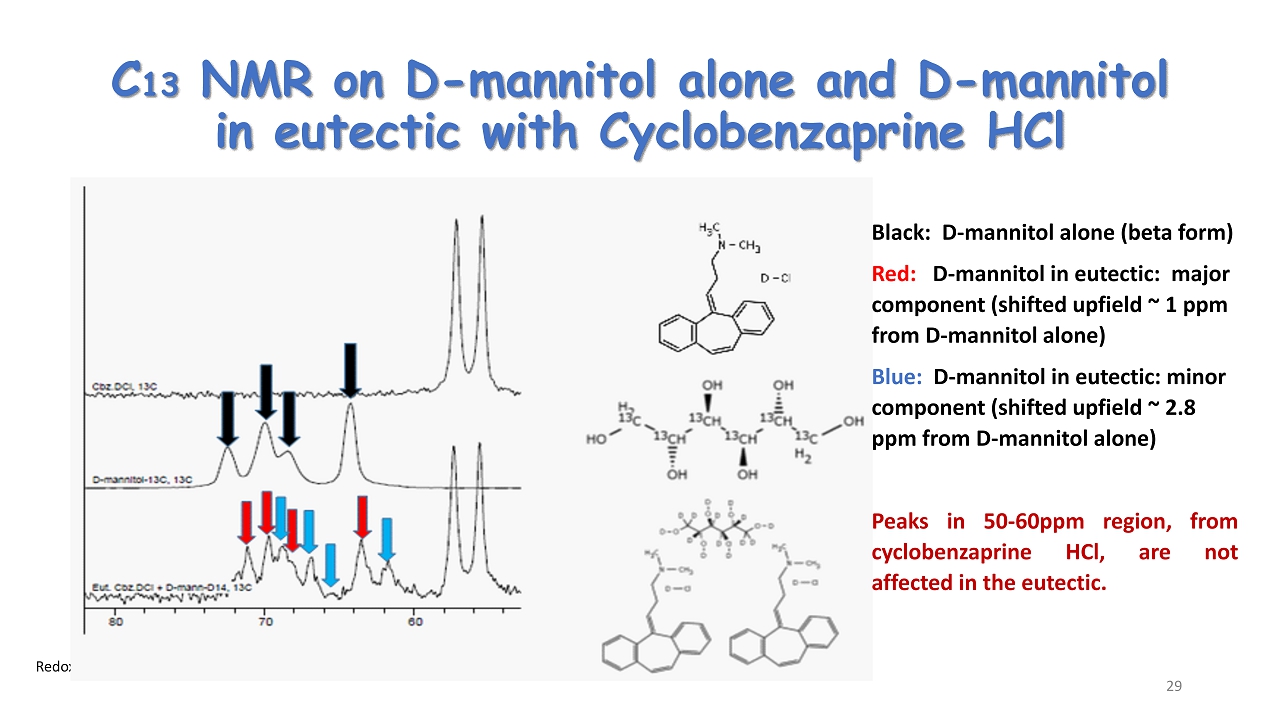
Redox ©2024 C 13 NMR on D - mannitol alone and D - mannitol in eutectic with Cyclobenzaprine HCl Black: D - mannitol alone (beta form) Red: D - mannitol in eutectic: major component (shifted upfield ~ 1 ppm from D - mannitol alone) Blue: D - mannitol in eutectic: minor component (shifted upfield ~ 2.8 ppm from D - mannitol alone) Peaks in 50 - 60 ppm region, from cyclobenzaprine HCl, are not affected in the eutectic . 29
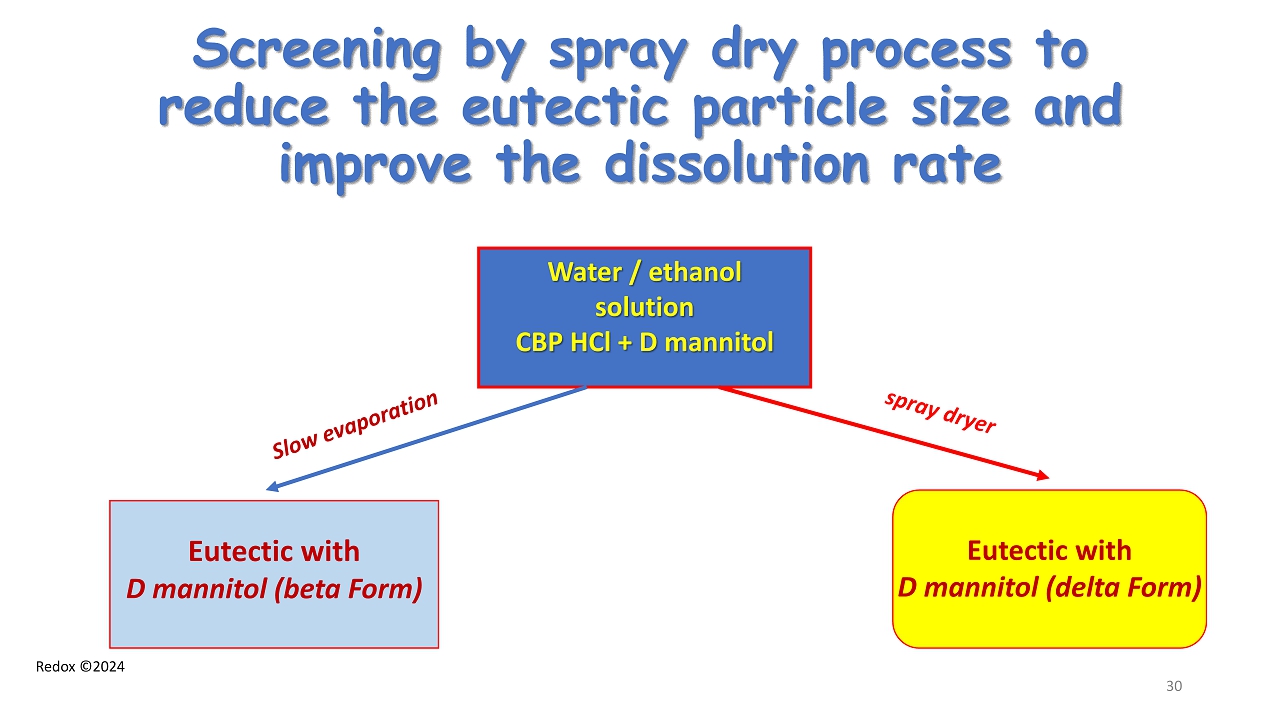
Redox ©2024 Screening by spray dry process to reduce the eutectic particle size and improve the dissolution rate Water / ethanol solution CBP HCl + D mannitol Eutectic with D mannitol (beta Form) Eutectic with D mannitol (delta Form) 30
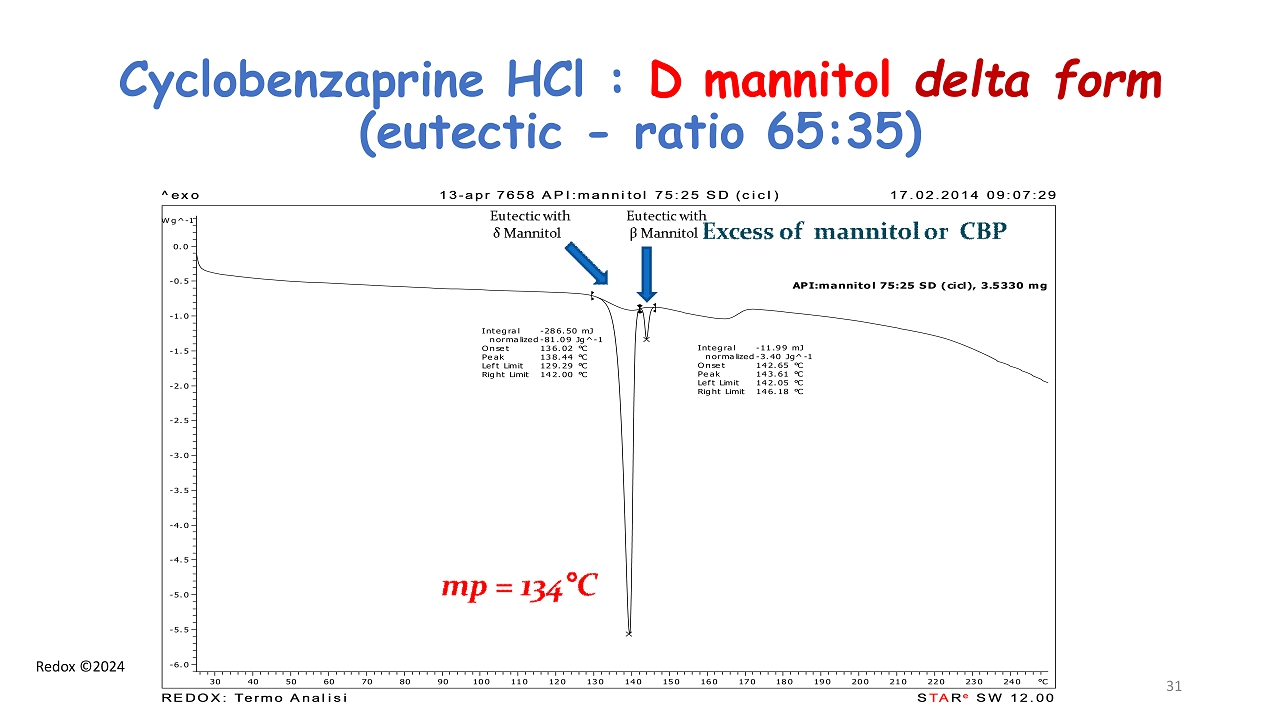
Redox ©2024 Cyclobenzaprine HCl : D mannitol delta form ( eutectic - ratio 65:35) API:mannitol 75:25 SD (cicl), 3.5330 mg Integral -11.99 mJ normalized -3.40 Jg^-1 Onset 142.65 °C Peak 143.61 °C Left Limit 142.05 °C Right Limit 146.18 °C Integral -286.50 mJ normalized -81.09 Jg^-1 Onset 136.02 °C Peak 138.44 °C Left Limit 129.29 °C Right Limit 142.00 °C Wg^-1 -6.0 -5.5 -5.0 -4.5 -4.0 -3.5 -3.0 -2.5 -2.0 -1.5 -1.0 -0.5 0.0 °C30 40 50 60 70 80 90 100 110 120 130 140 150 160 170 180 190 200 210 220 230 240 ^exo 13-apr 7658 API:mannitol 75:25 SD (cicl) 17.02.2014 09:07:29 STARe SW 12.00 REDOX: Termo Analisi 31
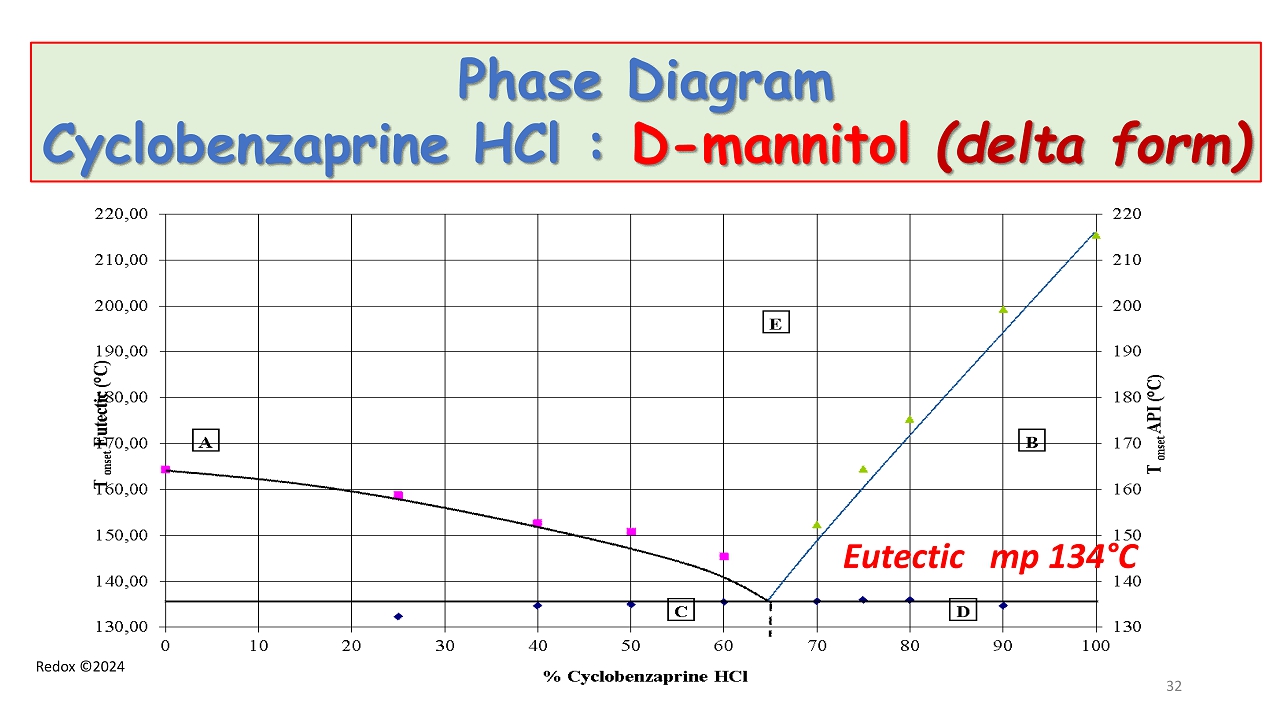
Redox ©2024 Phase Diagram Cyclobenzaprine HCl : D - mannitol (delta form ) Eutectic mp 134 ° C 32
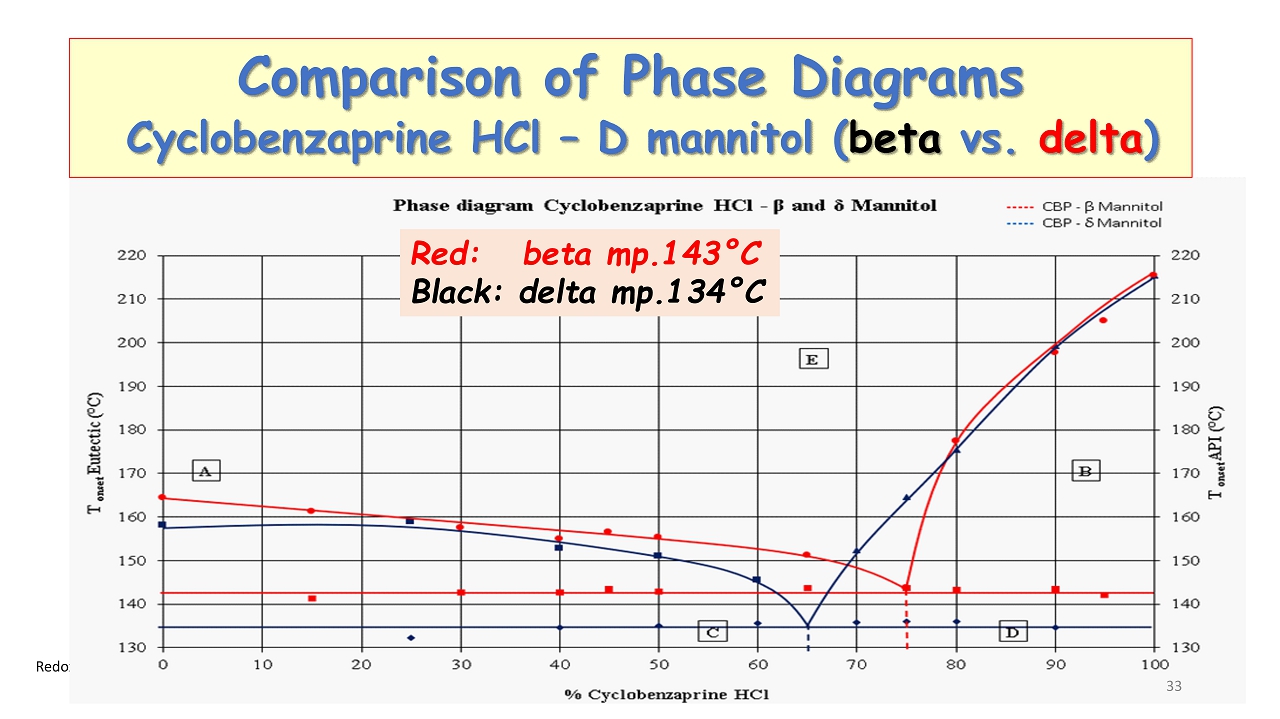
Redox ©2024 Comparison of Phase Diagrams Cyclobenzaprine HCl – D mannitol ( beta vs. delta ) Red: beta mp.143 ° C Black: delta mp.134 ° C 33
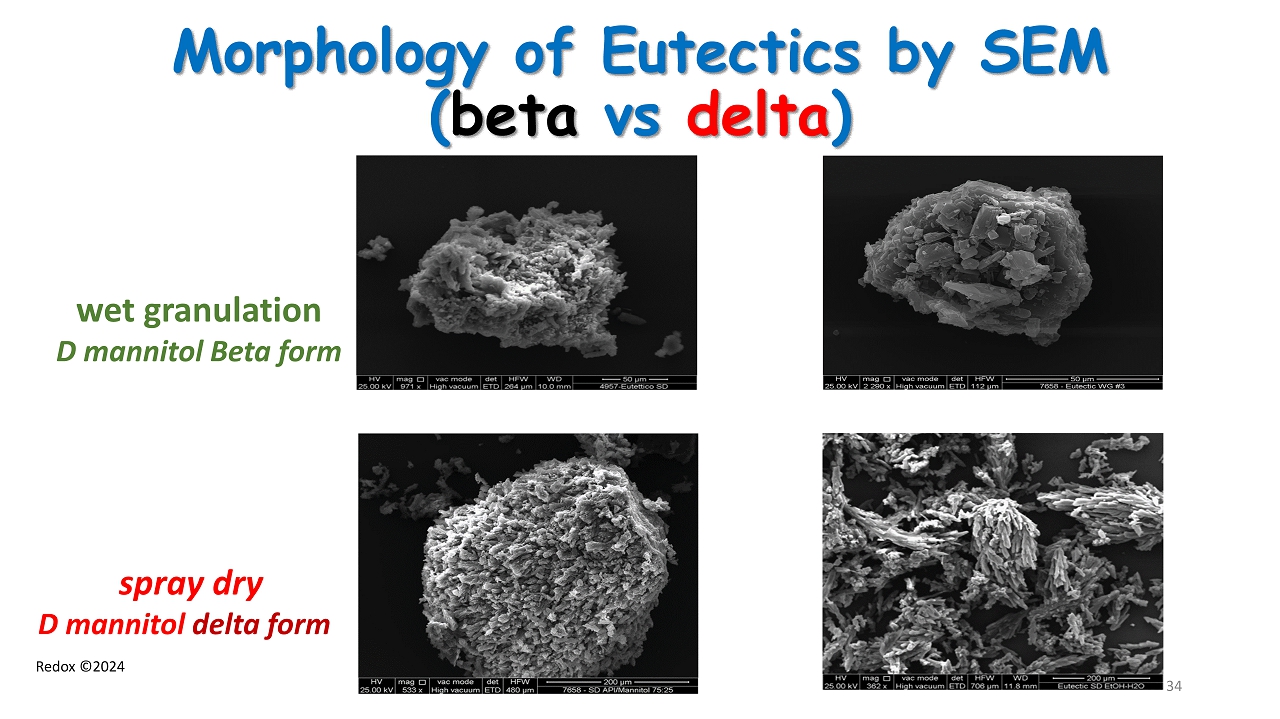
Redox ©2024 Morphology of Eutectics by SEM ( beta vs delta ) wet granulation D mannitol Beta form spray dry D mannitol delta form 34
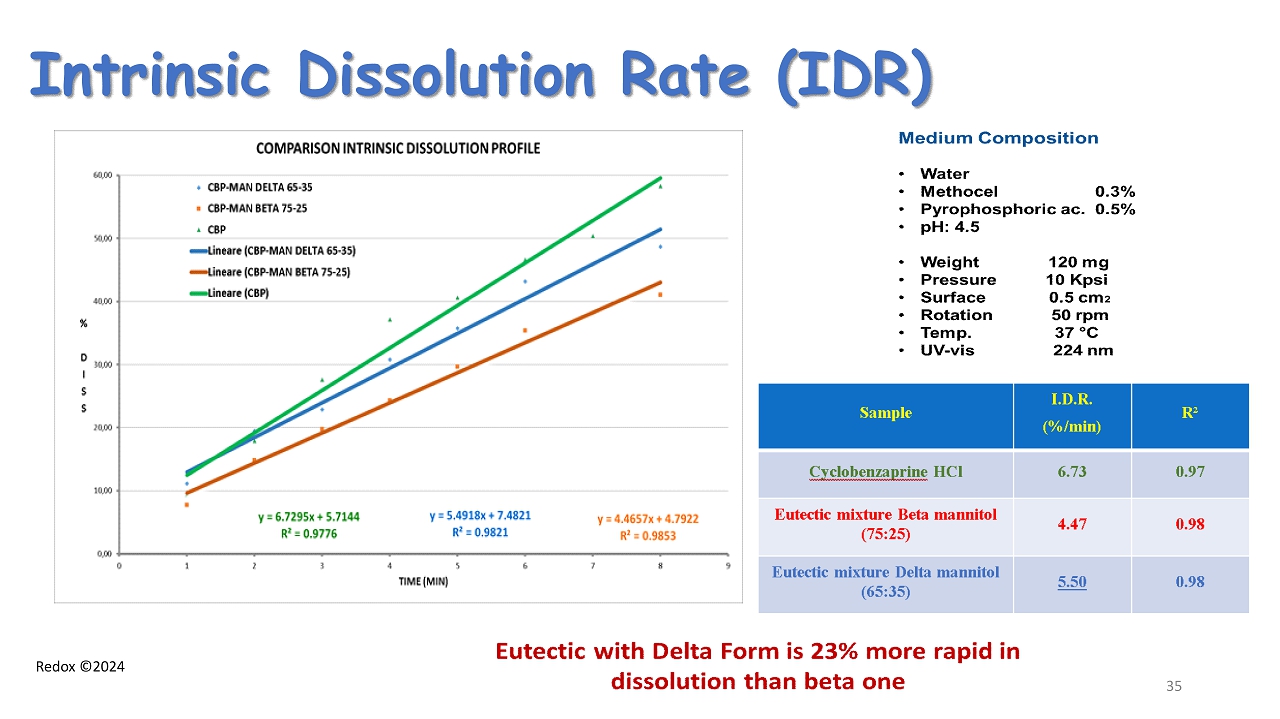
Redox ©2024 Intrinsic Dissolution Rate (IDR) 35
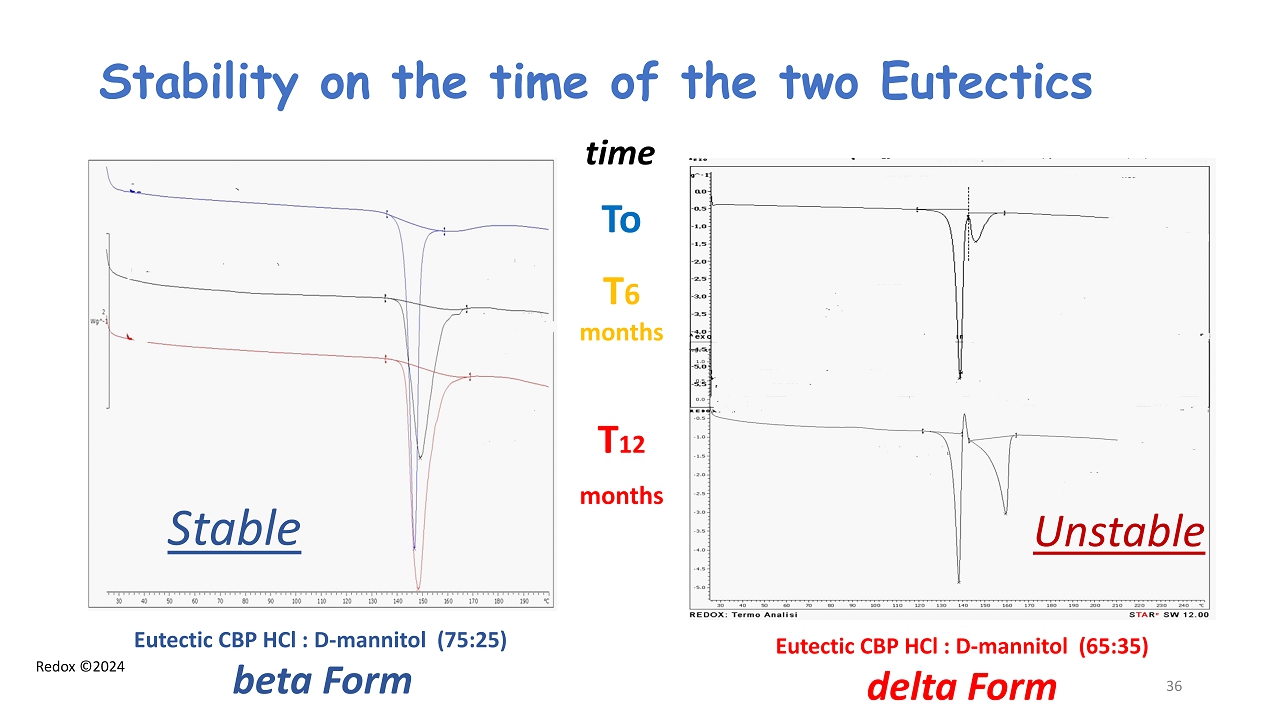
Redox ©2024 Stability on the time of the two Eutectics Eutectic CBP HCl : D - mannitol (75:25) beta Form To T 6 months T 12 months Eutectic CBP HCl : D - mannitol (65:35) delta Form time Stable Unstable 36
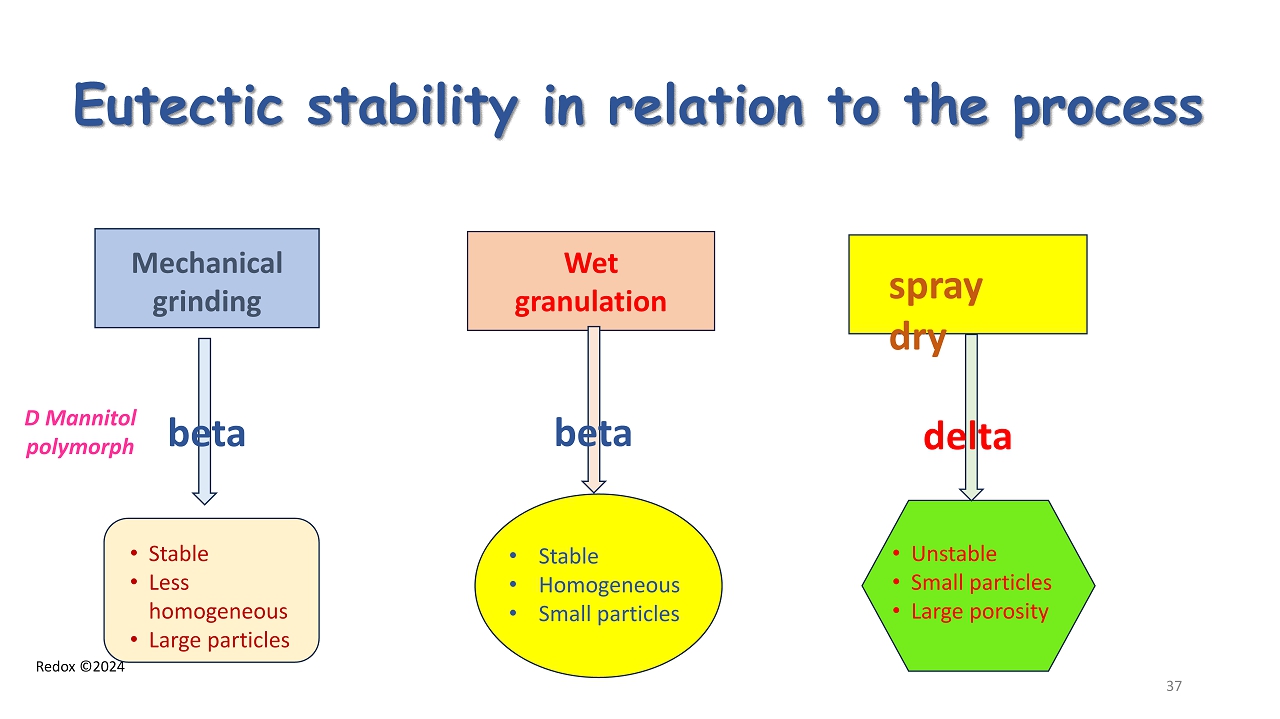
Redox ©2024 Eutectic stability in relation to the process Mechanical grinding Wet granulation spray dry D Mannitol polymorph • Stable • Less homogeneous • Large particles • Stable • Homogeneous • Small particles • Unstable • Small particles • Large porosity beta beta delta 37
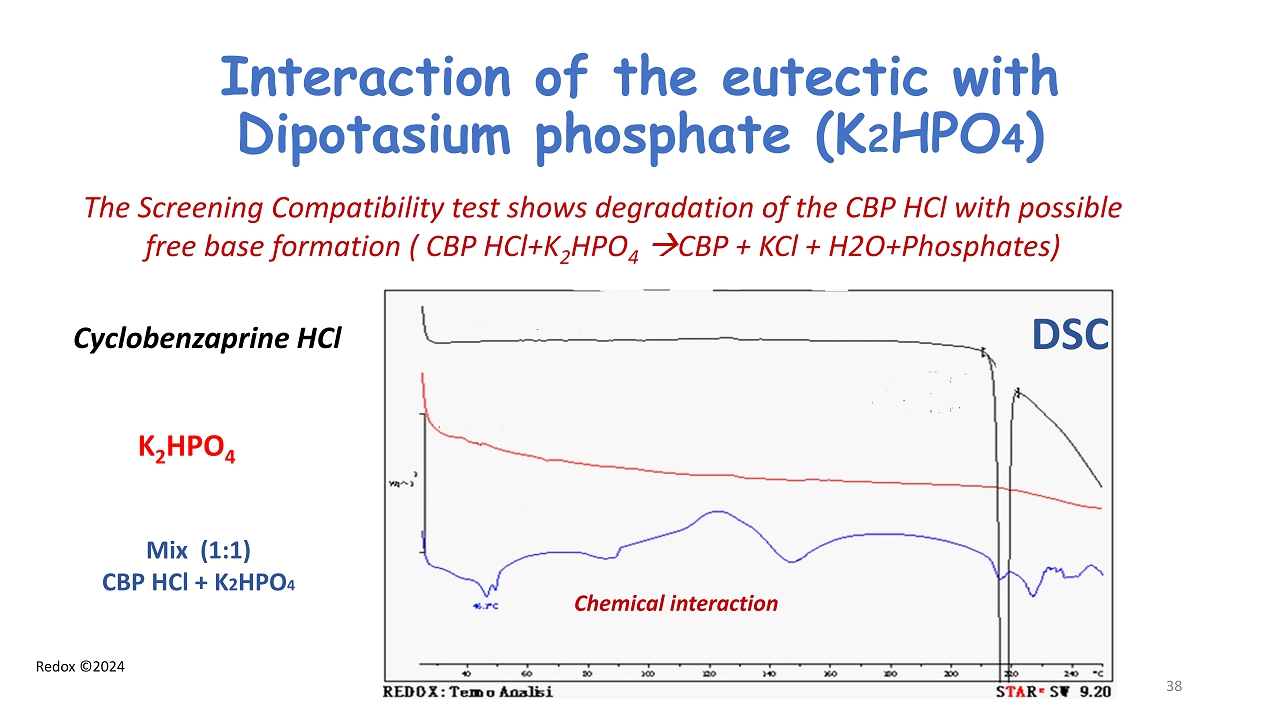
Redox ©2024 Interaction of the eutectic with Dipotasium phosphate (K 2 HPO 4 ) The Screening Compatibility test shows degradation of the CBP HCl with possible free base formation ( CBP HCl+K 2 HPO 4 CBP + KCl + H2O+Phosphates) Cyclobenzaprine HCl K 2 HPO 4 Mix (1:1) CBP HCl + K 2 HPO 4 Chemical interaction 38 DSC
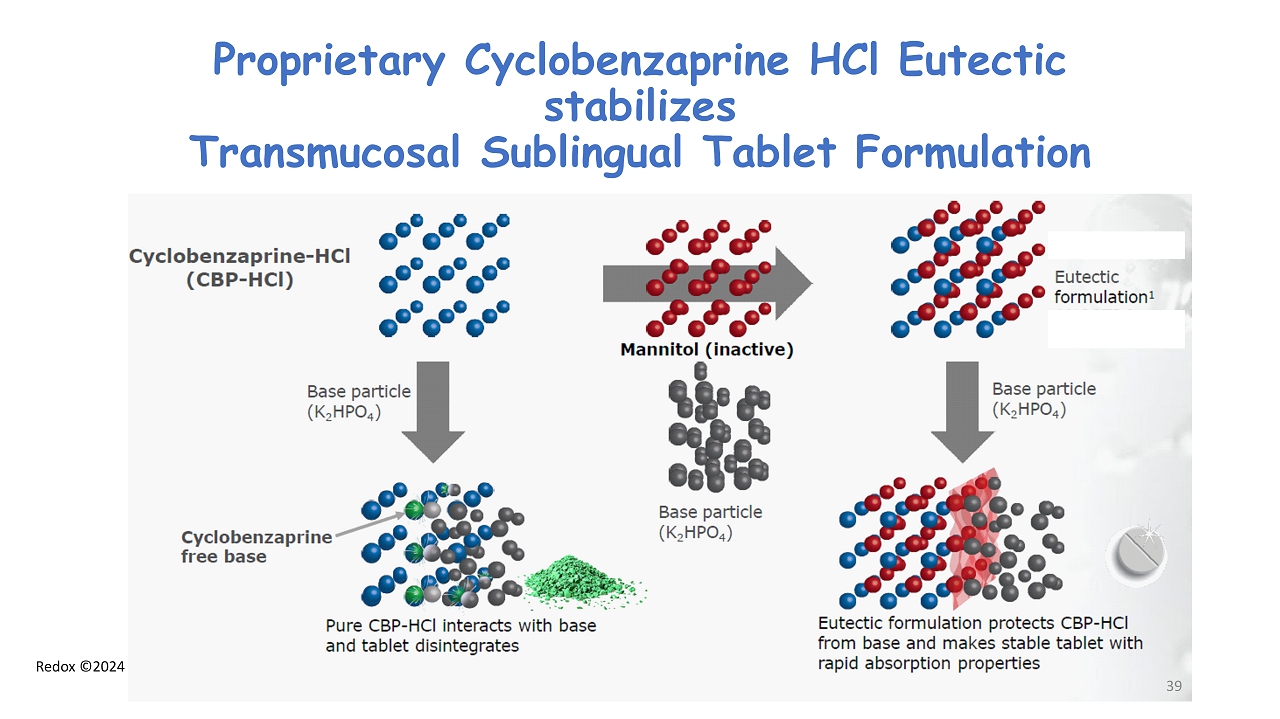
Redox ©2024 Proprietary Cyclobenzaprine HCl Eutectic stabilizes Transmucosal Sublingual Tablet Formulation 39
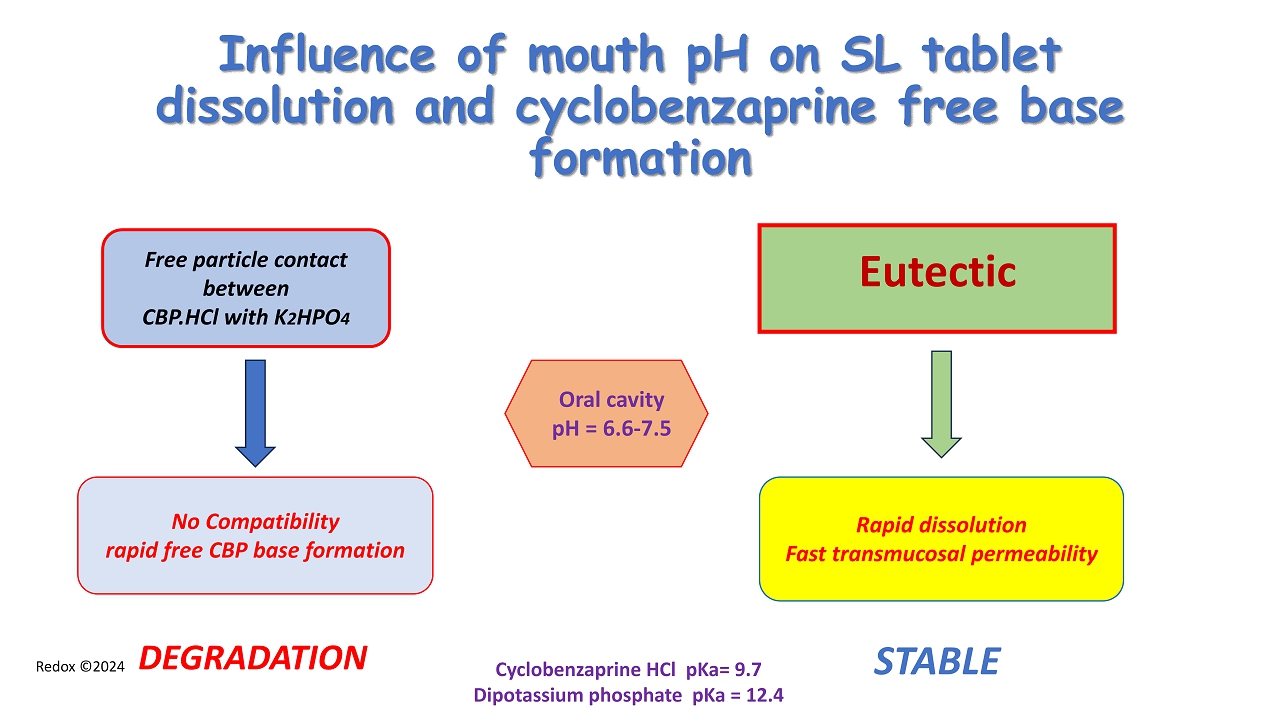
Redox ©2024 Influence of mouth pH on SL tablet dissolution and cyclobenzaprine free base formation No Compatibility rapid free CBP base formation Cyclobenzaprine HCl pKa = 9.7 Dipotassium phosphate pKa = 12.4 Oral cavity pH = 6.6 - 7.5 Eutectic Free particle contact between CBP.HCl with K 2 HPO 4 DEGRADATION Rapid dissolution Fast transmucosal permeability STABLE
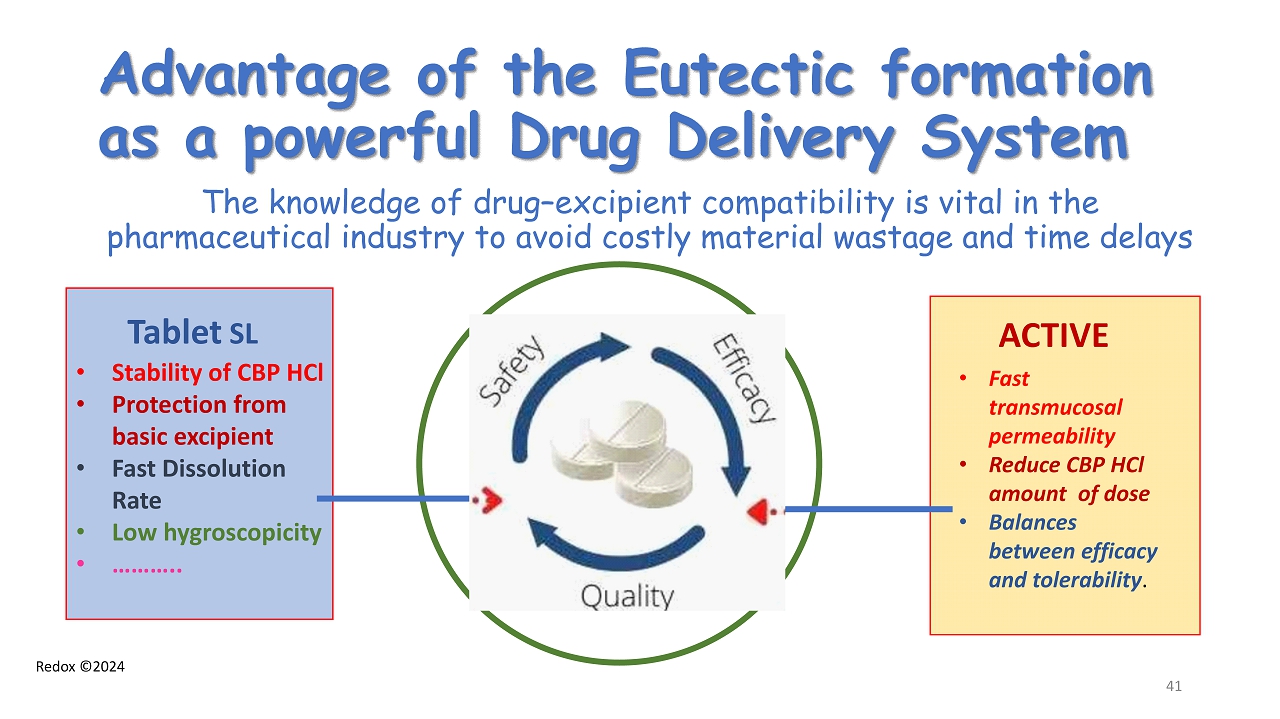
Redox ©2024 Advantage of the Eutectic formation as a powerful Drug Delivery System The knowledge of drug – excipient compatibility is vital in the pharmaceutical industry to avoid costly material wastage and time delays Tablet SL • Stability of CBP HCl • Protection from basic excipient • Fast Dissolution Rate • Low hygroscopicity • ……….. ACTIVE • Fast transmucosal permeability • Reduce CBP HCl amount of dose • Balances between efficacy and tolerability . 41
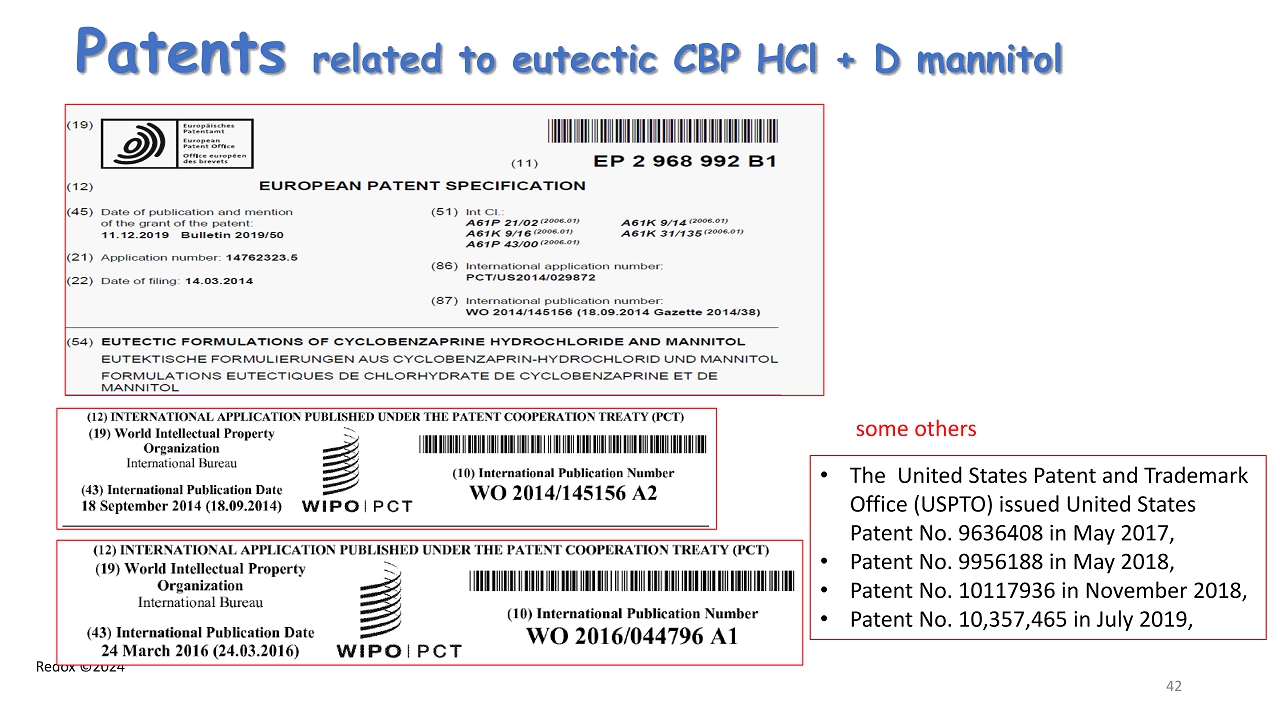
Redox ©2024 Patents related to eutectic CBP HCl + D mannitol 42 • The United States Patent and Trademark Office (USPTO) issued United States Patent No. 9636408 in May 2017, • Patent No. 9956188 in May 2018, • Patent No. 10117936 in November 2018, • Patent No. 10,357,465 in July 2019, some others
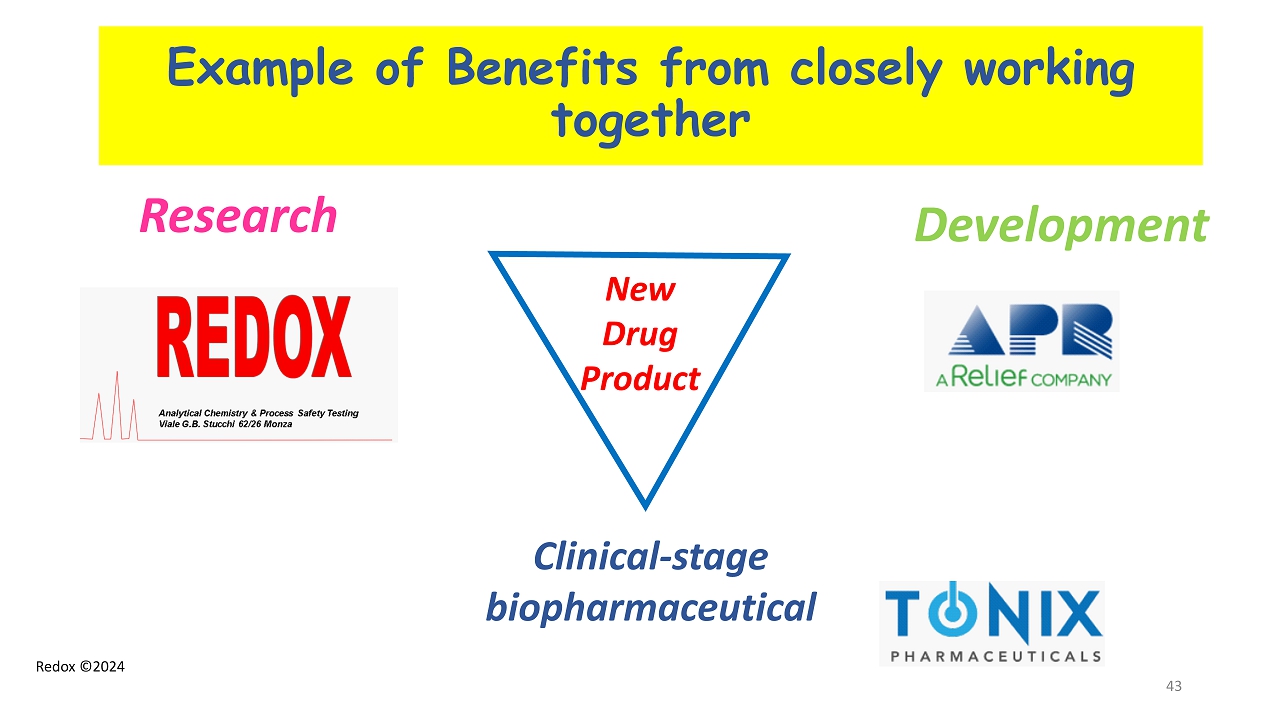
Redox ©2024 Example of Benefits from closely working together 43 Research Development Clinical - stage biopharmaceutic al New Drug Product
Redox ©2024 44

Redox ©2024 Contributors to the Project 45 REDOX – Monza - Italy P. Colombo M. Calvi M. Chirico APR Pharmaceutical – Balerna - Switzerland G. Reiner R. Marelli V. Reiner TONIX Pharmaceuticals – New York - USA S. Lederman S. Fogarty B. Daugherty M. Edgar

Redox ©2024 Thanks for your attention 46