
TONIX PHARMACEUTICALS HOLDING CORP. - 8-K
Exhibit 99.03

© 2024 Tonix Pharmaceuticals Holding Corp. Pharmacokinetic Properties of TNX - 102 SL, a Sublingual Formulation of Cyclobenzaprine Hydrochloride Bruce Daugherty, PhD 11 th Pharmaceutics and Novel Drug Delivery Systems (PDDS) Conference 2024 – Oral Presentation September 19, 2024 Rome, Italy Version 1513 September 12, 2024 (P0601)

2 © 2024 Tonix Pharmaceuticals Holding Corp. Cautionary Note on Forward - Looking Statements Certain statements in this presentation regarding strategic plans, expectations and objectives for future operations or results are “forward - looking statements” as defined by the Private Securities Litigation Reform Act of 1995. These statements may be identified by the use of forward - looking words such as “anticipate,” “believe,” “forecast,” “estimate” and “intend,” among others. These forward - looking statements are based on Tonix’s current expectations and actual results could differ materially. There are a number of factors that could cause actual events to differ materially from those indicated by such forward - looking statements. These factors include, but are not limited to, the risks related to failure to obtain FDA clearances or approvals and noncompliance with FDA regulations; risks related to the failure to successfully market any of our products; risks related to the timing and progress of clinical development of our product candidates; our need for additional financing; uncertainties of patent protection and litigation; uncertainties of government or third party payor reimbursement; limited research and development efforts and dependence upon third parties; and substantial competition. As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products. The forward - looking statements in this presentation are made as of the date of this presentation, even if subsequently made available by Tonix on its website or otherwise. Tonix does not undertake an obligation to update or revise any forward - looking statement, except as required by law. Investors should read the risk factors set forth in the Annual Report on Form 10 - K for the year ended December 31, 2023, as filed with the Securities and Exchange Commission (the “SEC”) on April 1, 2024, and periodic reports and current reports filed with the SEC on or after the date thereof. All of Tonix's forward - looking statements are expressly qualified by all such risk factors and other cautionary statements.

3 © 2024 Tonix Pharmaceuticals Holding Corp. Disclosure TONIX Seth Lederman* Gregory Sullivan* Mary Kelly* Jean Engels* Bruce Daugherty* Siobhan Fogarty* # # Poster : Friday, Sept 20 “The importance of in vitro discriminatory tests in the development of a sublingual dosage form of TNX - 102 SL (Cyclobenzaprine HCl) tablets *Own stock and/or stock options in Tonix UNIVERSITY OF TENNESSEE HEALTH SCIENCE CENTER Bernd Meibohm UNIVERSITY OF CINCINNATI COLLEGE OF MEDICINE Lesley Arnold RHO Ben Vaughn SYNEOS HEALTH PREMIER RESEARCH

4 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Outline □ Clinical Pharmacology of TNX - 102 SL □ Single dose □ Multiple dose □ Dose proportionality and food effect □ Phase 3 Efficacy and Safety of TNX - 102 SL in Fibromyalgia
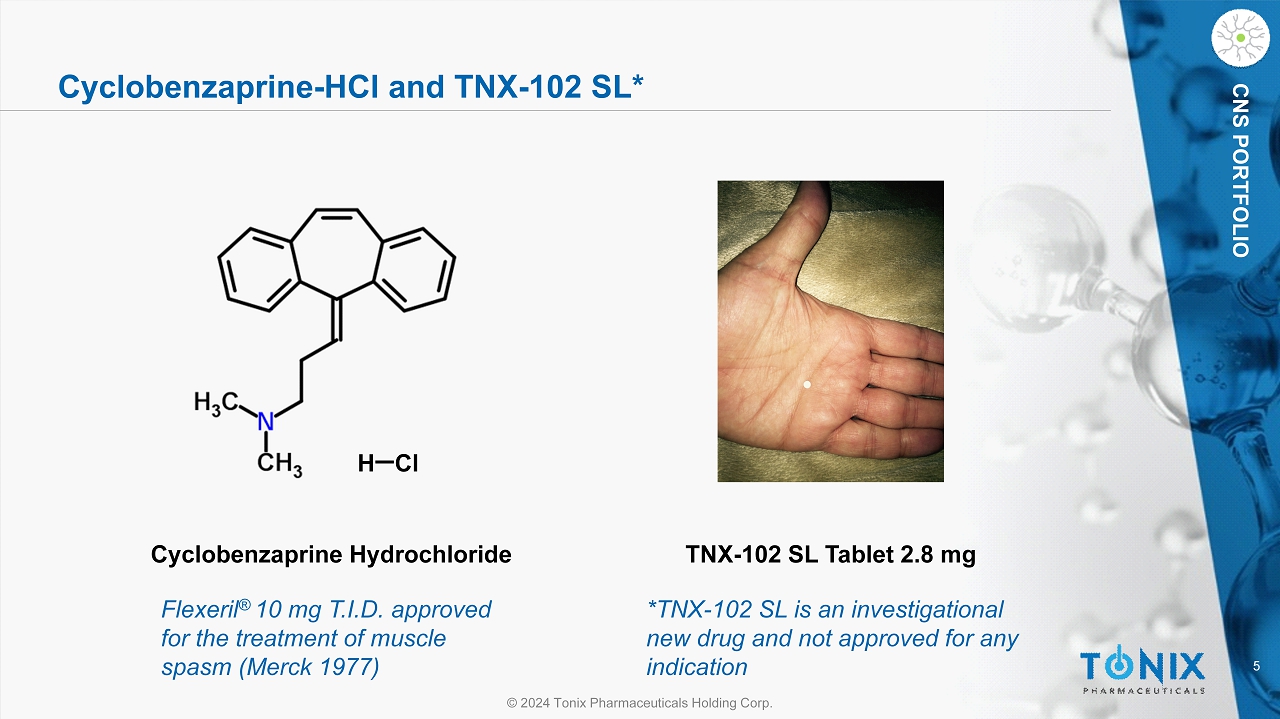
5 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Cyclobenzaprine - HCl and TNX - 102 SL* H Cl Cyclobenzaprine Hydrochloride TNX - 102 SL Tablet 2.8 mg Flexeril ® 10 mg T.I.D. approved for the treatment of muscle spasm (Merck 1977) *TNX - 102 SL is an investigational new drug and not approved for any indication
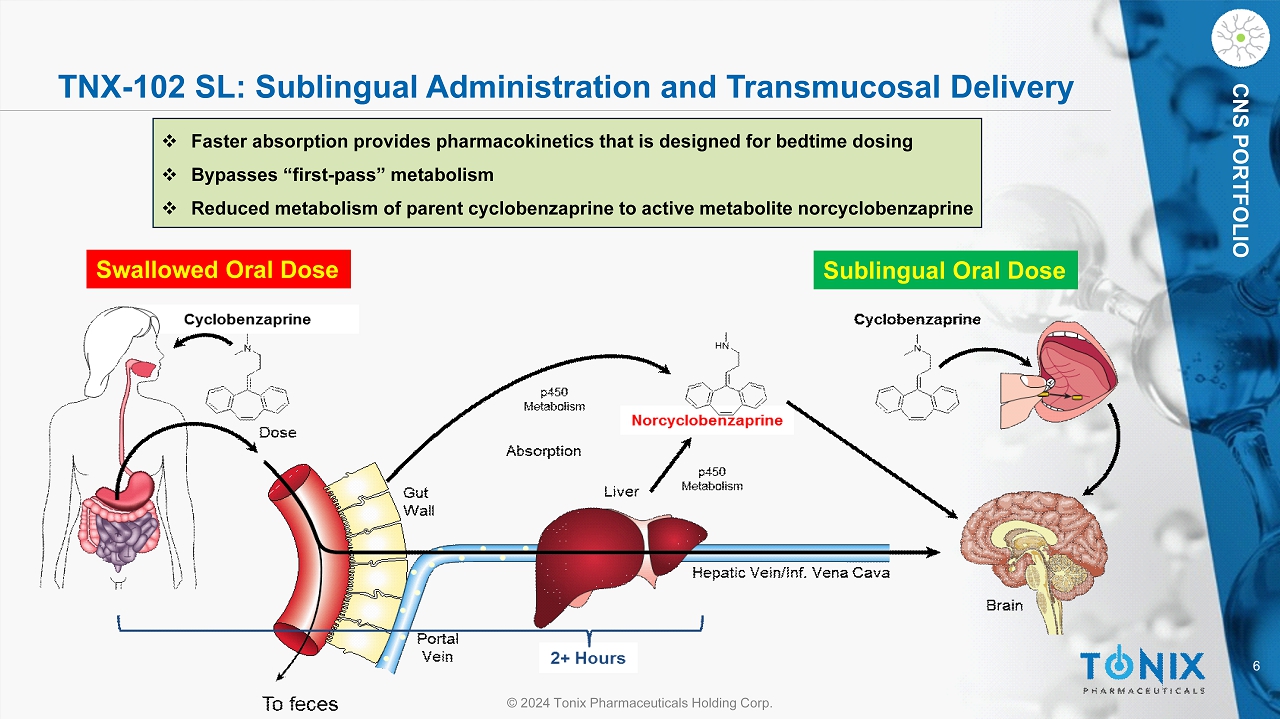
6 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 102 SL: Sublingual Administration and Transmucosal Delivery Swallowed Oral Dose Sublingual Oral Dose □ Faster absorption provides pharmacokinetics that is designed for bedtime dosing □ Bypasses “first - pass” metabolism □ Reduced metabolism of parent cyclobenzaprine to active metabolite norcyclobenzaprine
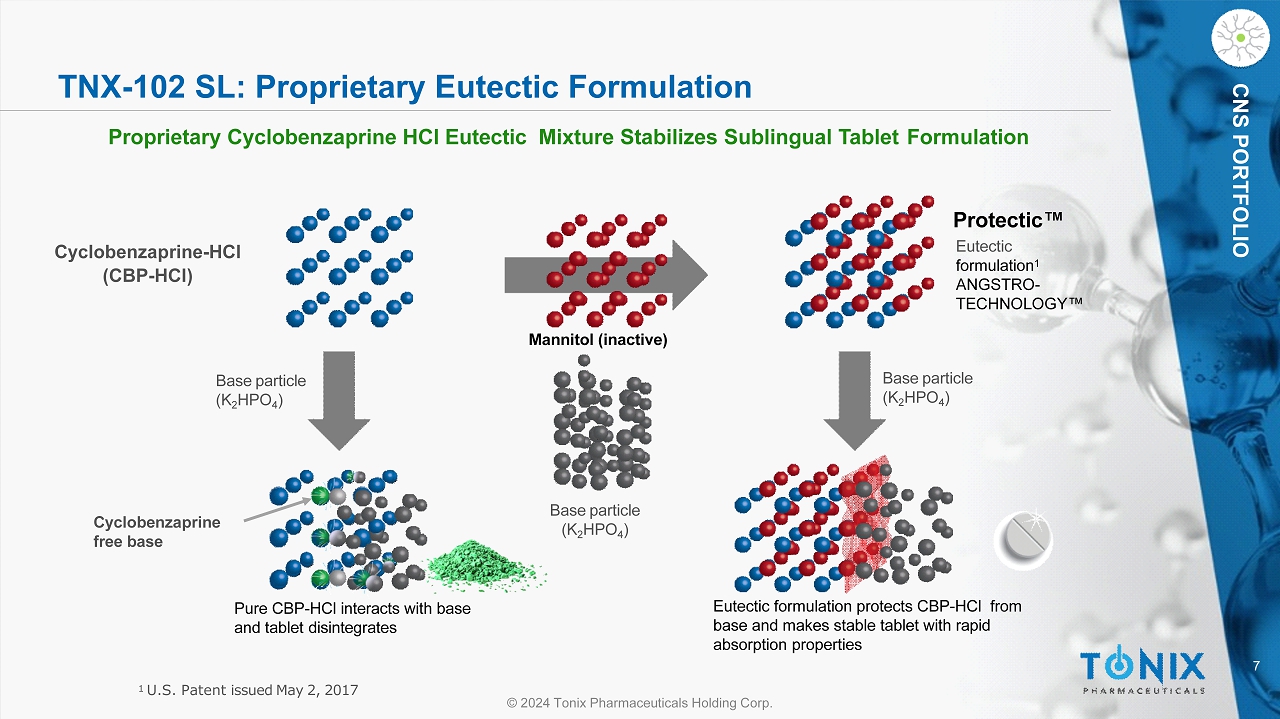
7 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 102 SL: Proprietary Eutectic Formulation Proprietary Cyclobenzaprine HCl Eutectic Mixture Stabilizes Sublingual Tablet Formulation Base particle (K 2 HPO 4 ) Base particle (K 2 HPO 4 ) Base particle (K 2 HPO 4 ) C y cl o be n z a p r i n e - HCl (CBP - HCl) Eutectic formulation protects CBP - HCl from base and makes stable tablet with rapid absorption properties Pure CBP - HCl interacts with base and tablet disintegrates Cy c l ob en zapr ine free base Protectic Œ Eutectic formulation 1 ANGSTRO - T E C H NO L O GY Œ Mannitol (inactive) 1 U.S. Patent issued May 2, 2017
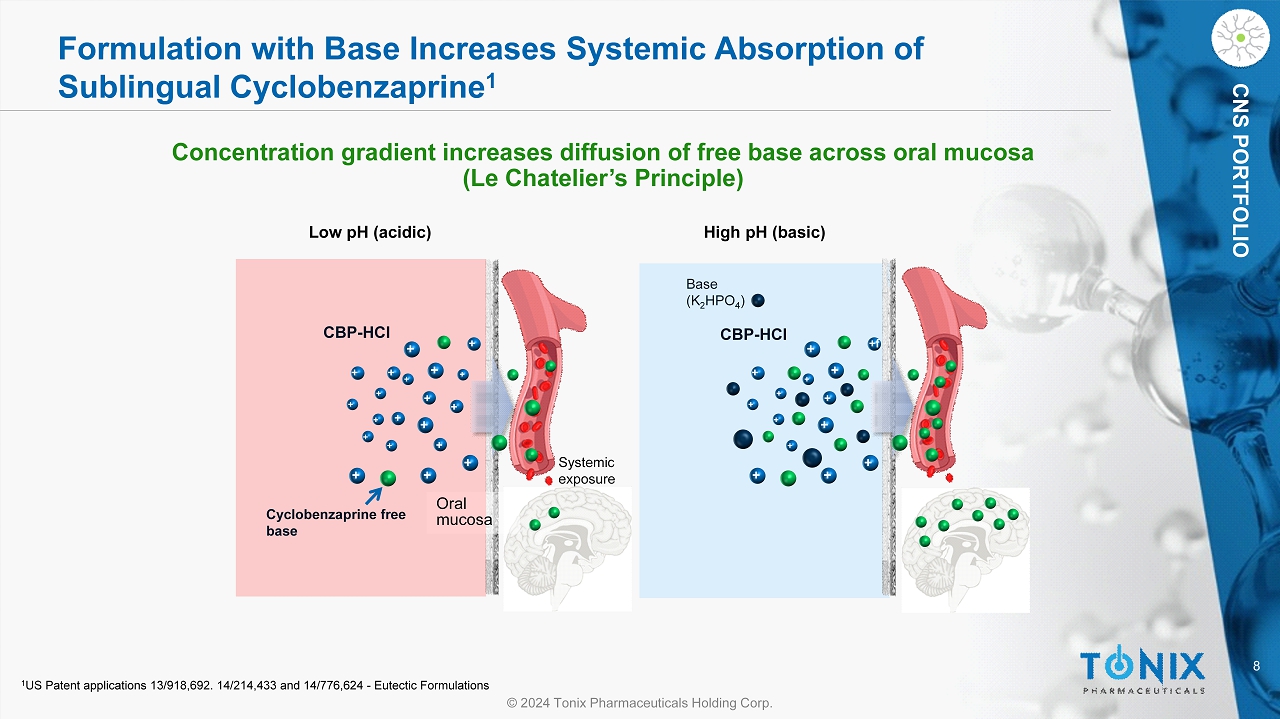
8 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Formulation with Base Increases Systemic Absorption of Sublingual Cyclobenzaprine 1 Concentration gradient increases diffusion of free base across oral mucosa (Le Chatelier’s Principle) CBP - HCl Cyclobenzaprine free base Oral mucosa CBP - HCl Low pH (acidic) High pH (basic) Systemic exposure Base (K 2 HPO 4 ) + + + + + + + + + + + + + + + + + + + + + + + + + + +f + + + + + + + 1 US Patent applications 13/918,692. 14/214,433 and 14/776,624 - Eutectic Formulations
9 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 102 SL Pharmacokinetic Study – Single Dose 2.8 mg Objectives • Single center, comparative, randomized, single - dose, open - label, parallel - design • Compare the rate and extent of absorption of 3 test formulations of TNX - 102 SL 2.8 mg tablets vs commercial cyclobenzaprine HCl 5 mg IR tablet • Assess safety and tolerability of TNX - 102 SL tablets (2.8 mg) vs commercial cyclobenzaprine HCl IR 5 mg tablet • Select optimal formulation for further clinical development Demographics • 58% Female • 96% White • 13% Hispanic • Aged 19 - 59 years (mean = 36.2 years) • N = 24
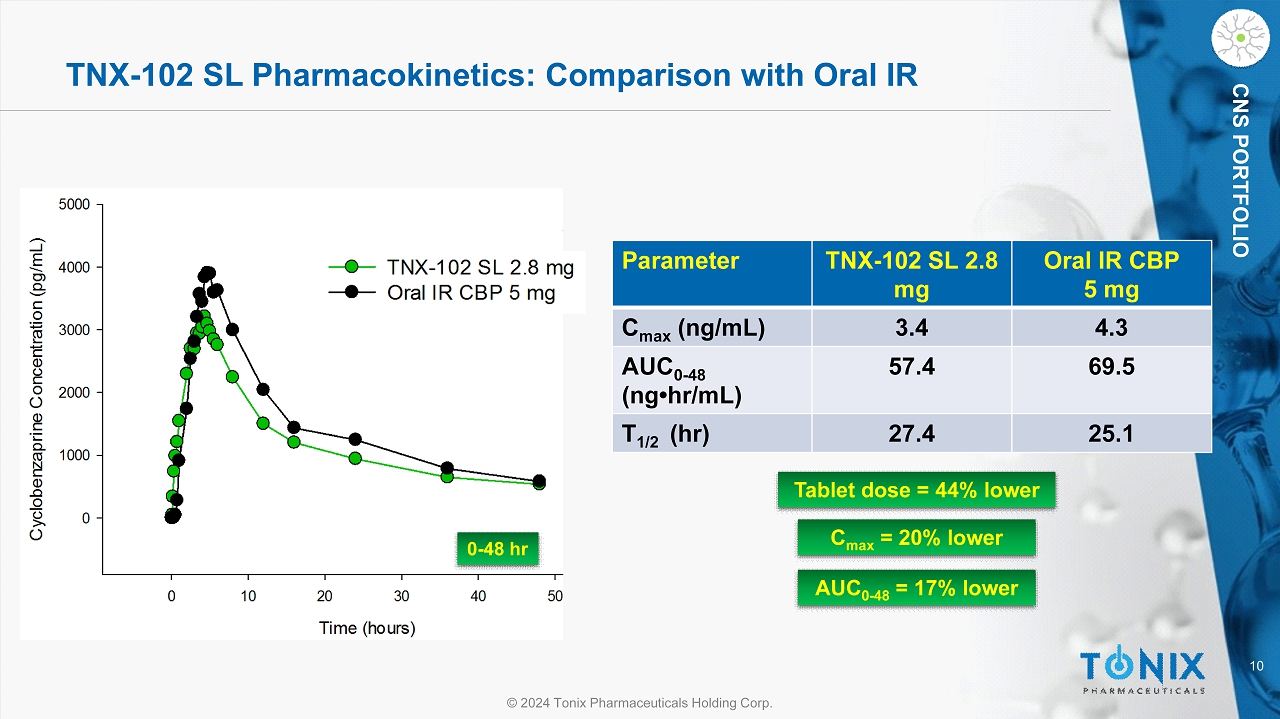
10 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 102 SL Pharmacokinetics: Comparison with Oral IR 0 - 48 hr Parameter TNX - 102 SL 2.8 mg Oral IR CBP 5 mg C max (ng/mL) 3.4 4.3 AUC 0 - 48 ( ng•hr /mL) 57.4 69.5 T 1/2 ( hr ) 27.4 25.1 Tablet dose = 44% lower C max = 20% lower AUC 0 - 48 = 17% lower
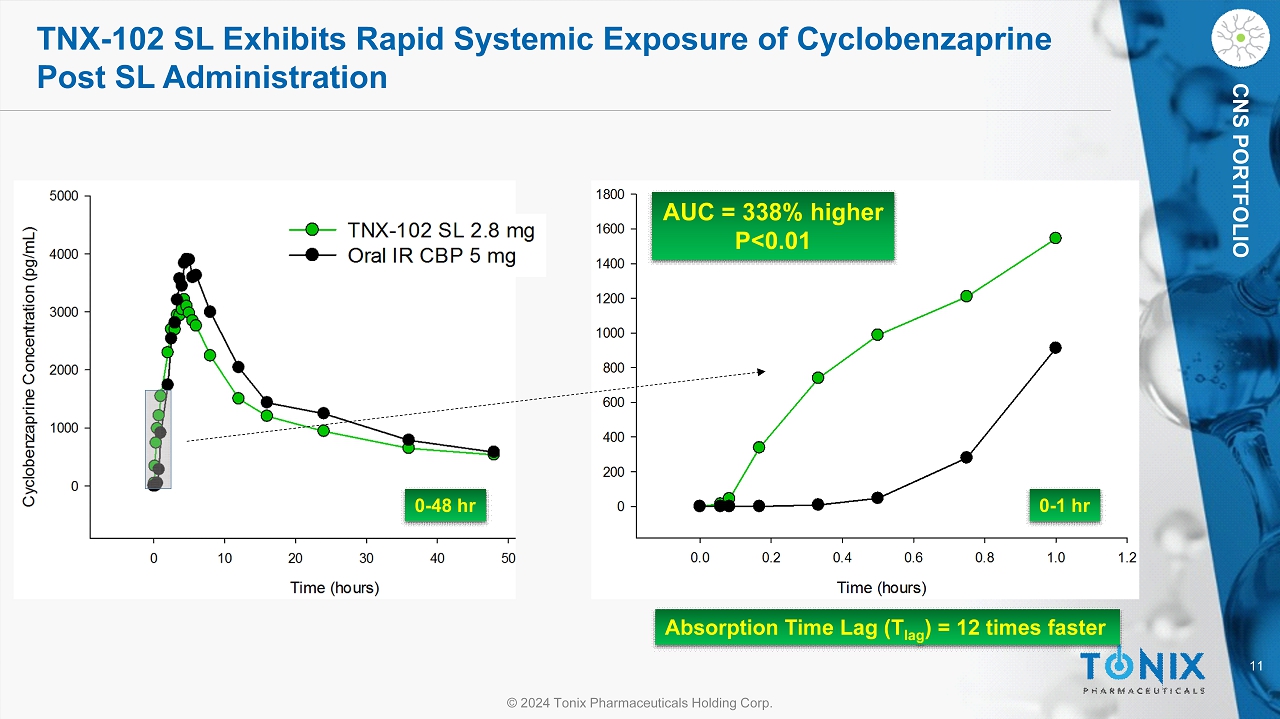
11 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 102 SL Exhibits Rapid Systemic Exposure of Cyclobenzaprine Post SL Administration Absorption Time Lag ( T lag ) = 12 times faster AUC = 338% higher P<0.01 0 - 1 hr 0 - 48 hr
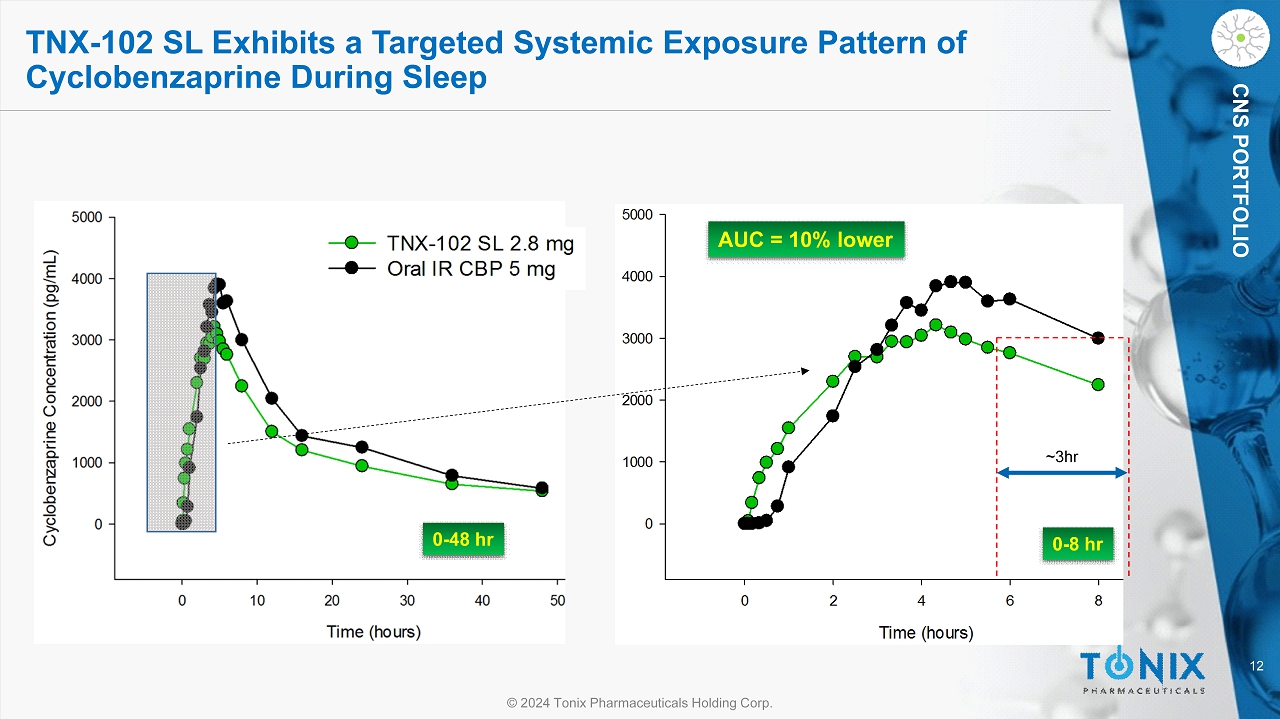
12 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO 0 - 48 hr TNX - 102 SL Exhibits a Targeted Systemic Exposure Pattern of Cyclobenzaprine During Sleep 0 - 8 hr AUC = 10% lower ~3hr
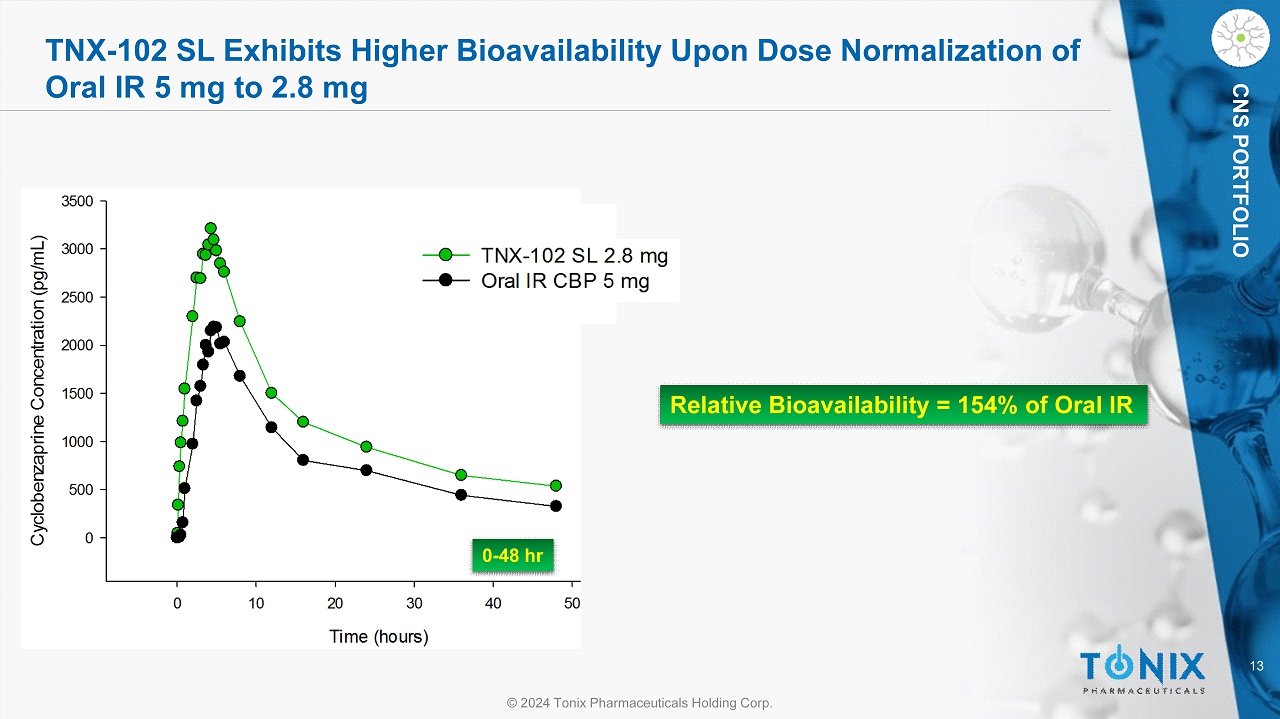
13 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 102 SL Exhibits Higher Bioavailability Upon Dose Normalization of Oral IR 5 mg to 2.8 mg Relative Bioavailability = 154% of Oral IR 0 - 48 hr
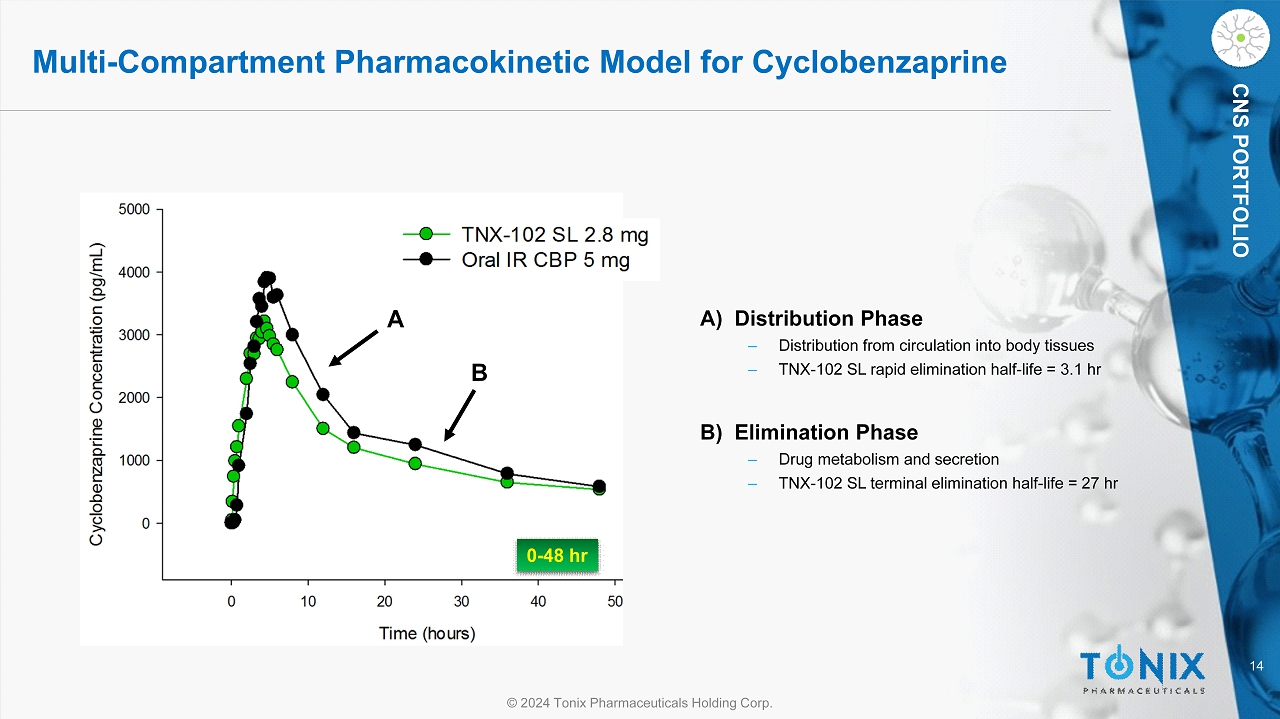
14 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Multi - Compartment Pharmacokinetic Model for Cyclobenzaprine 0 - 48 hr A B A) Distribution Phase ‒ Distribution from circulation into body tissues ‒ TNX - 102 SL rapid elimination half - life = 3.1 hr B) Elimination Phase ‒ Drug metabolism and secretion ‒ TNX - 102 SL terminal elimination half - life = 27 hr
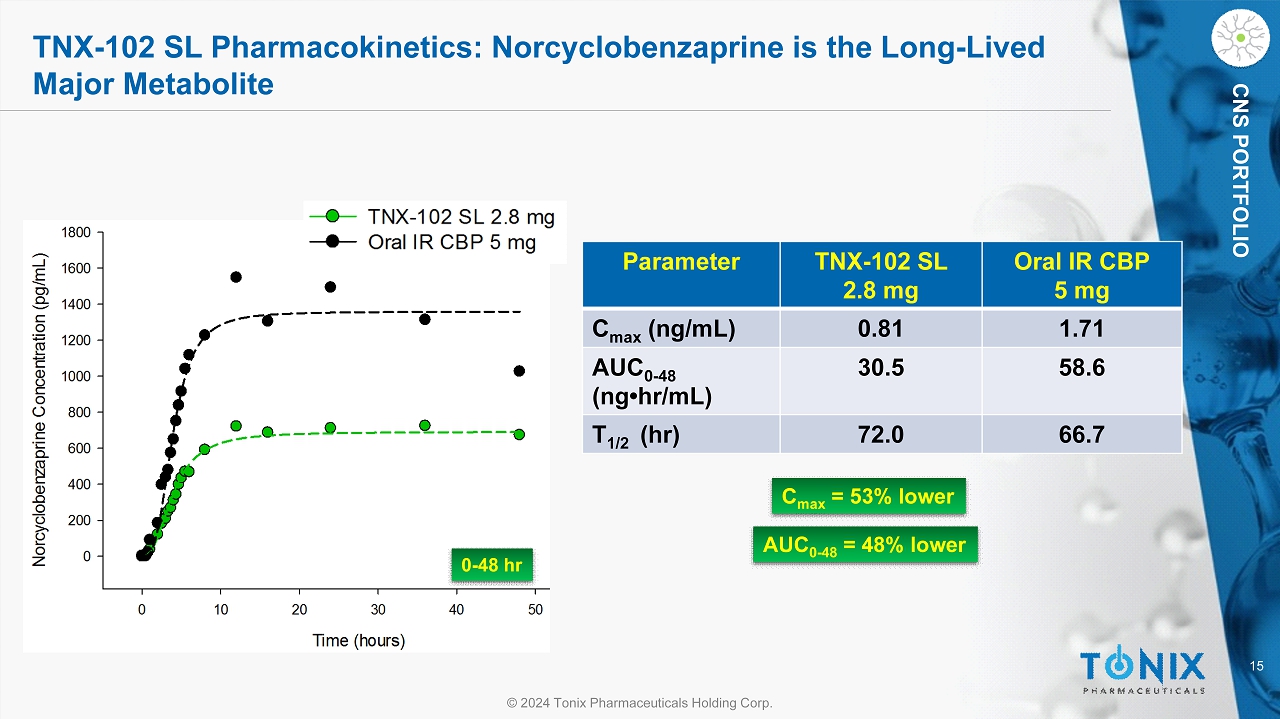
15 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 102 SL Pharmacokinetics: Norcyclobenzaprine is the Long - Lived Major Metabolite Parameter TNX - 102 SL 2.8 mg Oral IR CBP 5 mg C max (ng/mL) 0.81 1.71 AUC 0 - 48 ( ng•hr /mL) 30.5 58.6 T 1/2 ( hr ) 72.0 66.7 0 - 48 hr C max = 53% lower AUC 0 - 48 = 48% lower
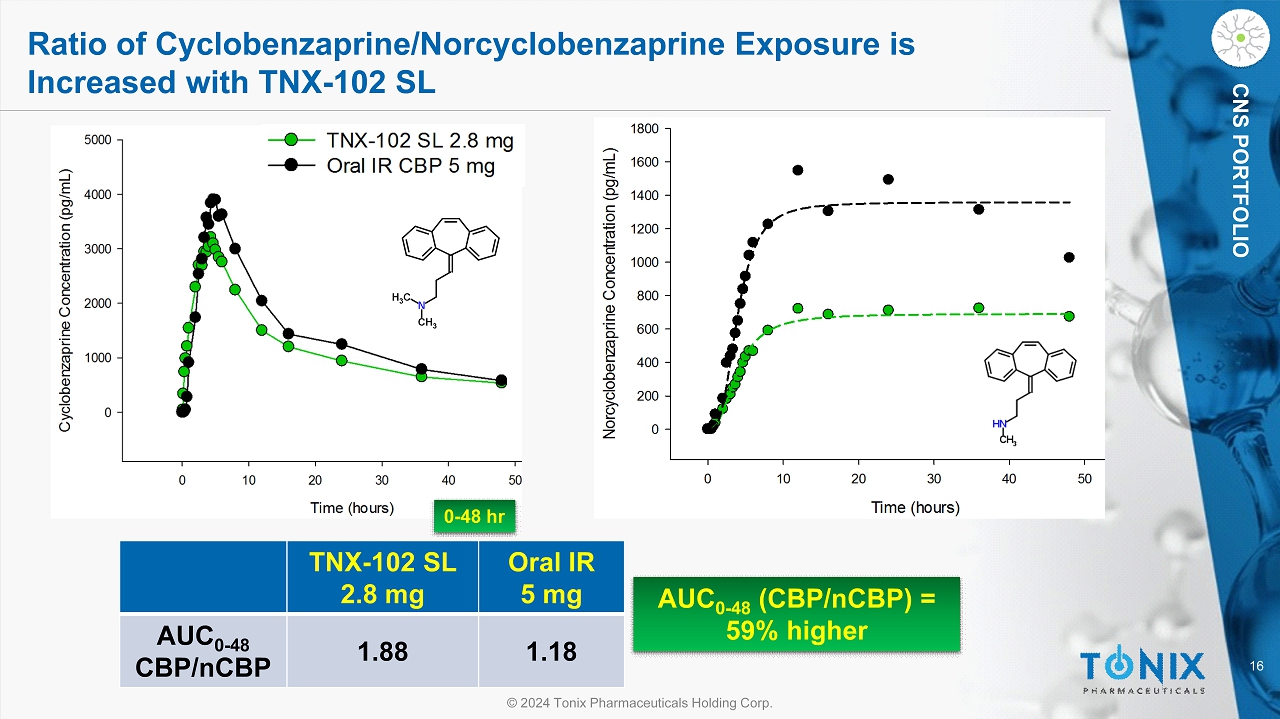
16 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Ratio of Cyclobenzaprine/ Norcyclobenzaprine Exposure is Increased with TNX - 102 SL AUC 0 - 48 (CBP/ nCBP ) = 59% higher TNX - 102 SL 2.8 mg Oral IR 5 mg AUC 0 - 48 CBP/ nCBP 1.88 1.18 0 - 48 hr
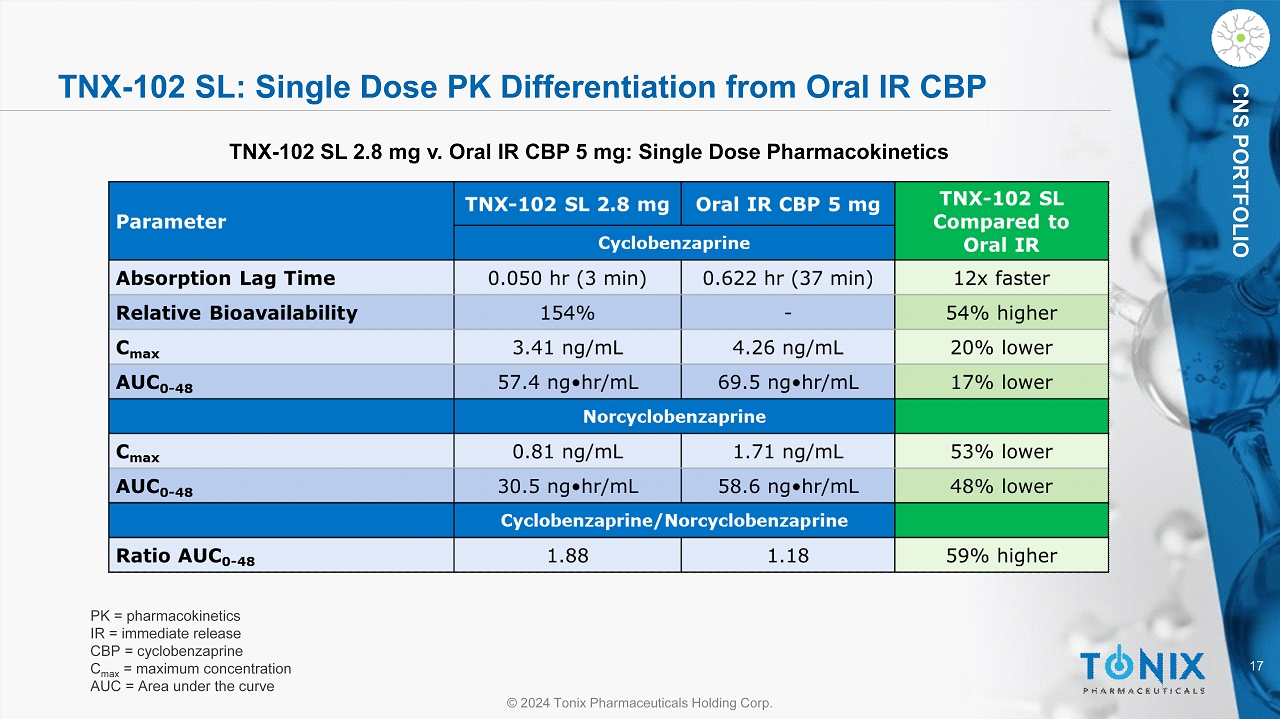
17 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 102 SL: Single Dose PK Differentiation from Oral IR CBP TNX - 102 SL 2.8 mg v. Oral IR CBP 5 mg: Single Dose Pharmacokinetics PK = pharmacokinetics IR = immediate release CBP = cyclobenzaprine C max = maximum concentration AUC = Area under the curve
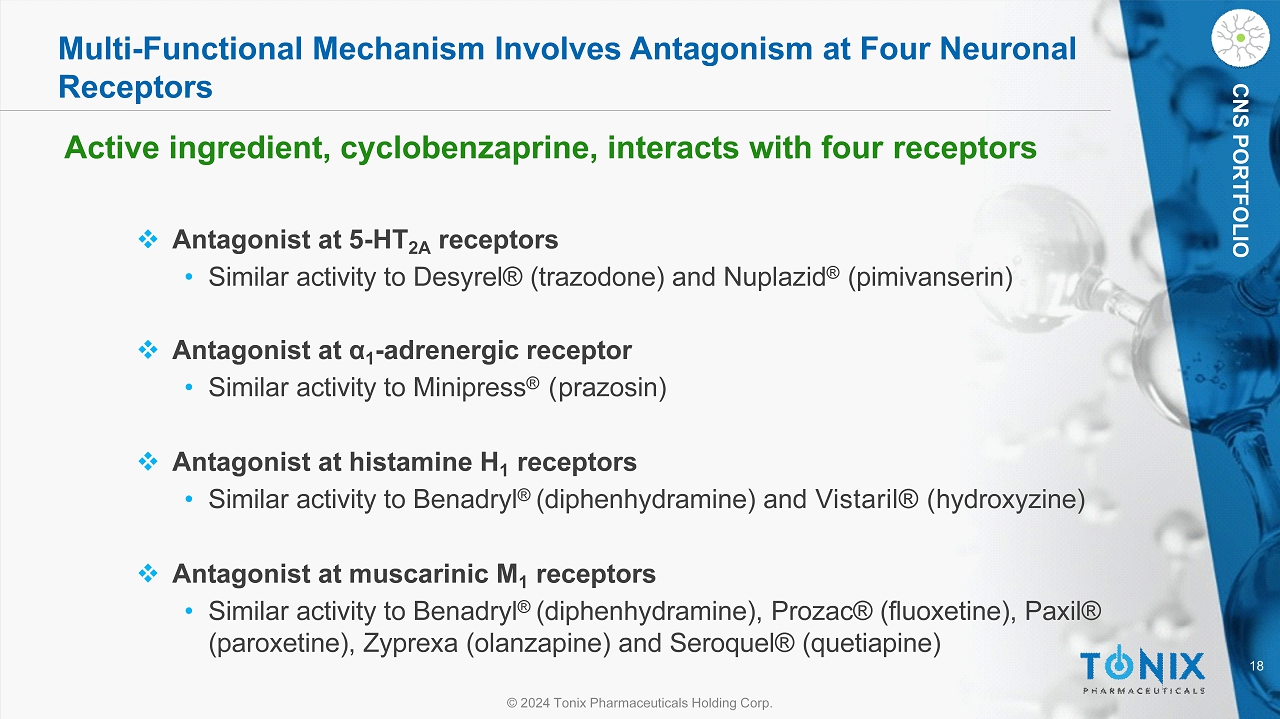
18 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Multi - Functional Mechanism Involves Antagonism at Four Neuronal Receptors Active ingredient, cyclobenzaprine, interacts with four receptors □ Antagonist at 5 - HT 2A receptors • Similar activity to Desyrel® ( t razodone ) and Nuplazid ® ( pimivanserin ) □ Antagonist at α 1 - adrenergic receptor • Similar activity to Minipress ® ( prazosin) □ Antagonist at histamine H 1 receptors • Similar activity to Benadryl ® (diphenhydramine) and Vistaril® ( hydroxyzine) □ Antagonist at muscarinic M 1 receptors • Similar activity to Benadryl ® (diphenhydramine), Prozac® (fluoxetine), Paxil® (paroxetine), Zyprexa (olanzapine) and Seroquel® (quetiapine)
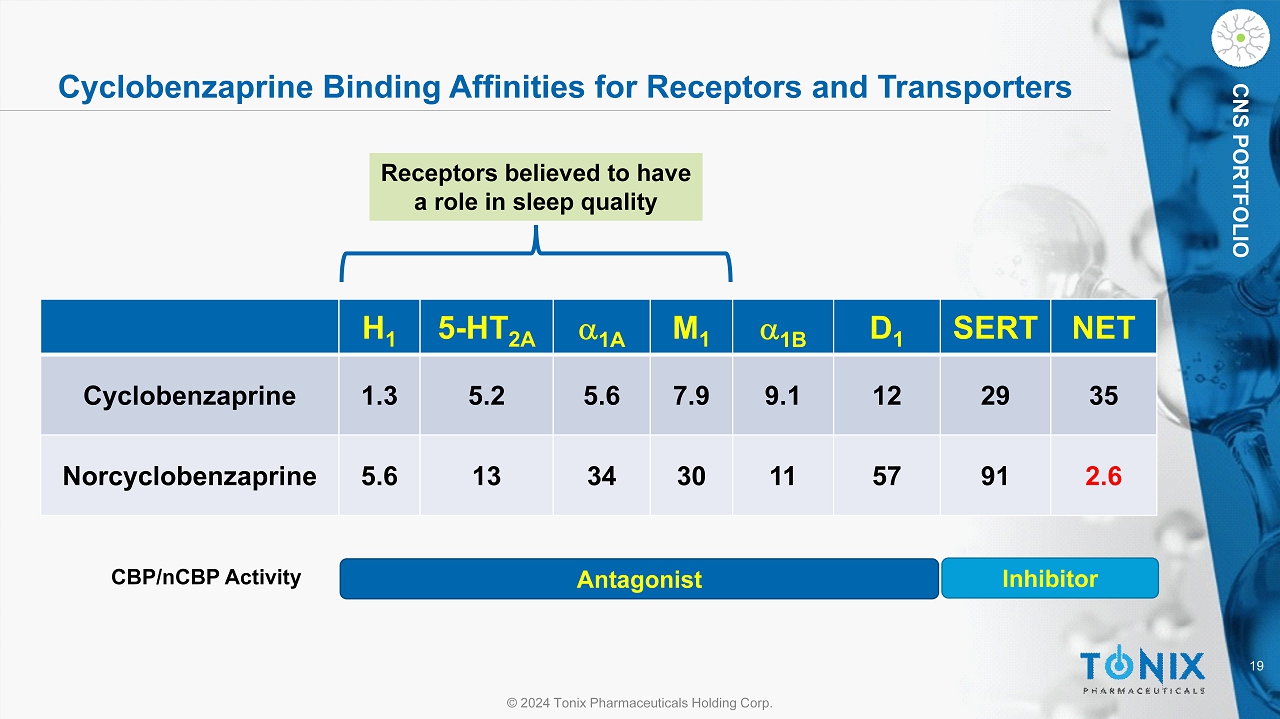
19 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Cyclobenzaprine Binding Affinities for Receptors and Transporters Antagonist Inhibitor CBP/ nCBP Activity H 1 5 - HT 2A a 1A M 1 a 1B D 1 SERT NET Cyclobenzaprine 1.3 5.2 5.6 7.9 9.1 12 29 35 Norcyclobenzaprine 5.6 13 34 30 11 57 91 2.6 Receptors believed to have a role in sleep quality
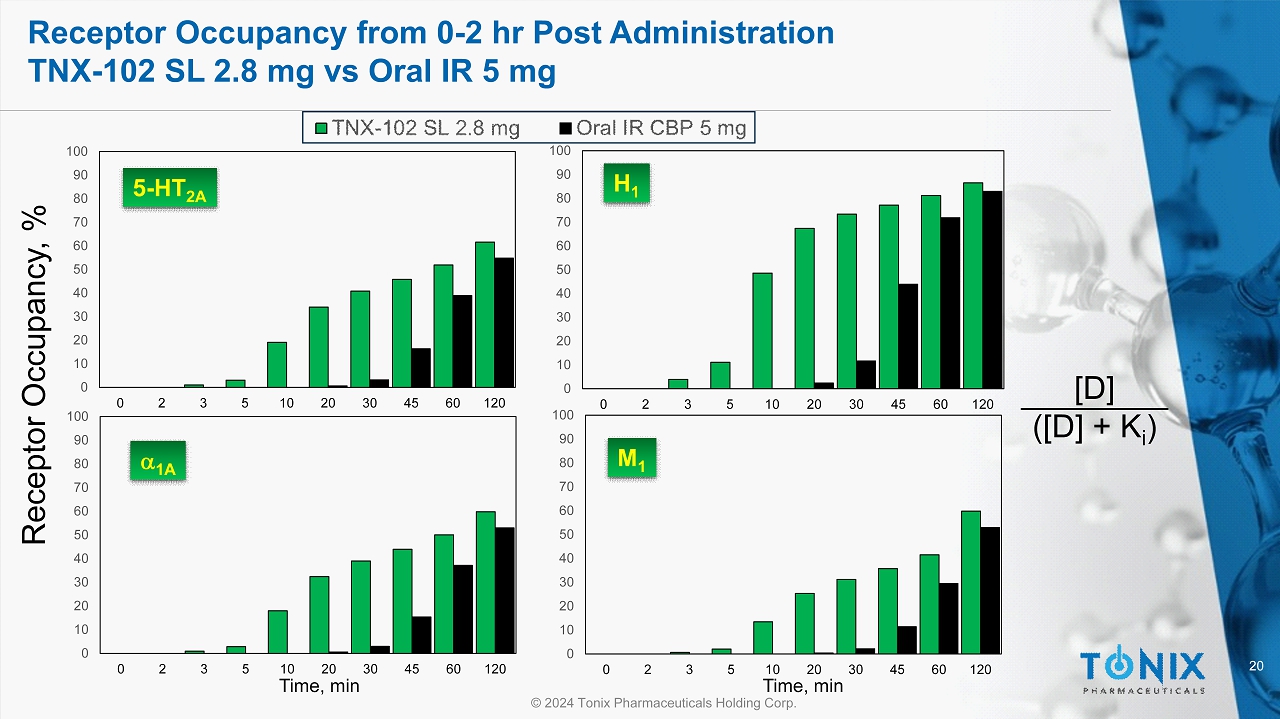
20 © 2024 Tonix Pharmaceuticals Holding Corp. Receptor Occupancy from 0 - 2 hr Post Administration TNX - 102 SL 2.8 mg vs Oral IR 5 mg 0 10 20 30 40 50 60 70 80 90 100 0 2 3 5 10 20 30 45 60 120 0 10 20 30 40 50 60 70 80 90 100 0 2 3 5 10 20 30 45 60 120 0 10 20 30 40 50 60 70 80 90 100 0 2 3 5 10 20 30 45 60 120 0 10 20 30 40 50 60 70 80 90 100 0 2 3 5 10 20 30 45 60 120 Time, min Time, min Receptor Occupancy, % [D] ([D] + K i ) 5 - HT 2A H 1 a 1A M 1
21 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Objectives • Single center, comparative, randomized, multiple - dose, open - label, parallel - design • Compare the rate and extent of absorption of TNX - 102 SL tablets (2 x 2.8 mg) vs AMRIX ® 30 mg extended - release capsule once daily for 20 days • Assess safety and tolerability of TNX - 102 SL tablets (2 x 2.8 mg) vs AMRIX ® 30 mg extended release • Compare systemic absorption and metabolic profile of the 2 products at steady state Demographics • 47 % Female • 95% White • 15% Hispanic • Aged 23 - 75 years (mean = 43.4 years) • N = 60 TNX - 102 SL Pharmacokinetic Study – Multiple Dose 5.6 mg
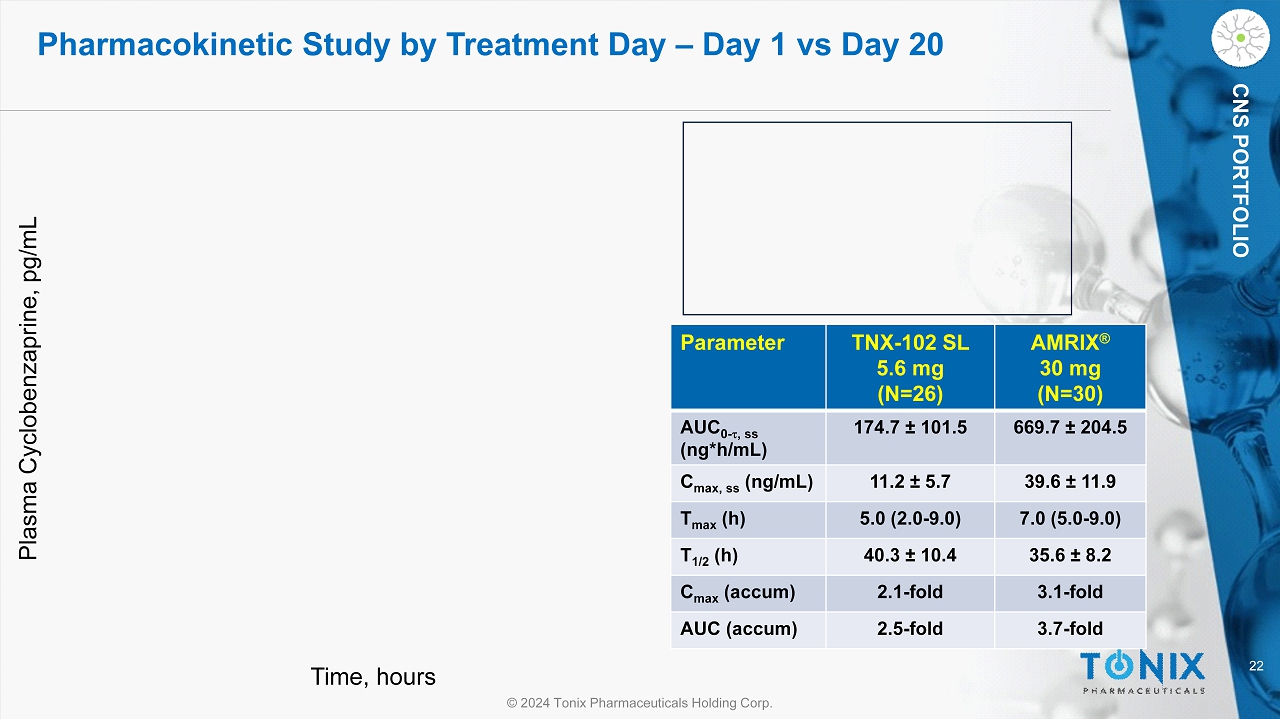
22 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Time, hours Plasma Cyclobenzaprine, pg /mL Parameter TNX - 102 SL 5.6 mg (N=26) AMRIX ® 30 mg (N=30) AUC 0 - t , ss (ng*h/mL) 174.7 ± 101.5 669.7 ± 204.5 C max , ss (ng/mL) 11.2 ± 5.7 39.6 ± 11.9 T max (h) 5.0 (2.0 - 9.0) 7.0 (5.0 - 9.0) T 1/2 (h) 40.3 ± 10.4 35.6 ± 8.2 C max ( accum ) 2.1 - fold 3.1 - fold AUC ( accum ) 2.5 - fold 3.7 - fold Pharmacokinetic Study by Treatment Day – Day 1 vs Day 20
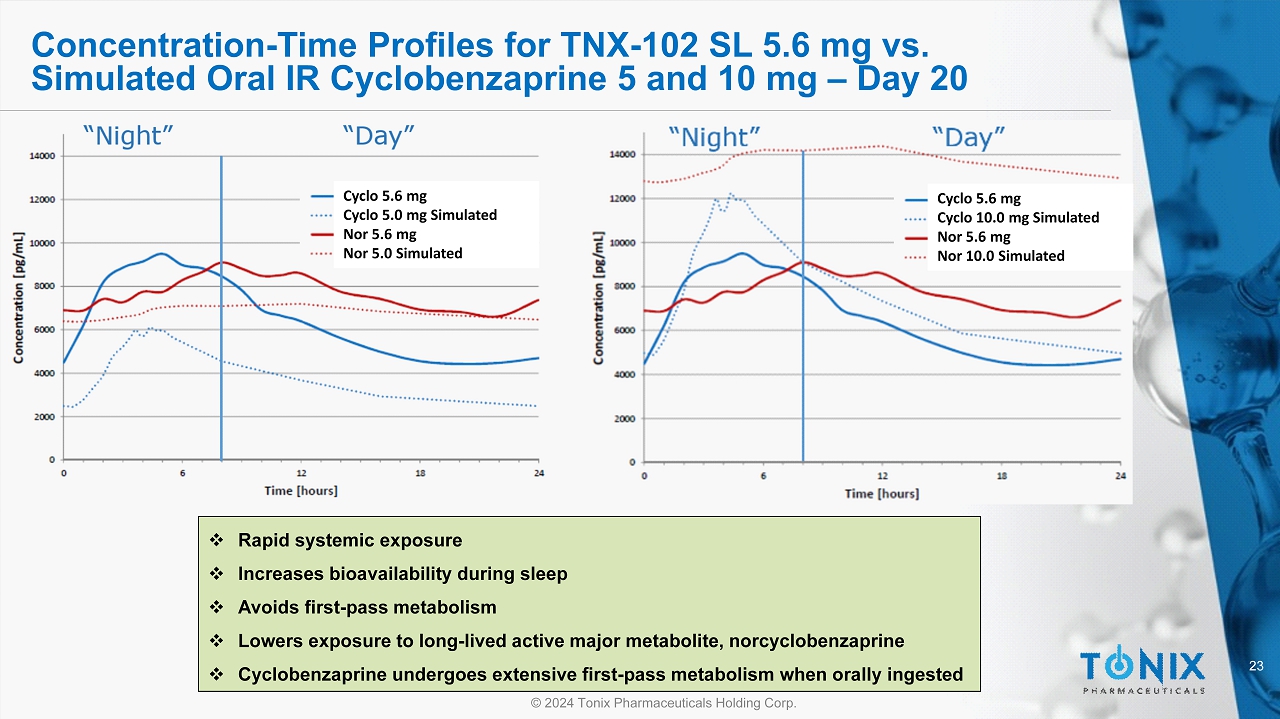
23 © 2024 Tonix Pharmaceuticals Holding Corp. Concentration - Time Profiles for TNX - 102 SL 5.6 mg vs. Simulated Oral IR Cyclobenzaprine 5 and 10 mg – Day 20 Cyclo 5.6 mg Cyclo 10.0 mg Simulated Nor 5.6 mg Nor 10.0 Simulated Cyclo 5.6 mg Cyclo 5.0 mg Simulated Nor 5.6 mg Nor 5.0 Simulated □ Rapid systemic exposure □ Increases bioavailability during sleep □ Avoids first - pass metabolism □ Lowers exposure to long - lived active major metabolite, norcyclobenzaprine □ Cyclobenzaprine undergoes extensive first - pass metabolism when orally ingested
24 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 102 SL Pharmacokinetic Study - Dose Proportionality and Food Effect Objectives • Single center, comparative, randomized, single - dose, 3 - period, 6 - sequence crossover • Evaluate the dose proportionality of TNX - 102 SL 2.8 mg vs 5.6 mg under fasted conditions • Evaluate the effect of food on TNX - 102 SL 5.6 mg • Assess safety and tolerability of TNX - 102 SL tablets in healthy subjects Demographics • 50% Female • 100% White • 12.5% Hispanic • Aged 36 - 62 years (mean = 52.7 years) • N = 15
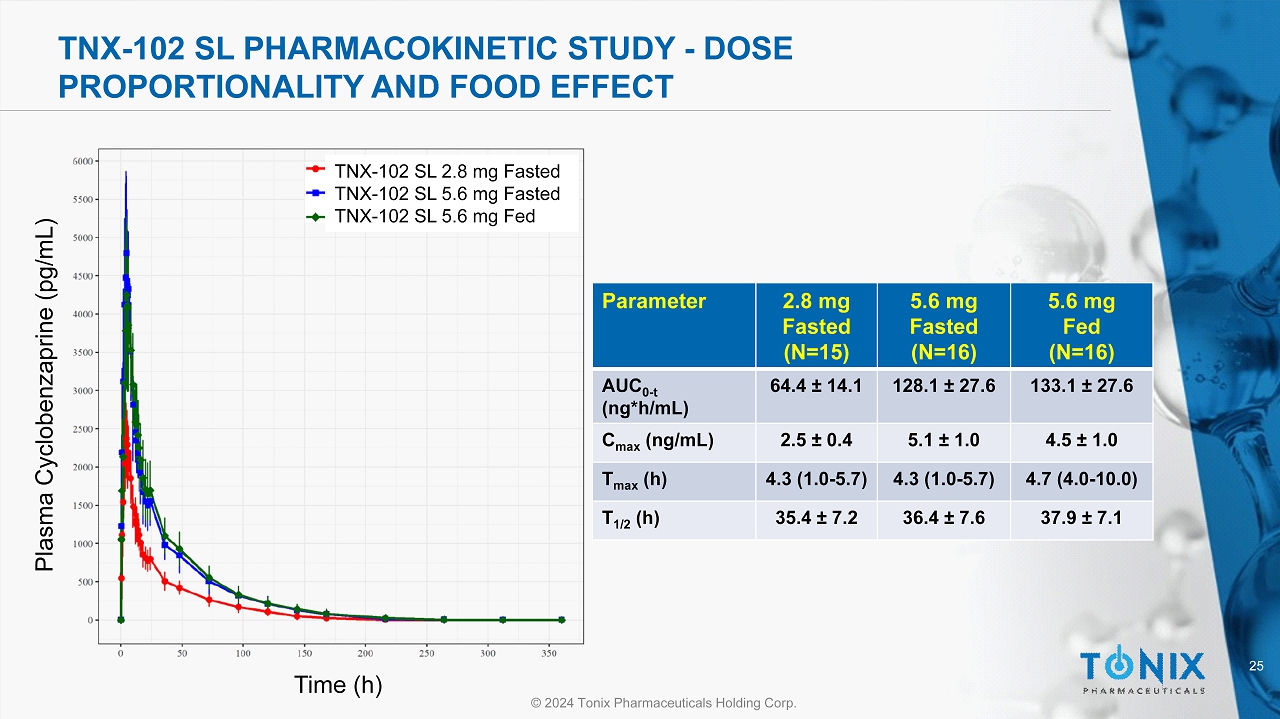
25 © 2024 Tonix Pharmaceuticals Holding Corp. TNX - 102 SL PHARMACOKINETIC STUDY - DOSE PROPORTIONALITY AND FOOD EFFECT Parameter 2.8 mg Fasted (N=15) 5.6 mg Fasted (N=16) 5.6 mg Fed (N=16) AUC 0 - t (ng*h/mL) 64.4 ± 14.1 128.1 ± 27.6 133.1 ± 27.6 C max (ng/mL) 2.5 ± 0.4 5.1 ± 1.0 4.5 ± 1.0 T max (h) 4.3 (1.0 - 5.7) 4.3 (1.0 - 5.7) 4.7 (4.0 - 10.0) T 1/2 (h) 35.4 ± 7.2 36.4 ± 7.6 37.9 ± 7.1 TNX - 102 SL 2.8 mg Fasted TNX - 102 SL 5.6 mg Fasted TNX - 102 SL 5.6 mg Fed Time (h) Plasma Cyclobenzaprine ( pg /mL)

26 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Fibromyalgia is a Large, Underserved and Dissatisfied Population • More than 10 million U.S. adults are affected – predominantly women 1,2 ‒ Debilitating and life altering condition ‒ Significant economic impact • Patients are dissatisfied, despite three FDA approved drugs 3,4 ‒ 85% of patients fail first - line therapy 5 : efficacy variability, tolerability issues especially when used long - term and lack of broad - spectrum activity ‒ Typical for patients to rotate between drugs and be on multiple drugs at the same time; 79% of FM patients are on multiple therapies 5 • ~2.7 million FM patients diagnosed and treated 6 ‒ >22 million prescriptions are issued for the treatment of fibromyalgia (on - and off - label usage) each year 7,8 • No new R x product since 2009 • The treatment objective is to restore functionality and quality of life by broadly improving symptoms while avoiding significant side effects 1 American College of Rheumatology ( www.ACRPatientInfo.org accessed May 7, 2019) – prevalence rate of 2 - 4% for U.S. adult population (~250 million) 2 Vincent A, et al. Arthritis Care Res (Hoboken) . 2013 65(5):786 - 92. doi : 10.1002 ; diagnosed prevalence rate was 1.1% of adult population or 50% of the prevalent population 3 Robinson RL, et al. Pain Med . 2012 13(10):1366 - 76. doi : 10.1111; 85% received drug treatment 4 The three drugs with FDA approval for the treatment of fibromyalgia: Pregabalin (Lyrica); Duloxetine (Cymbalta); Milnacipran ( Savella ) 5 EVERSANA primary physician research, May 2024; commissioned by Tonix 6 EVERSANA analysis of claims database, May 2024; commissioned by Tonix 7 Product sales derived from IMS MIDAS; IMS NDTI used to factor usage for fibromyalgia; data accessed April 2015. 8 Market research by Frost & Sullivan, commissioned by Tonix, 2011
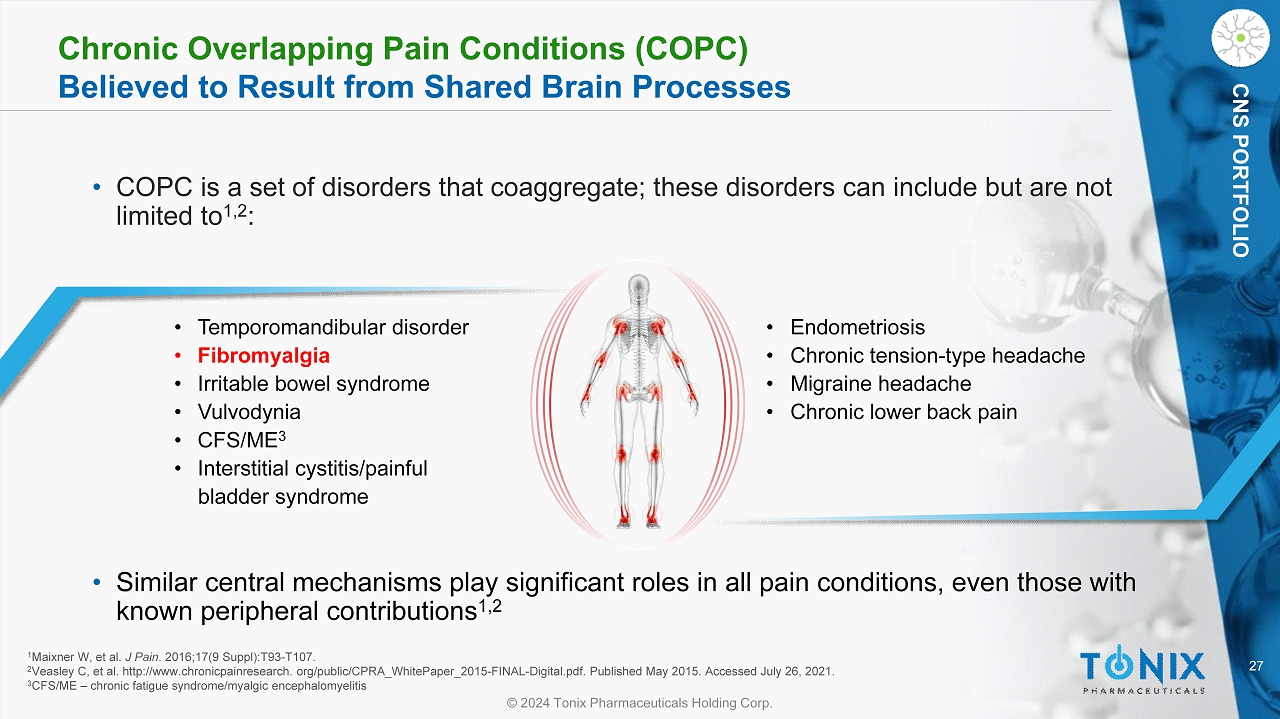
27 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Chronic Overlapping Pain Conditions (COPC) Believed to Result from Shared Brain Processes • COPC is a set of disorders that coaggregate ; these disorders can include but are not limited to 1,2 : • Temporomandibular disorder • Fibromyalgia • Irritable bowel syndrome • Vulvodynia • CFS/ME 3 • Interstitial cystitis/painful bladder syndrome • Endometriosis • Chronic tension - type headache • Migraine headache • Chronic lower back pain 1 Maixner W, et al. J Pain . 2016;17(9 Suppl):T93 - T107. 2 Veasley C, et al. http://www.chronicpainresearch. org/public/CPRA_WhitePaper_2015 - FINAL - Digital.pdf. Published May 2015. Accesse d July 26, 2021. 3 CFS/ME – chronic fatigue syndrome/ myalgic encephalomyelitis • Similar central mechanisms play significant roles in all pain conditions, even those with known peripheral contributions 1,2
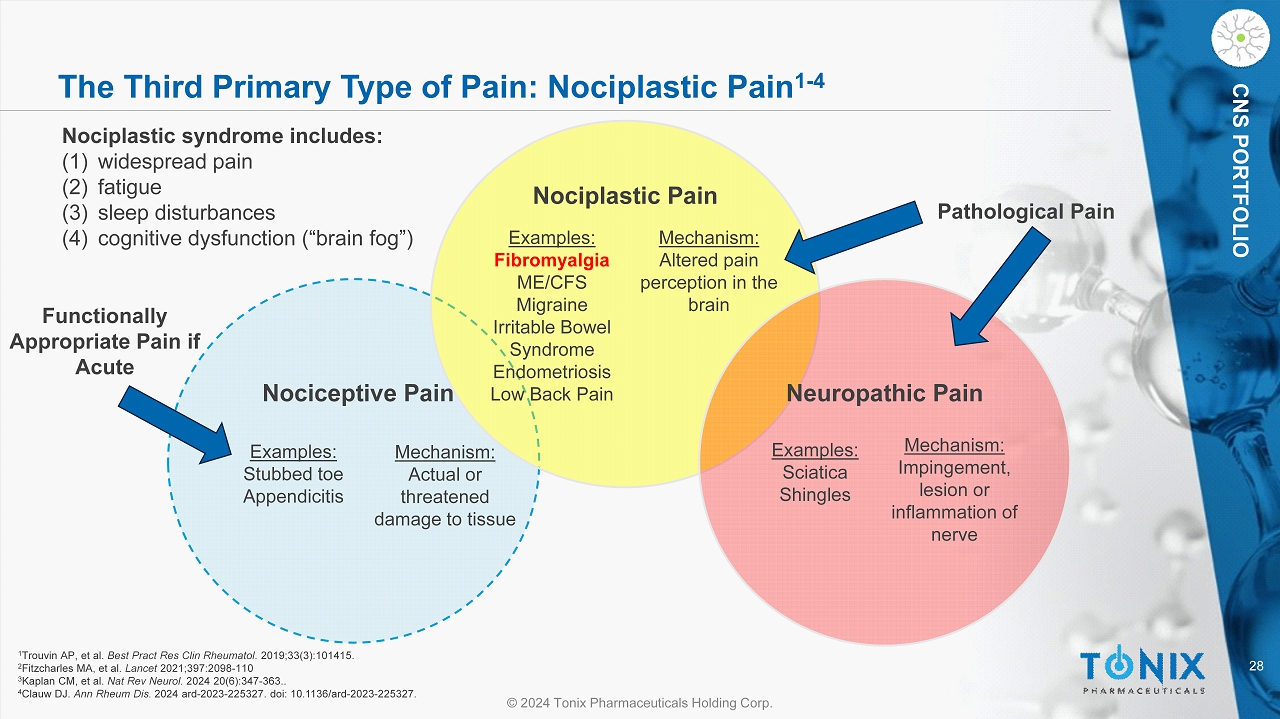
28 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO The Third Primary Type of Pain: Nociplastic Pain 1 - 4 Nociplastic syndrome includes: (1) widespread pain (2) fatigue (3) sleep disturbances (4) cognitive dysfunction (“brain fog”) Nociplastic Pain Examples: Fibromyalgia ME/CFS Migraine Irritable Bowel Syndrome Endometriosis Low Back Pain Examples: Stubbed toe Appendicitis Examples: Sciatica Shingles Mechanism: Altered pain perception in the brain Mechanism: Impingement, lesion or inflammation of nerve Mechanism: Actual or threatened damage to tissue Nociceptive Pain Neuropathic Pain Pathological Pain Functionally Appropriate Pain if Acute 1 Trouvin AP, et al. Best Pract Res Clin Rheumatol . 2019;33(3):101415. 2 Fitzcharles MA, et al. Lancet 2021;397:2098 - 110 3 Kaplan CM, et al. Nat Rev Neurol. 2024 20(6):347 - 363.. 4 Clauw DJ. Ann Rheum Dis. 2024 ard - 2023 - 225327. doi : 10.1136/ard - 2023 - 225327.
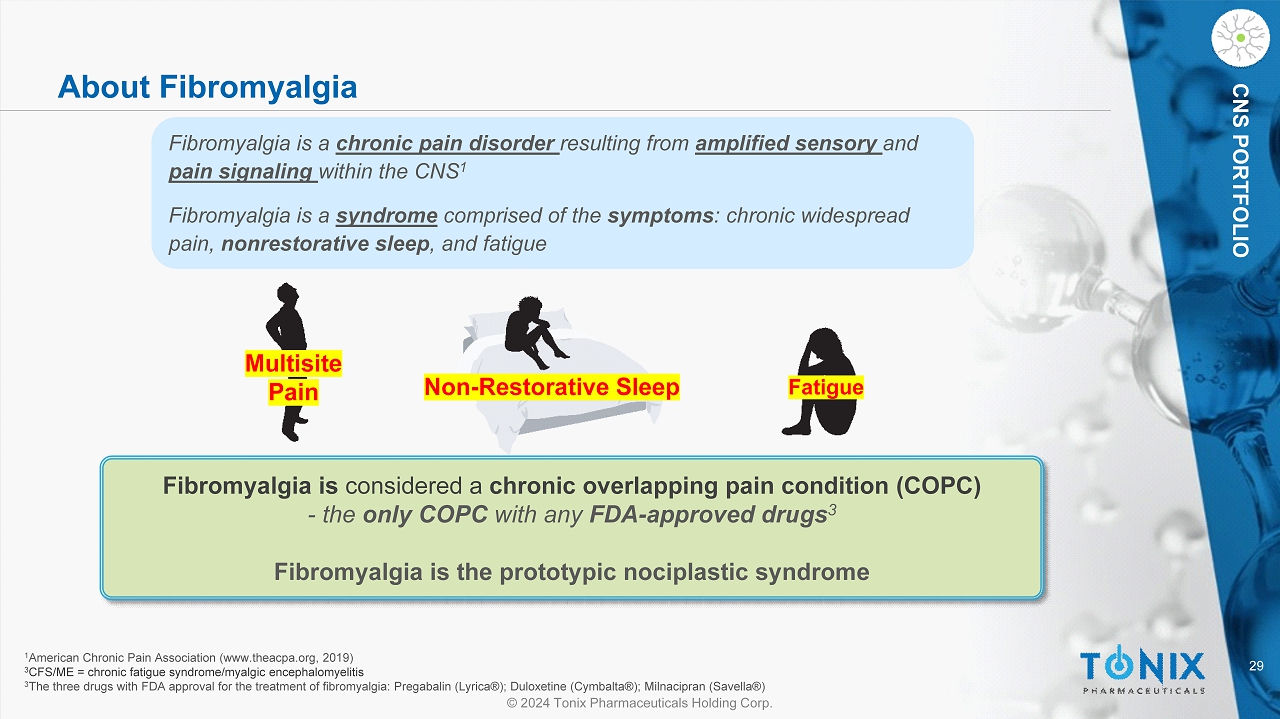
29 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO About Fibromyalgia Fibromyalgia is considered a chronic overlapping pain condition (COPC) - the only COPC with any FDA - approved drugs 3 Fibromyalgia is the prototypic nociplastic syndrome Fibromyalgia is a chronic pain disorder resulting from amplified sensory and pain signaling within the CNS 1 Fibromyalgia is a syndrome comprised of the symptoms : chronic widespread pain, nonrestorative sleep , and fatigue 1 American Chronic Pain Association (www.theacpa.org, 2019) 3 CFS/ME = chronic fatigue syndrome/ myalgic encephalomyelitis 3 The three drugs with FDA approval for the treatment of fibromyalgia: Pregabalin (Lyrica®); Duloxetine (Cymbalta®); Milnacipra n ( Savella ®) Multisite Pain Non - Restorative Sleep Fatigue

30 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Fibromyalgia: Unrefreshing Sleep and Cyclobenzaprine Treatment 1 Moldofsky H et al. Psychosom Med. 1975. 37:341 - 51. 2 Moldofsky H and Scarisbrick P. Psychosom Med. 1976. 38:35 - 44. 3 Bennett RM, et al. Arthritis Rheum 1988 . 31 : 1535 – 42 . 4 Quimby LG, et al . J Rheumatol Suppl, 1989 Nov ; 19 : 140 – 3 . 5 Reynolds WJ, et al. J Rheumatol . 1991 . 18 : 452 – 4 . 6 Santandrea S, et al . J Int Med Res. 1993 . 21 : 74 – 80 . • Non - restorative sleep 1,2 ‒ Harvey Moldofsky – recognition of unrefreshing/non - restorative sleep: ▪ Symptom ▪ Potential causative or potentiating factor • Cyclobenzaprine 3,9 ‒ Potentially the earliest drug studied in fibromyalgia as an oral swallowed agent ‒ Studies showed equivocal effects and tolerability issues at “muscle spasm” doses • Bedtime, low - dose cyclobenzaprine targeting non - restorative sleep 10 - 11 ‒ Recognition of unrefreshing sleep as a target of therapy ‒ Primitive oral, swallowed formulation – “flat” pharmacokinetics • Bedtime, sublingual transmucosal cyclobenzaprine targeting non - restorative sleep 12 ‒ Dynamic pharmacokinetic profile, rapid absorption, decrease in major metabolite ‒ Two studies (Phase 2 and Phase 3) at 2.8 mg; three Phase 3 studies at 5.6 mg. 7 Cantini F, et al . Minerva Med. 1994 . 85 : 97 – 100 . 8 Carette S, et al. Arthritis Rheum. 1994 . 37 : 32 – 40 . 9 Tofferi JK, et al . Arthritis Rheum. 2004 . 51 : 9 – 13 . 1 10 Iglehart IW. 2003; US Patent 6,541,523. 11 Moldofsky et al. J Rheumatol . 2011. 38:2653 - 2663 12 Lederman S et al. Arthritis Care Res . 2023. 75:2359 - 2368.
31 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Cyclobenzaprine Long - Term Utilization • Flexeril® approved in 1977 by Merck for the treatment of muscle spasm • 10 mg T.I.D. for acute use (2 - 3 weeks) • Original NDA included “ 8 long term safety studies in which patients with various neurologic disorders received cyclobenzaprine up to 80 mg per day for 1 month up to 3 years .” 1 • 6 published studies in fibromyalgia 2 - 8 • N=246, placebo controlled, 4 - 24 week treatment period • Generally well tolerated, no new or unexpected AEs • Extensive safety record in humans for over 30 years • Widely used in the U.S., ~20 million prescriptions and ~ 1 billion tablets dispensed per year 9 • Chronic cyclobenzaprine use is common ( ~12% of users) 9 • Post - marketing surveillance program 1 • 7,607 patients included 297 patients treated with 10 mgs for > 30 days • Incidence of most common AEs was much lower than in controlled studies 1 1999 Merck OTC AdCom Briefing Package 2 Bennett RM, et al. Arthritis Rheum 1988 . 31 : 1535 – 42 . 3 Quimby LG, et al . J Rheumatol Suppl, 1989 Nov ; 19 : 140 – 3 . 4 Reynolds WJ, et al. J Rheumatol . 1991 . 18 : 452 – 4 . 5 Santandrea S, et al . J Int Med Res. 1993 . 21 : 74 – 80 . 6 Cantini F, et al . Minerva Med. 1994 . 85 : 97 – 100 . 7 Carette S, et al. Arthritis Rheum. 1994 . 37 : 32 – 40 . 8 Tofferi JK, et al . Arthritis Rheum. 2004 . 51 : 9 – 13 . 1 9 IMS report 2011 of cyclobenzaprine use in 2009 – Data on File
32 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 102 SL: P hase 3 RESILIENT Study Design General s tudy c haracteristics: • Randomized, double - blind, multicenter, placebo - controlle d study in fibromyalgia • 33 U.S. sites enrolled 457 participants with fibromyalgia as defined by 2016 Revisions to the 2010/2011 FM Diagnostic Criteria 1 • Aged: 18 - 65 years (mean = 49.4 years); FM diagnosis: mean = 9.2 years; 94.5% female; 84.6% white • Average self - reported pain at baseline = 5.9 out of 10 • 366 (80.1%) completed the trial Primary Endpoint : • Change from baseline to Week 14 (TNX - 102 SL vs. placebo) in weekly averages of daily diary average pain severity score Placebo once - daily at bedtime TNX - 102 SL once - daily at bedtime Two - week run - in at 2.8 mg dose at bedtime followed by 12 weeks at 5.6 mg (2 x 2.8 mg) dose Study Title : A Phase 3 Study to Evaluate the Efficacy and Safety of TNX - 102 SL Taken Daily in Patients With Fibromyalgia (RESILIENT) Trial ID : TNY - CY - F307 (‘ RESILIENT ’) ClinicalTrials.gov Identifier : NCT05273749 14 weeks 1 Wolfe F, et al. Semin Arthritis Rheum . 2016 46(3):319 - 329. doi : 10.1016

33 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Weekly Average of Daily Diary NRS Ratings of Average Pain Over Prior 24 Hours RESILIENT Primary Outcome Measure Reduction in Widespread Pain **p<0.001; ***p<0.0001 Week 14 LS mean (SE) change from baseline for TNX - 102 SL - 1.82 (0.12) and for placebo - 1.16 (0.12); LSMD from placebo - 0.65 (0.1 6) # Based on Mixed Model Repeated Measures with Multiple Imputation, with fixed categorical effects of treatment, center, study w eek , and treatment by study week interaction, as well as baseline value and baseline value - by - study week interaction. Abbreviations: LS, least squares; LSMD, least squares mean differe nce; NRS, numerical rating scale; SE, standard error Week of Study LS Mean (SE) Change in NRS Pain Score N = 225 Effect Size = 0.38 p = 0.00005 # N = 231
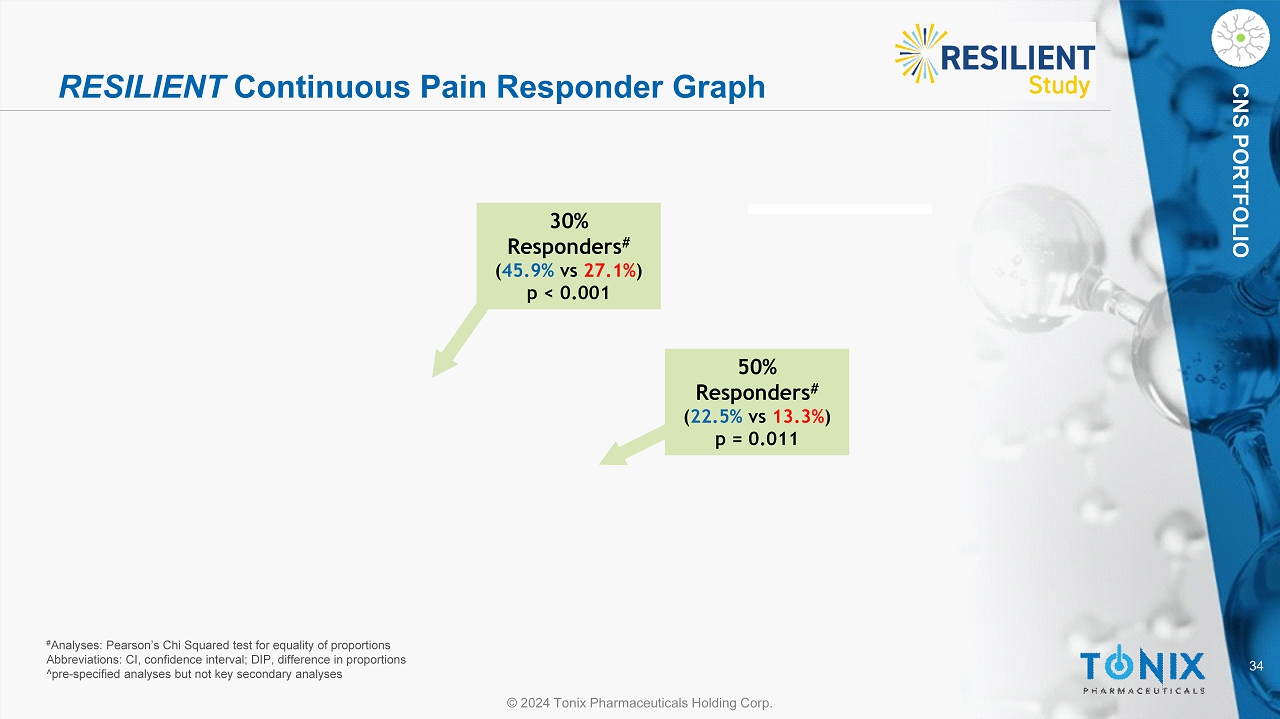
34 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO RESILIENT Continuous Pain Responder Graph P e r c e n t o f S u b j e c t s 0 10 20 30 40 50 60 70 80 90 100 Percentage Redution in Pain ≥ 0 ≥ 10% ≥ 20% ≥ 30% ≥ 40% ≥ 50% ≥ 60% ≥ 70% ≥ 80% ≥ 90% ≥ 100% Placebo TNX-102 SL 30% Responders # ( 45.9% vs 27.1% ) p < 0.001 50% Responders # ( 22.5% vs 13.3% ) p = 0.011 # Analyses: Pearson’s Chi Squared test for equality of proportions Abbreviations: CI, confidence interval; DIP, difference in proportions ^pre - specified analyses but not key secondary analyses
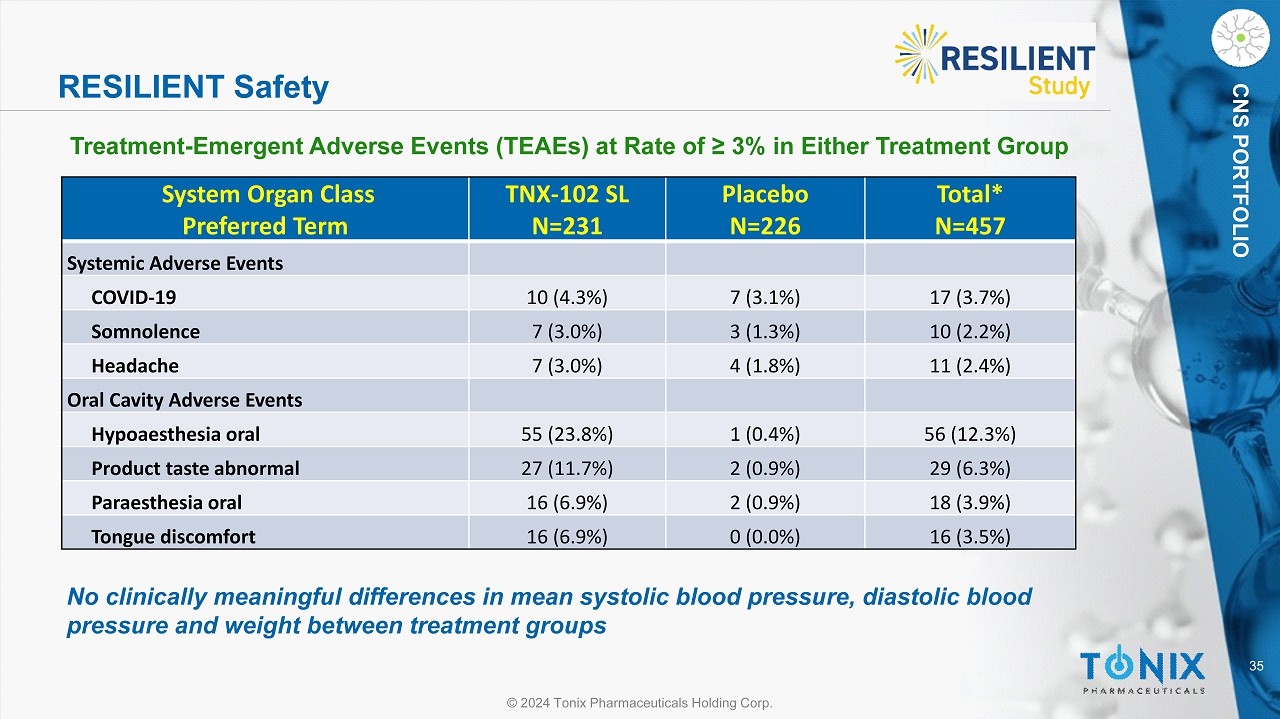
35 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO RESILIENT Safety System Organ Class Preferred Term TNX - 102 SL N=231 Placebo N=226 Total* N=457 Systemic Adverse Events COVID - 19 10 (4.3%) 7 (3.1%) 17 (3.7%) Somnolence 7 (3.0%) 3 (1.3%) 10 (2.2%) Headache 7 (3.0%) 4 (1.8%) 11 (2.4%) Oral Cavity Adverse Events Hypoaesthesia oral 55 (23.8%) 1 (0.4%) 56 (12.3%) Product taste abnormal 27 (11.7%) 2 (0.9%) 29 (6.3%) Paraesthesia oral 16 (6.9%) 2 (0.9%) 18 (3.9%) Tongue discomfort 16 (6.9%) 0 (0.0%) 16 (3.5%) Treatment - Emergent Adverse Events (TEAEs) at Rate of ≥ 3% in Either Treatment Group No clinically meaningful differences in mean systolic blood pressure, diastolic blood pressure and weight between treatment groups
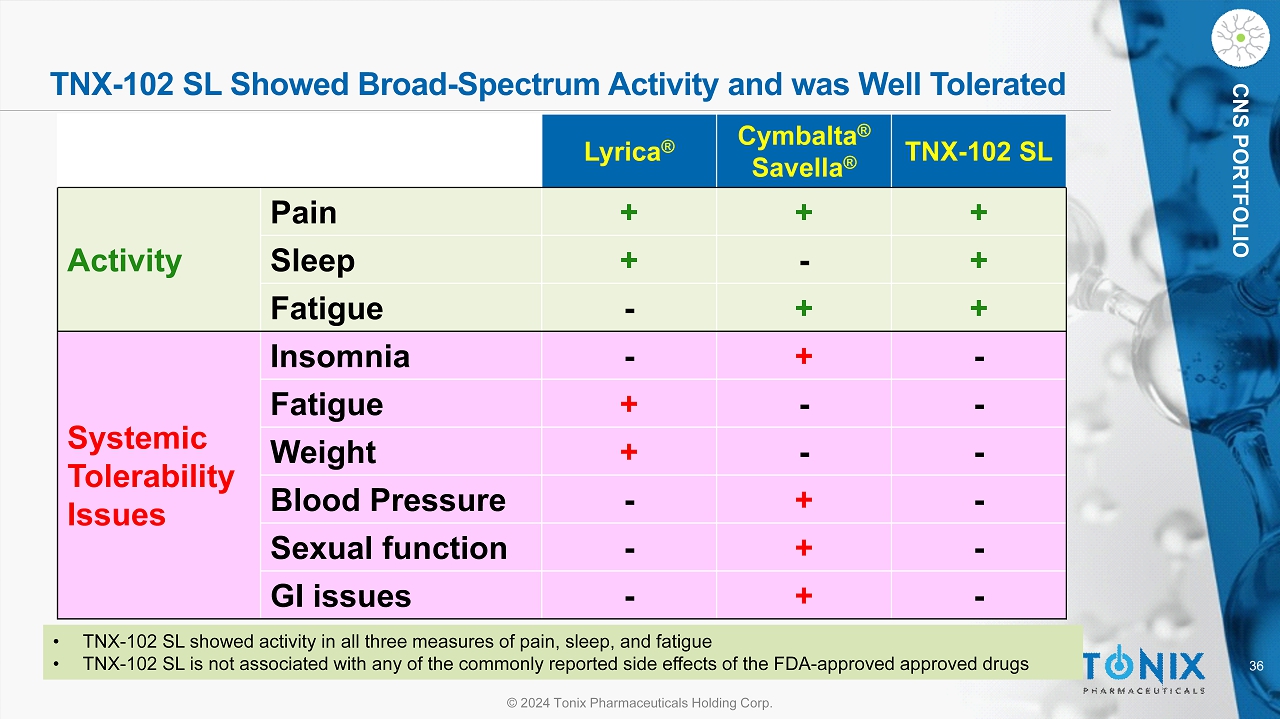
36 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 102 SL Showed Broad - Spectrum Activity and was Well Tolerated Lyrica ® Cymbalta ® Savella ® TNX - 102 SL Activity Pain + + + Sleep + - + Fatigue - + + Systemic Tolerability Issues Insomnia - + - Fatigue + - - Weight + - - Blood Pressure - + - Sexual function - + - GI issues - + - • TNX - 102 SL showed activity in all three measures of pain, sleep, and fatigue • TNX - 102 SL is not associated with any of the commonly reported side effects of the FDA - approved approved drugs
37 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 102 SL: A unique, sublingual formulation of cyclobenzaprine designed to optimize delivery and absorption □ Clinical Pharmacology of TNX - 102 SL • Rapid systemic exposure during the first 1 - 2 hr • Increased bioavailability during sleep • Reduced exposure to active metabolite • Dose proportional • Absence of food effect □ Efficacy and Safety of TNX - 102 SL in Fibromyalgia • Reduction in widespread pain in 14 - week study • Rapid onset of action: p - values <0.01 at each weekly time point, including Week 1 • Well - tolerated, side effects limited to the oral cavity • N on - opioid, centrally - acting analgesic that could provide a new therapeutic option for fibromyalgia patients • NDA fling in October 2024 with US FDA Conclusions

© 2023 Tonix Pharmaceuticals Holding Corp. THANK YOU