
TONIX PHARMACEUTICALS HOLDING CORP. 8-KA
EXHIBIT 99.03

TNX - 102 SL Lead Indication : Fibromyalgia Additional Indication: Acute Stress Disorder NASDAQ: TNXP December 2024 © 2024 Tonix Pharmaceuticals Holding Corp. PO6027 December 3, 2024 (Doc 1539)

Fibromyalgia Status: Two statistically significant Phase 3 studies completed; FDA granted Fast Track Designation • First pivotal Phase 3 study (RELIEF) completed • Second Phase 3 study (RALLY) missed primary endpoint • Confirmatory pivotal Phase 3 study (RESILIENT) completed • Submitted New Drug Application (NDA) to FDA in October 2024 Next Steps: FDA decision on NDA expected December 2024; If accepted, FDA decision on NDA approval expected August 2025 for standard review *TNX - 102 SL has not been approved for any indication. **5mg once - daily at bedtime. TNX - 102 SL* Cyclobenzaprine HCl Non - opiate analgesic A unique, sublingual formulation of cyclobenzaprine designed for bedtime dosing with sublingual delivery and transmucosal absorption, bypassing 1 st pass metabolism** Potent binding and antagonist activities at the serotonergic - 5 - HT 2A , adrenergic - α 1 , histaminergic - H 1 , and muscarinic - M 1 cholinergic receptors to facilitate restorative sleep Rapid drug exposure following once nightly sublingual administration Differentiators: Relative to Oral Cyclobenzaprine • Lower daytime exposure • Avoids first - pass metabolism • Reduces risk of pharmacological interference from major metabolite Relative to Standard of Care • Potential for better tolerability while maintaining efficacy • Not scheduled, without recognized abuse potential Indications with Active Programs Acute Stress Reaction/ Acute Stress Disorder • Phase 2 ready investigator - initiated study • U.S. Department of Defense funded / UNC will perform study Next Steps: Expect to start Phase 2 in 1Q 2025 © 2024 Tonix Pharmaceuticals Holding Corp.
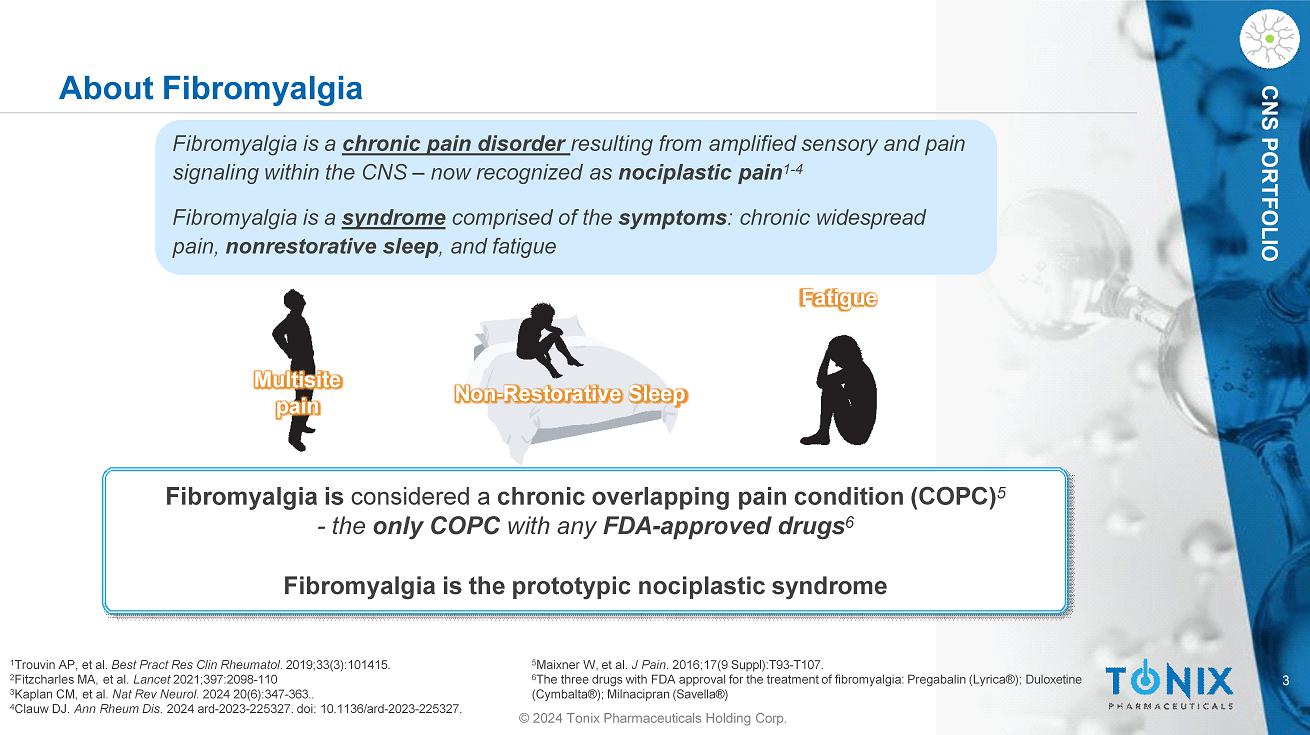
3 CNS PORTFOLIO About Fibromyalgia Fibromyalgia is considered a chronic overlapping pain condition (COPC) 5 - the only COPC with any FDA - approved drugs 6 Fibromyalgia is the prototypic nociplastic syndrome Multisite pain Non - Restorative Sleep Fibromyalgia is a chronic pain disorder resulting from amplified sensory and pain signaling within the CNS – now recognized as nociplastic pain 1 - 4 Fibromyalgia is a syndrome comprised of the symptoms : chronic widespread pain, nonrestorative sleep , and fatigue Fatigue 4 Clauw DJ. Ann Rheum Dis. 2024 ard - 2023 - 225327. doi: 10.1136/ard - 2023 - 225327. © 2024 Tonix Pharmaceuticals Holding Corp. 1 Trouvin AP, et al. Best Pract Res Clin Rheumatol . 2019;33(3):101415. 2 Fitzcharles MA, et al. Lancet 2021;397:2098 - 110 3 Kaplan CM, et al. Nat Rev Neurol. 2024 20(6):347 - 363.. 5 Maixner W, et al. J Pain . 2016;17(9 Suppl):T93 - T107. 6 The three drugs with FDA approval for the treatment of fibromyalgia: Pregabalin (Lyrica®); Duloxetine (Cymbalta®); Milnacipran (Savella®)
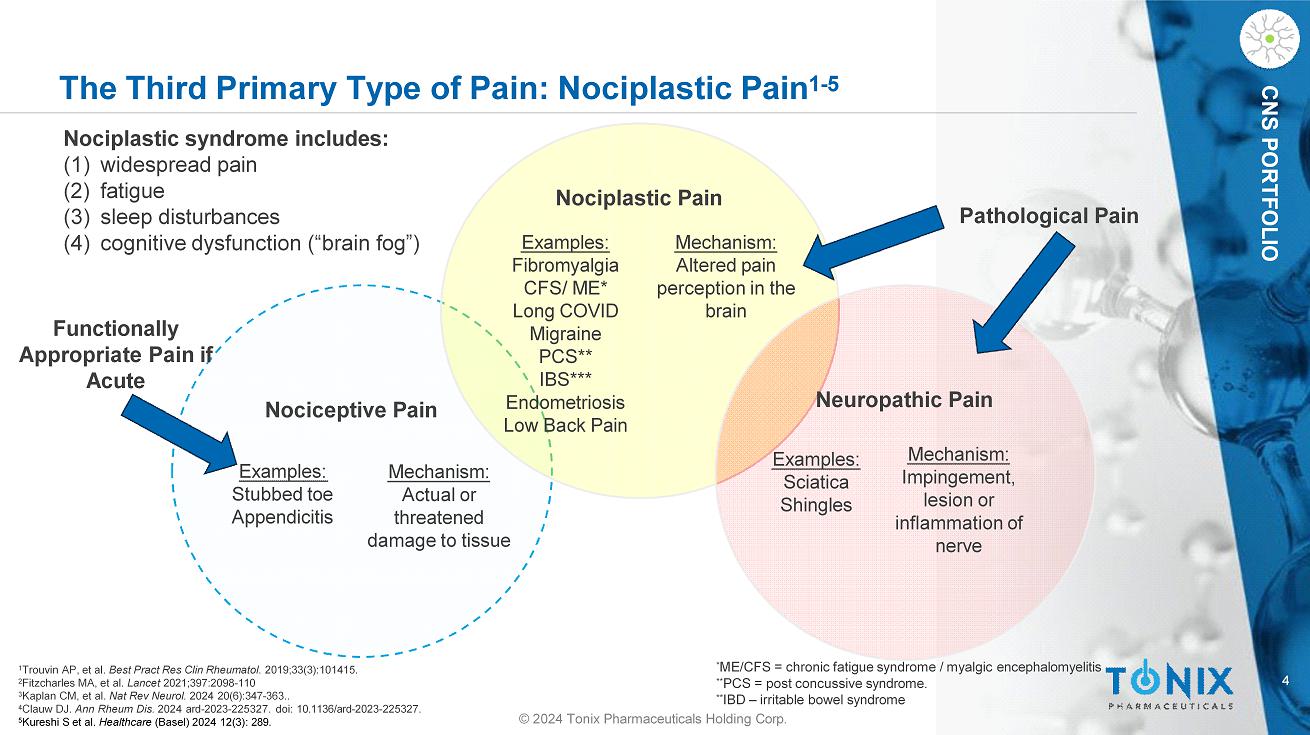
4 CNS PORTFOLIO The Third Primary Type of Pain: Nociplastic Pain 1 - 5 Nociplastic syndrome includes: (1) widespread pain (2) fatigue (3) sleep disturbances (4) cognitive dysfunction (“brain fog”) Nociplastic Pain Examples: Fibromyalgia CFS/ ME* Long COVID Migraine PCS** IBS*** Endometriosis Low Back Pain Examples: Stubbed toe Appendicitis Examples: Sciatica Shingles Mechanism: Altered pain perception in the brain Mechanism: Impingement, lesion or inflammation of nerve Mechanism: Actual or threatened damage to tissue Nociceptive Pain Neuropathic Pain Pathological Pain Functionally Appropriate Pain if Acute 1 Trouvin AP, et al. Best Pract Res Clin Rheumatol . 2019;33(3):101415. 2 Fitzcharles MA, et al. Lancet 2021;397:2098 - 110 3 Kaplan CM, et al. Nat Rev Neurol. 2024 20(6):347 - 363.. 4 Clauw DJ. Ann Rheum Dis. 2024 ard - 2023 - 225327. doi: 10.1136/ard - 2023 - 225327. 5 Kureshi S et al. Healthcare (Basel) 2024 12(3): 289. * ME/CFS = chronic fatigue syndrome / myalgic encephalomyelitis ** PCS = post concussive syndrome. ** IBD – irritable bowel syndrome © 2024 Tonix Pharmaceuticals Holding Corp.
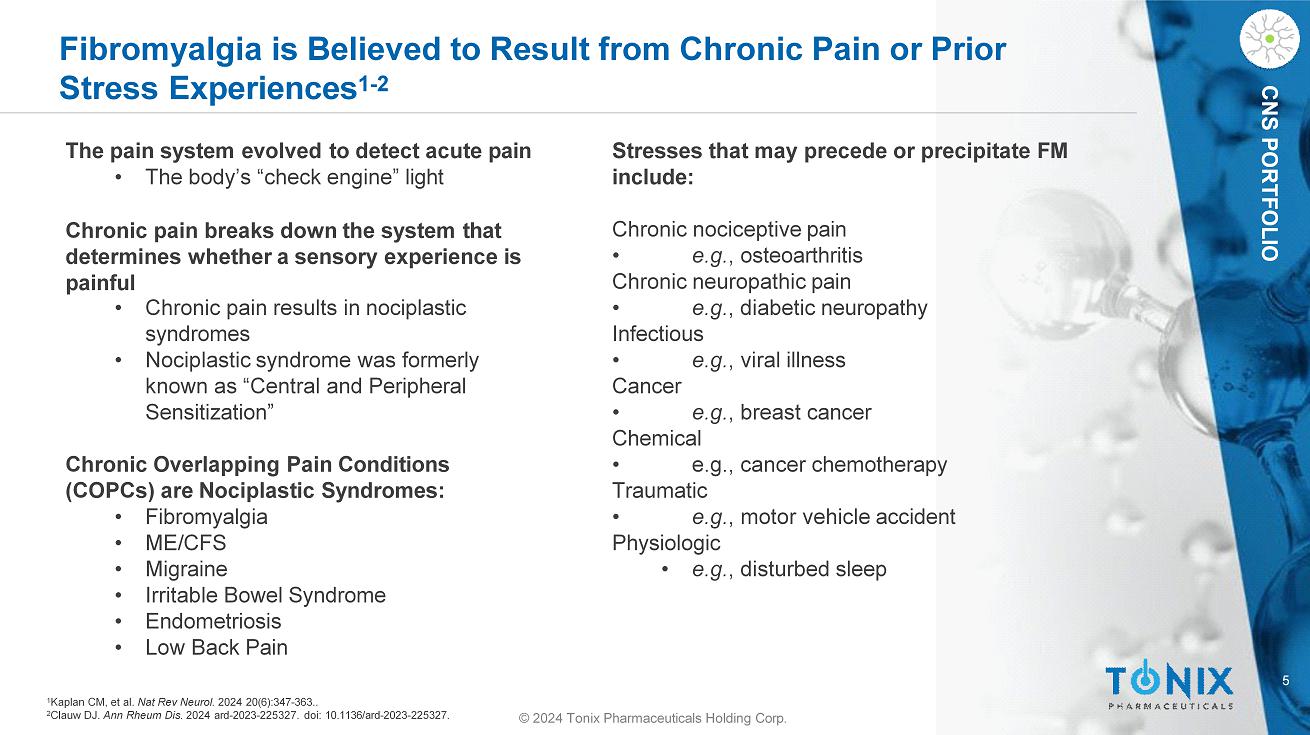
5 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Fibromyalgia is Believed to Result from Chronic Pain or Prior Stress Experiences 1 - 2 Stresses that may precede or precipitate FM include: Chronic nociceptive pain • e.g. , osteoarthritis Chronic neuropathic pain • e.g. , diabetic neuropathy Infectious • e.g. , viral illness Cancer • e.g. , breast cancer Chemical • e.g., cancer chemotherapy Traumatic • e.g. , motor vehicle accident Physiologic • e.g. , disturbed sleep The pain system evolved to detect acute pain • The body’s “check engine” light Chronic pain breaks down the system that determines whether a sensory experience is painful • Chronic pain results in nociplastic syndromes • Nociplastic syndrome was formerly known as “Central and Peripheral Sensitization” Chronic Overlapping Pain Conditions (COPCs) are Nociplastic Syndromes: • Fibromyalgia • ME/CFS • Migraine • Irritable Bowel Syndrome • Endometriosis • Low Back Pain 1 Kaplan CM, et al. Nat Rev Neurol. 2024 20(6):347 - 363.. 2 Clauw DJ. Ann Rheum Dis. 2024 ard - 2023 - 225327. doi: 10.1136/ard - 2023 - 225327.

6 CNS PORTFOLIO Common Chronic Conditions are a Challenge for Pharma © 2024 Tonix Pharmaceuticals Holding Corp. Fibromyalgia is a common chronic disease 1 - 3 • Chronic pain syndrome that persists for years or decades No animal model is recognized for nociplastic syndromes or its component symptoms • Widespread pain • Fatigue • Sleep disturbance • Cognitive impairment Nociplastic symptoms are subjective • Humans need to report symptoms using scales Clinical trials measuring subjective symptoms are challenging • Placebo response is typically observed • Long - term therapy means requires long - term tolerability 1 The U.S. Centers for Disease Control defines chronic diseases as “conditions that last 1 year or more and require ongoing medical attention or limit activities of daily living or both.” www.cdc.gov/chronicdisease/about/index.htm . (accessed Jan 28, 2024) 2 Kaplan CM, et al. Nat Rev Neurol. 2024 20(6):347 - 363.. 3 Clauw DJ. Ann Rheum Dis. 2024 ard - 2023 - 225327. doi: 10.1136/ard - 2023 - 225327 .

7 CNS PORTFOLIO Common Chronic Conditions are a Challenge for Society © 2024 Tonix Pharmaceuticals Holding Corp. The Opiate Crisis in the U.S. was driven by mistreatment of chronic pain, which was often nociplastic pain • The epidemic of prescription pain killers was addressed by regulations which limited the availability of opiates • Mandy individuals who are opiate dependent have transitioned to illegal street heroin and fentanyl • Illegal drugs contribute to homelessness There is an unmet need for non - opiate analgesics that address nociplastic pain • No new drug for fibromyalgia has been approved since 2009

8 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Fibromyalgia is a Large, Underserved and Dissatisfied Population • More than 10 million U.S. adults are affected – predominantly women 1,2 ‒ Debilitating and life altering condition ‒ Significant economic impact • Patients have expressed dissatisfaction, despite three FDA approved drugs 3,4 ‒ 85% of patients fail first - line therapy 5 : efficacy variability, tolerability issues especially when used long - term and lack of broad - spectrum activity ‒ Typical for patients to rotate between drugs and be on multiple drugs at the same time; 79% of FM patients are on multiple therapies 5 • ~2.7 million FM patients diagnosed and treated 6 ‒ >22 million prescriptions are issued for the treatment of fibromyalgia (on - and off - label usage) each year 7,8 • No new R x product since 2009 • The treatment objective is to restore functionality and quality of life while avoiding significant side effects 1 American College of Rheumatology ( www.ACRPatientInfo.org accessed May 7, 2019) – prevalence rate of 2 - 4% for U.S. adult population (~250 million) 2 Vincent A, et al. Arthritis Care Res (Hoboken) . 2013 65(5):786 - 92. doi: 10.1002; diagnosed prevalence rate was 1.1% of adult population or 50% of the prevalent population 3 Robinson RL, et al. Pain Med . 2012 13(10):1366 - 76. doi: 10.1111; 85% received drug treatment 4 The three drugs with FDA approval for the treatment of fibromyalgia: Pregabalin (Lyrica); Duloxetine (Cymbalta); Milnacipran (Savella) 5 EVERSANA primary physician research, May 2024; commissioned by Tonix 6 EVERSANA analysis of claims database, May 2024; commissioned by Tonix 7 Product sales derived from IMS MIDAS; IMS NDTI used to factor usage for fibromyalgia;data accessedApril 2015. 8 Market research by Frost & Sullivan, commissioned by Tonix, 2011
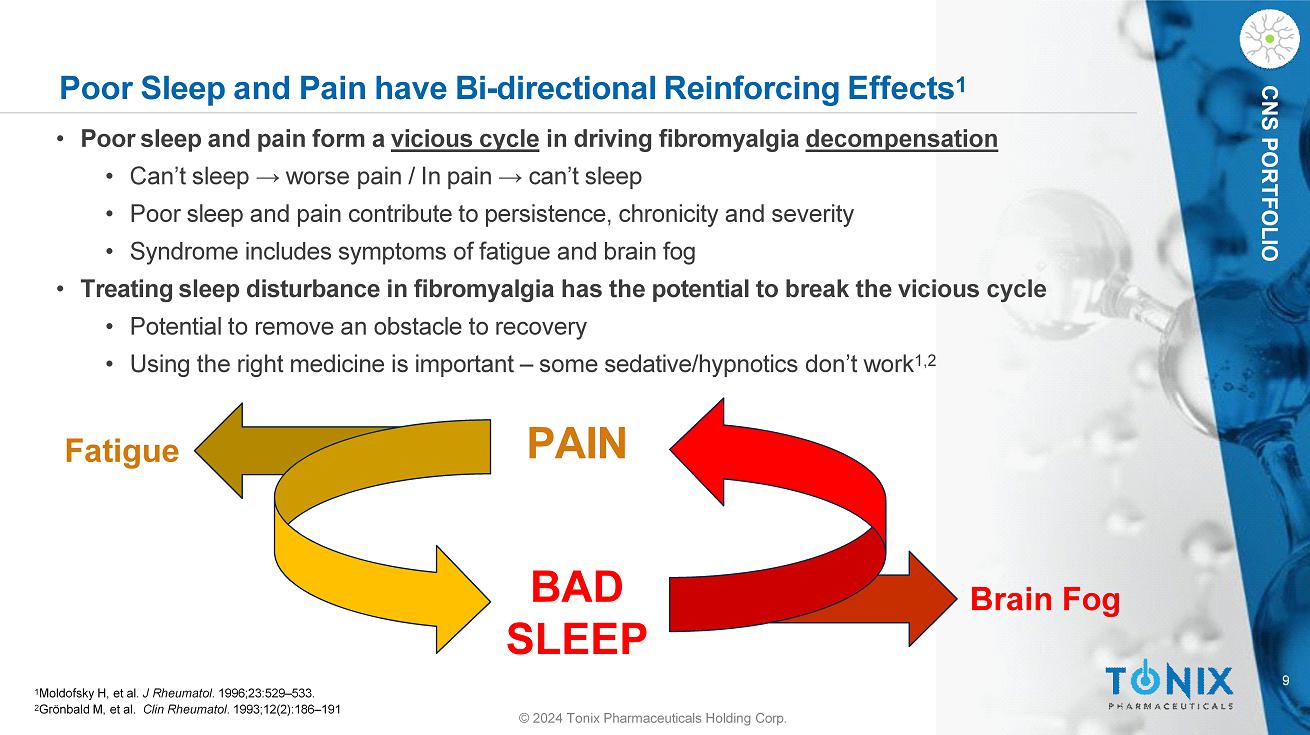
9 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Poor Sleep and Pain have Bi - directional Reinforcing Effects 1 1 Moldofsky H, et al. J Rheumatol . 1996;23:529 – 533. 2 Grönbald M, et al. Clin Rheumatol . 1993;12(2):186 – 191 • Poor sleep and pain form a vicious cycle in driving fibromyalgia decompensation • Can’t sleep → worse pain / In pain → can’t sleep • Poor sleep and pain contribute to persistence, chronicity and severity • Syndrome includes symptoms of fatigue and brain fog • Treating sleep disturbance in fibromyalgia has the potential to break the vicious cycle • Potential to remove an obstacle to recovery • Using the right medicine is important – some sedative/hypnotics don’t work 1,2 PAIN BAD SLEEP Fatigue Brain Fog

10 CNS PORTFOLIO Fibromyalgia: Unrefreshing Sleep and Cyclobenzaprine Treatment 1 Moldofsky H et al. Psychosom Med. 1975. 37:341 - 51. 2 Moldofsky H and Scarisbrick P. Psychosom Med. 1976. 38:35 - 44. 3 Bennett RM, et al. Arthritis Rheum 1988. 31:1535 – 42. 4 Quimby LG, et al . J Rheumatol Suppl, 1989 Nov ; 19 : 140 – 3 . 5 Reynolds WJ, et al. J Rheumatol. 1991 . 18 : 452 – 4 . 6 Santandrea S, et al . J Int Med Res. 1993 . 21 : 74 – 80 . • Non - restorative sleep 1,2 ‒ Harvey Moldofsky – recognition of unrefreshing/non - restorative sleep: ▪ Symptom ▪ Potential causative or potentiating factor • Cyclobenzaprine 3 - 9 ‒ Potentially the earliest drug studied in fibromyalgia as an oral swallowed agent ‒ Studies showed equivocal effects and tolerability issues at “muscle spasm” doses • Bedtime, low - dose cyclobenzaprine targeting non - restorative sleep 10 - 11 ‒ Recognition of unrefreshing sleep as a target of therapy ‒ Primitive oral, swallowed formulation – “flat” pharmacokinetics • Bedtime, sublingual transmucosal cyclobenzaprine targeting non - restorative sleep 12 ‒ Dynamic pharmacokinetic profile, rapid absorption, decrease in major metabolite ‒ Two studies (Phase 2 and Phase 3) at 2.8 mg; three Phase 3 studies at 5.6 mg. 7 Cantini F, et al . Minerva Med. 1994 . 85 : 97 – 100 . 8 Carette S, et al. Arthritis Rheum. 1994 . 37 : 32 – 40 . 9 Tofferi JK, et al . Arthritis Rheum. 2004 . 51 : 9 – 13 . 1 10 Iglehart IW. 2003; US Patent 6,541,523. 11 Moldofsky et al. J Rheumatol . 2011. 38:2653 - 2663 12 Lederman S et al. Arthritis Care Res . 2023. 75:2359 - 2368. © 2024 Tonix Pharmaceuticals Holding Corp.
11 CNS PORTFOLIO Fibromyalgia Program Status Fibromyalgia TNX - 102 SL Cyclobenzaprine Sublingual Tablets NDA Submitted to FDA in October 2024 1 Lederman S, et al. Arthritis Care Res (Hoboken) . 2023 Nov;75(11):2359 - 2368. doi: 10.1002. © 2024 Tonix Pharmaceuticals Holding Corp. First pivotal Phase 3 study ( RELIEF ) reported – December 2020 1 Second Phase 3 study ( RALLY ) missed primary endpoint – July 2021 Confirmatory pivotal Phase 3 study ( RESILIENT ) reported – December 2023 Granted FDA Fast Track Designation Submitted NDA to FDA in October 2024 Next Steps: • FDA decision on acceptance of NDA for review expected December 2024 • If accepted, FDA decision on NDA approval expected August 2025 for standard review

12 Phase 3 RESILIENT Study Population © 2024 Tonix Pharmaceuticals Holding Corp.

13 CNS PORTFOLIO TNX - 102 SL: P hase 3 RESILIENT Study Design General study characteristics: • Randomized, double - blind, multicenter, placebo - controlled study in fibromyalgia • 33 U.S. sites enrolled 457 participants with fibromyalgia as defined by 2016 Revisions to the 2010/2011 FM Diagnostic Criteria 1 Primary Endpoint: • Change from baseline to Week 14 (TNX - 102 SL vs. placebo) in weekly averages of daily diary average pain severity score • Primary Endpoint, p - value = 0.00005 Placebo once - daily at bedtime TNX - 102 SL once - daily at bedtime 5.6 mg (2 x 2.8 mg tablets) * ClinicalTrials.gov Identifier: NCT05273749 Study Title: A Phase 3 Study to Evaluate the Efficacy and Safety of TNX - 102 SL Taken Daily in Patients With Fibromyalgia (RESILIENT) Trial ID: TNY - CY - F307 (‘RESILIENT’) 14 weeks * Two - week run - in at 2.8 mg dose at bedtime followed by 12 weeks at 5.6 mg dose 1 Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Häuser W, Katz RL, Mease PJ, Russell AS, Russell IJ, Walitt B. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin Arthritis Rheum. 2016; 46(3):319 - 329. © 2024 Tonix Pharmaceuticals Holding Corp.

14 CNS PORTFOLIO RESILIENT Demographics and Baseline Characteristics © 2024 Tonix Pharmaceuticals Holding Corp. Placebo (N=225) TNX - 102 SL (N=231) 49.5 (11.35)* 49.3 (10.45)* Age (years) 211 (93.8%) † 224 (97.0%) † Female 35 (15.6%) † 36 (15.6%) † Hispanic or Latino 192 (85.3%) † 194 (84.0%) † White 26 (11.6%) † 32 (13.9%) † Black 5.9 (1.08)* 5.9 (1.05)* Pain Score (0 - 10 NRS) 150 (66.7%) † 147 (63.6%) † Employed Yes 9.9 (9.53)* 8.6 (8.44)* FM Duration (years) 31.1 (6.32)* 31.1 (6.34)* BMI (kg/m 2 ) * Mean (standard deviation) † N (%)
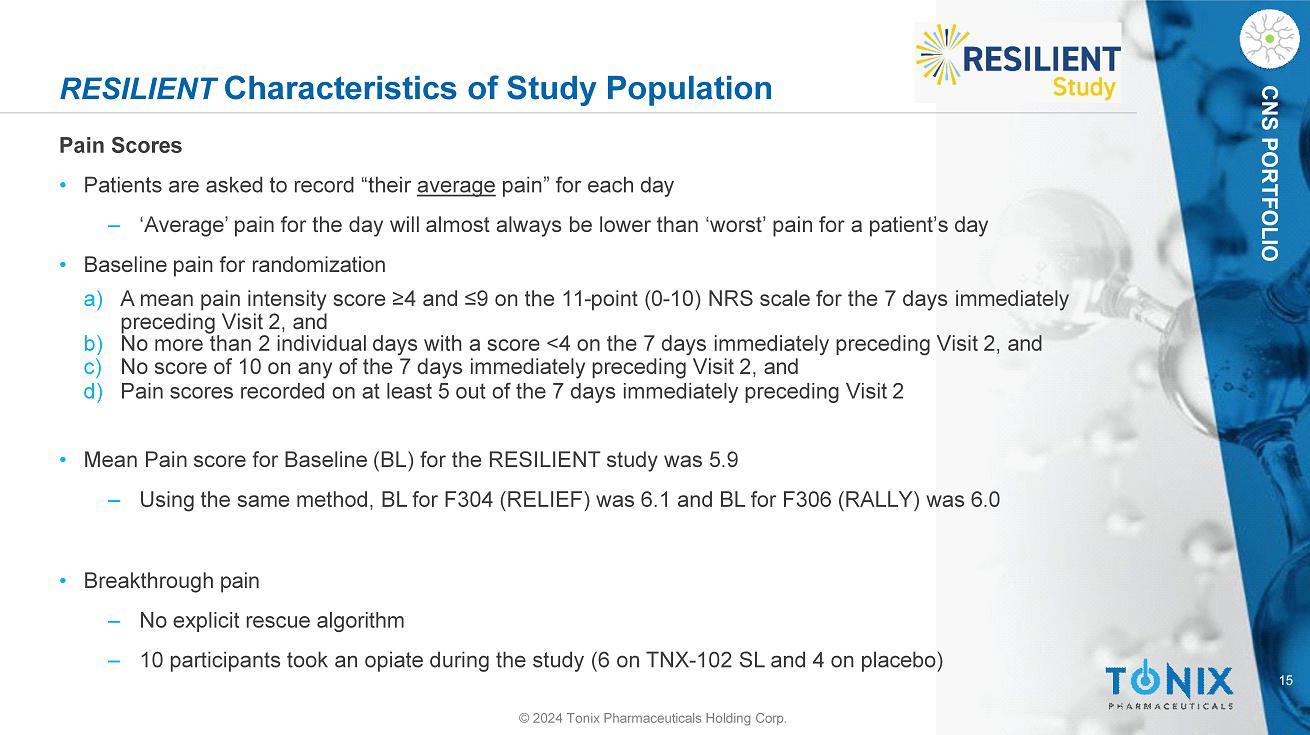
CNS PORTFOLIO RESILIENT Characteristics of Study Population Pain Scores • Patients are asked to record “their average pain” for each day ‒ ‘Average’ pain for the day will almost always be lower than ‘worst’ pain for a patient’s day • Baseline pain for randomization a) A mean pain intensity score ≥4 and ≤9 on the 11 - point (0 - 10) NRS scale for the 7 days immediately preceding Visit 2, and b) No more than 2 individual days with a score <4 on the 7 days immediately preceding Visit 2, and c) No score of 10 on any of the 7 days immediately preceding Visit 2, and d) Pain scores recorded on at least 5 out of the 7 days immediately preceding Visit 2 • Mean Pain score for Baseline (BL) for the RESILIENT study was 5.9 ‒ Using the same method, BL for F304 (RELIEF) was 6.1 and BL for F306 (RALLY) was 6.0 • Breakthrough pain ‒ No explicit rescue algorithm ‒ 10 participants took an opiate during the study (6 on TNX - 102 SL and 4 on placebo) 15 © 2024 Tonix Pharmaceuticals Holding Corp.

RESILIENT Study Efficacy Findings 16 © 2024 Tonix Pharmaceuticals Holding Corp.
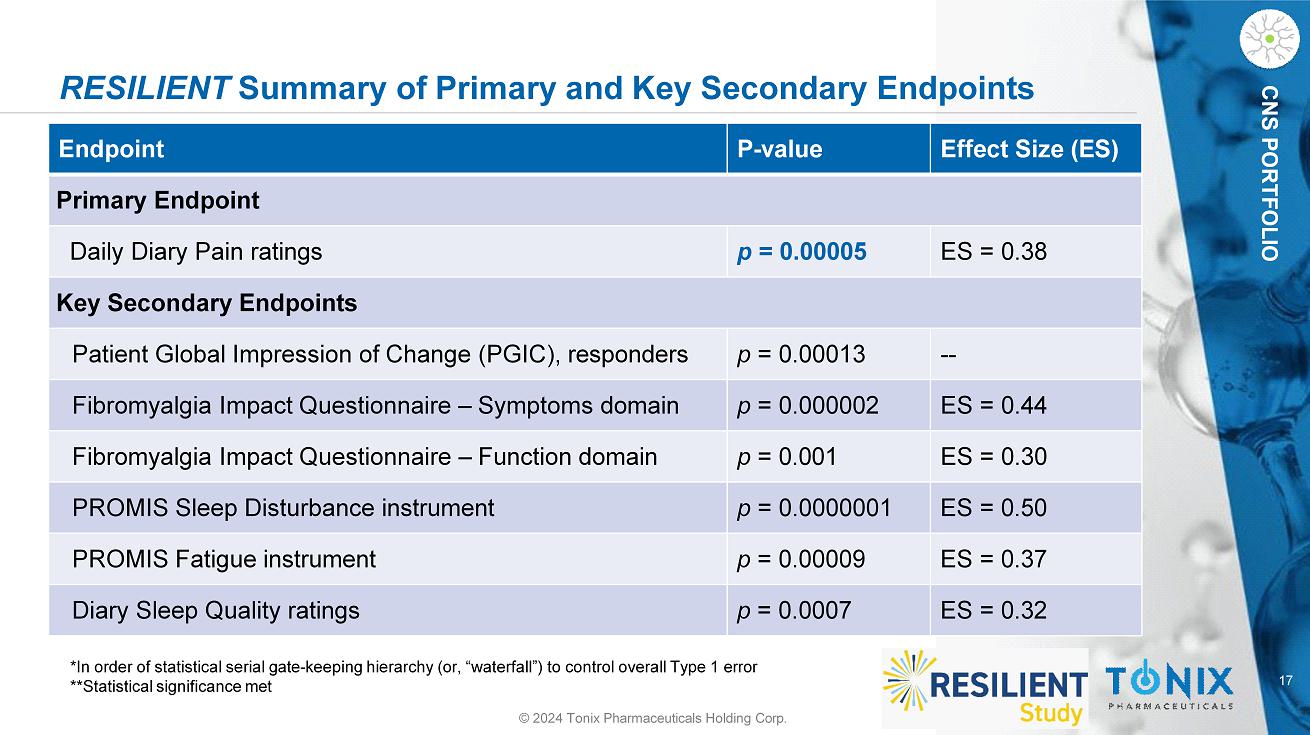
17 CNS PORTFOLIO RESILIENT Summary of Primary and Key Secondary Endpoints © 2024 Tonix Pharmaceuticals Holding Corp. *In order of statistical serial gate - keeping hierarchy (or, “waterfall”) to control overall Type 1 error **Statistical significance met Effect Size (ES) P - value Endpoint Primary Endpoint ES = 0.38 p = 0.00005 Daily Diary Pain ratings Key Secondary Endpoints - - p = 0.00013 Patient Global Impression of Change (PGIC), responders ES = 0.44 p = 0.000002 Fibromyalgia Impact Questionnaire – Symptoms domain ES = 0.30 p = 0.001 Fibromyalgia Impact Questionnaire – Function domain ES = 0.50 p = 0.0000001 PROMIS Sleep Disturbance instrument ES = 0.37 p = 0.00009 PROMIS Fatigue instrument ES = 0.32 p = 0.0007 Diary Sleep Quality ratings
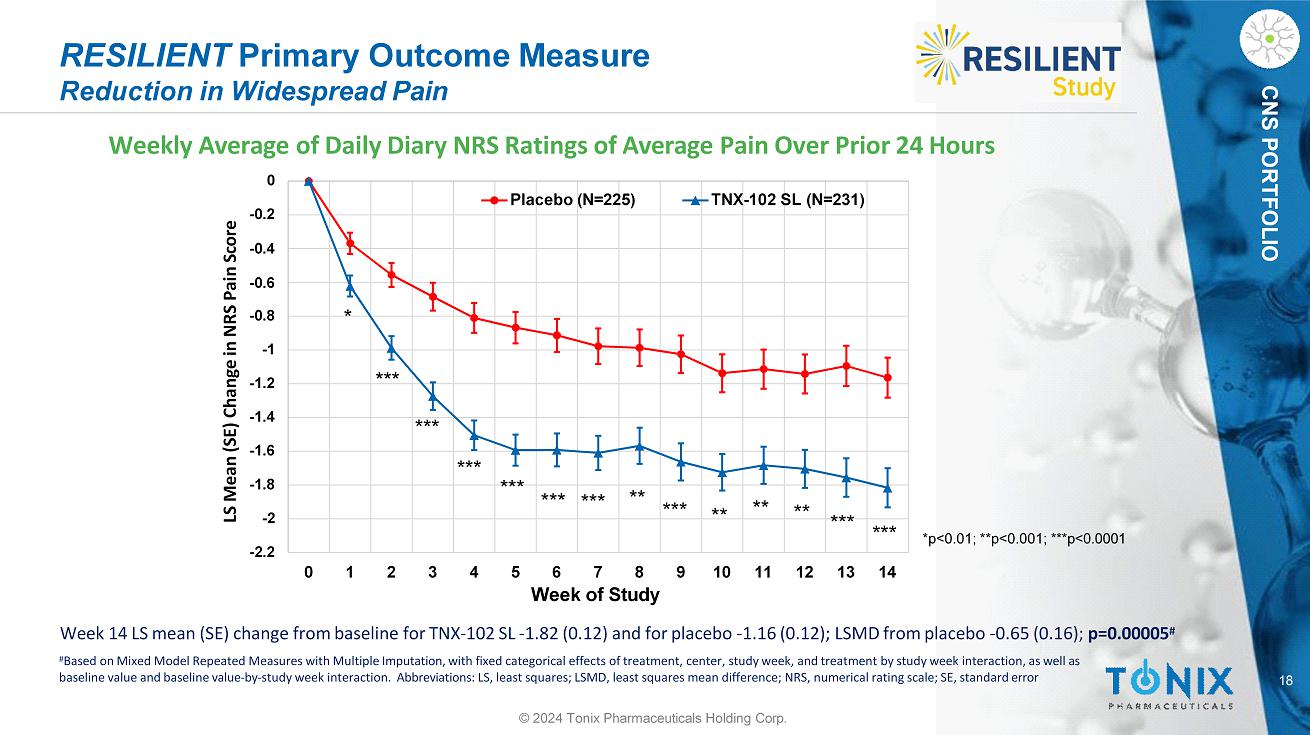
18 CNS PORTFOLIO RESILIENT Primary Outcome Measure Reduction in Widespread Pain Weekly Average of Daily Diary NRS Ratings of Average Pain Over Prior 24 Hours - 2 - 1.8 - 1.6 - 1.4 - 1.2 - 1 - 0.8 - 0.2 - 0.4 - 0.6 0 LS Mean (SE) Change in NRS Pain Score - 2.2 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Week of Study Placebo (N=225) TNX - 102 SL (N=231) * *** *** ** ** ** *** *** *** *** *** *** *** ** *p<0.01; **p<0.001; ***p<0.0001 Week 14 LS mean (SE) change from baseline for TNX - 102 SL - 1.82 (0.12) and for placebo - 1.16 (0.12); LSMD from placebo - 0.65 (0.16); p=0.00005 # # Based on Mixed Model Repeated Measures with Multiple Imputation, with fixed categorical effects of treatment, center, study week, and treatment by study week interaction, as well as baseline value and baseline value - by - study week interaction. Abbreviations: LS, least squares; LSMD, least squares mean difference; NRS, numerical rating scale; SE, standard error © 2024 Tonix Pharmaceuticals Holding Corp.

19 CNS PORTFOLIO RESILIENT Continuous Pain Responder Graph # Analyses: Pearson’s Chi Squared test for equality of proportions Abbreviations: CI, confidence interval; DIP, difference in proportions ^pre - specified analyses but not key secondary analyses Percent of Subjects 100 90 80 70 60 50 40 30 20 10 0 ≥ 0 ≥ 10% ≥ 20% ≥ 30% ≥ 40% ≥ 50% ≥ 60% ≥ 70% ≥ 80% ≥ 90% Percentage Redution in Pain ≥ 100% Placebo TNX - 102 SL 30% Responders # ( 45.9% vs 27.1% ) p < 0.001 50% Responders # ( 22.5% vs. 13.3% ) p = 0.011 © 2024 Tonix Pharmaceuticals Holding Corp.
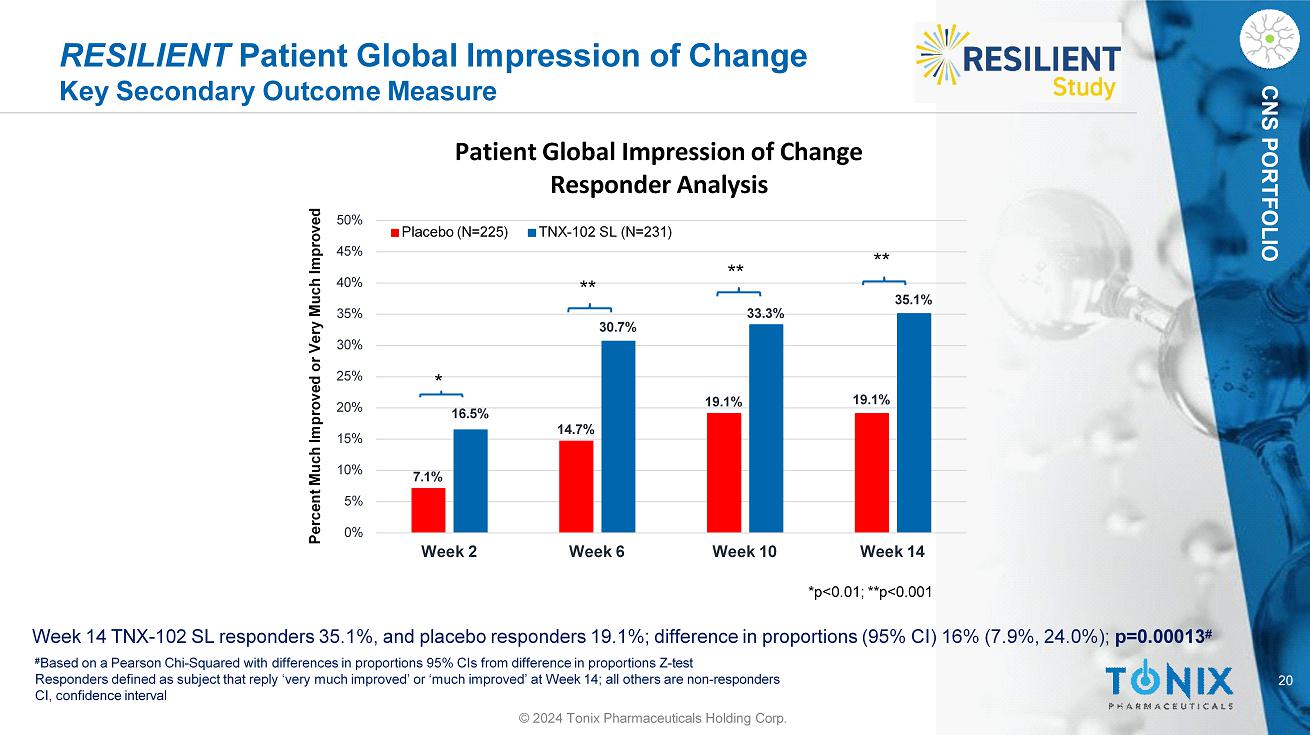
20 CNS PORTFOLIO RESILIENT Patient Global Impression of Change Key Secondary Outcome Measure Week 14 TNX - 102 SL responders 35.1%, and placebo responders 19.1%; difference in proportions (95% CI) 16% (7.9%, 24.0%); p=0.00013 # # Based on a Pearson Chi - Squared with differences in proportions 95% CIs from difference in proportions Z - test Responders defined as subject that reply ‘very much improved’ or ‘much improved’ at Week 14; all others are non - responders CI, confidence interval 7.1% 14.7% 19.1% 19.1% 16.5% 30.7% 33.3% 35.1% 5% 0% 10% 15% 20% 25% 30% 35% 40% 45% 50% Week 2 Week 6 Week 10 Week 14 Patient Global Impression of Change Responder Analysis Placebo (N=225) TNX - 102 SL (N=231) Percent Much Improved or Very Much Improved *p<0.01; **p<0.001 * ** ** ** © 2024 Tonix Pharmaceuticals Holding Corp.
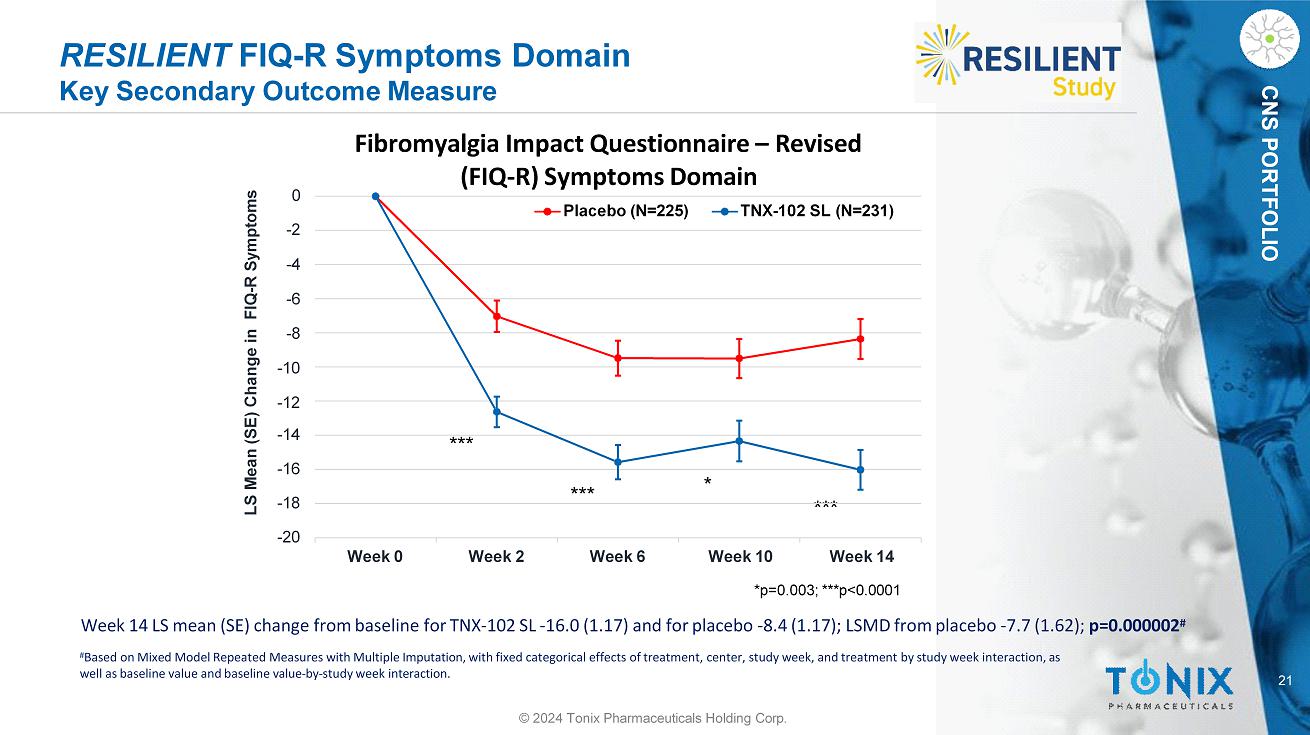
21 CNS PORTFOLIO RESILIENT FIQ - R Symptoms Domain Key Secondary Outcome Measure * *** *** *** Week 14 LS mean (SE) change from baseline for TNX - 102 SL - 16.0 (1.17) and for placebo - 8.4 (1.17); LSMD from placebo - 7.7 (1.62); p=0.000002 # # Based on Mixed Model Repeated Measures with Multiple Imputation, with fixed categorical effects of treatment, center, study week, and treatment by study week interaction, as well as baseline value and baseline value - by - study week interaction. - 20 - 18 - 16 - 14 - 2 - 4 - 6 - 8 - 10 - 12 0 Week 0 Week 2 Week 6 LS Mean (SE) Change in FIQ - R Symptoms Fibromyalgia Impact Questionnaire – Revised (FIQ - R) Symptoms Domain Placebo (N=225) TNX - 102 SL (N=231) Week 10 Week 14 *p=0.003; ***p<0.0001 © 2024 Tonix Pharmaceuticals Holding Corp.

CNS PORTFOLIO RESILIENT FIQ - R Function Domain Key Secondary Outcome Measure Week 14 LS mean (SE) change from baseline for TNX - 102 SL - 12.2 (1.19) and for placebo - 6.8 (1.21); LSMD from placebo - 5.4 (1.66); p=0.001 # # Based on Mixed Model Repeated Measures with Multiple Imputation, with fixed categorical effects of treatment, center, study week, and treatment by study week interaction, as - 16 - 14 - 12 - 2 - 4 - 6 - 8 - 10 0 Week 0 Week 2 Week 6 Week 14 LS Mean (SE) Change in FIQ - R Function Fibromyalgia Impact Questionnaire – Revised (FIQ - R) Function Domain Placebo (N=225) TNX - 102 SL (N=231) Week 10 **p<0.01 ** ** ** well as baseline value and baseline value - by - study week interaction. 22 © 2024 Tonix Pharmaceuticals Holding Corp.
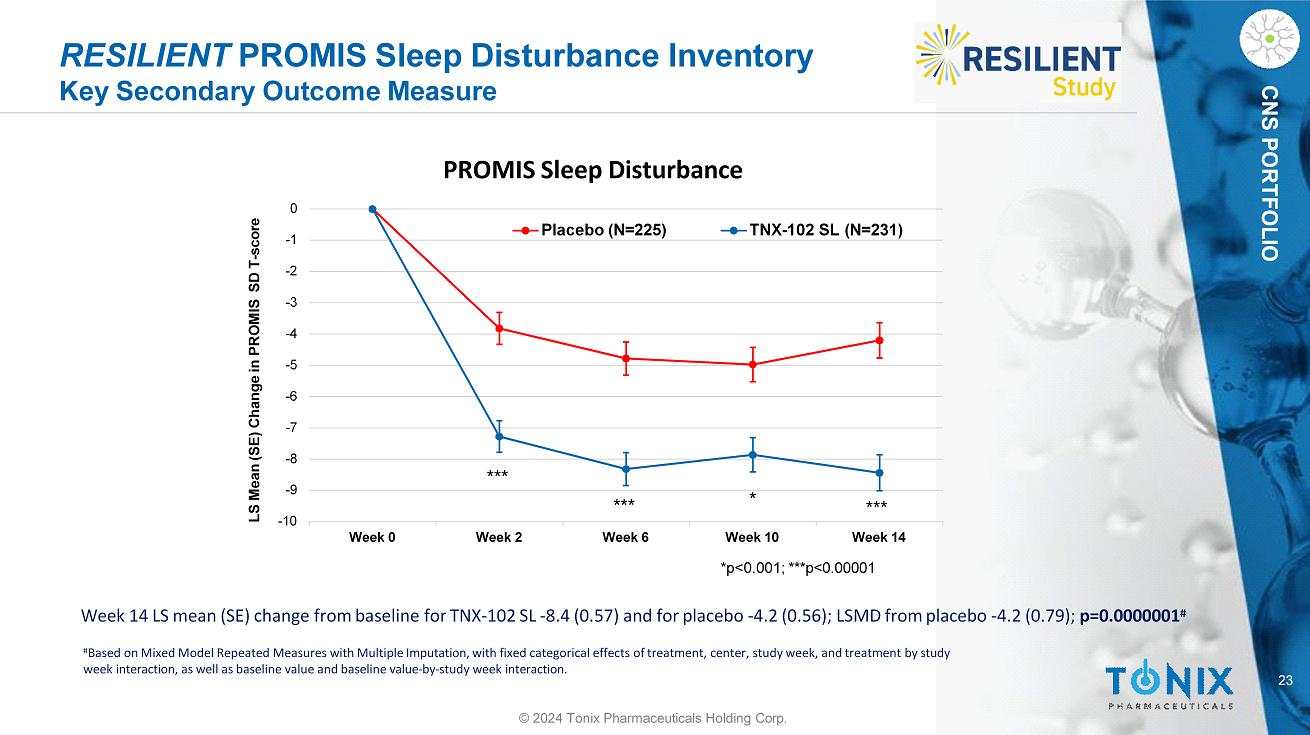
CNS PORTFOLIO RESILIENT PROMIS Sleep Disturbance Inventory Key Secondary Outcome Measure Week 14 LS mean (SE) change from baseline for TNX - 102 SL - 8.4 (0.57) and for placebo - 4.2 (0.56); LSMD from placebo - 4.2 (0.79); p=0.0000001 # # Based on Mixed Model Repeated Measures with Multiple Imputation, with fixed categorical effects of treatment, center, study week, and treatment by study week interaction, as well as baseline value and baseline value - by - study week interaction. - 10 - 9 - 2 - 3 - 4 - 5 - 6 - 7 - 8 - 1 0 Week 0 Week 2 Week 6 LS Mean (SE) Change in PROMIS SD T - score PROMIS Sleep Disturbance Placebo (N=225) TNX - 102 SL (N=231) Week 10 Week 14 *p<0.001; ***p<0.00001 * *** *** *** 23 © 2024 Tonix Pharmaceuticals Holding Corp.
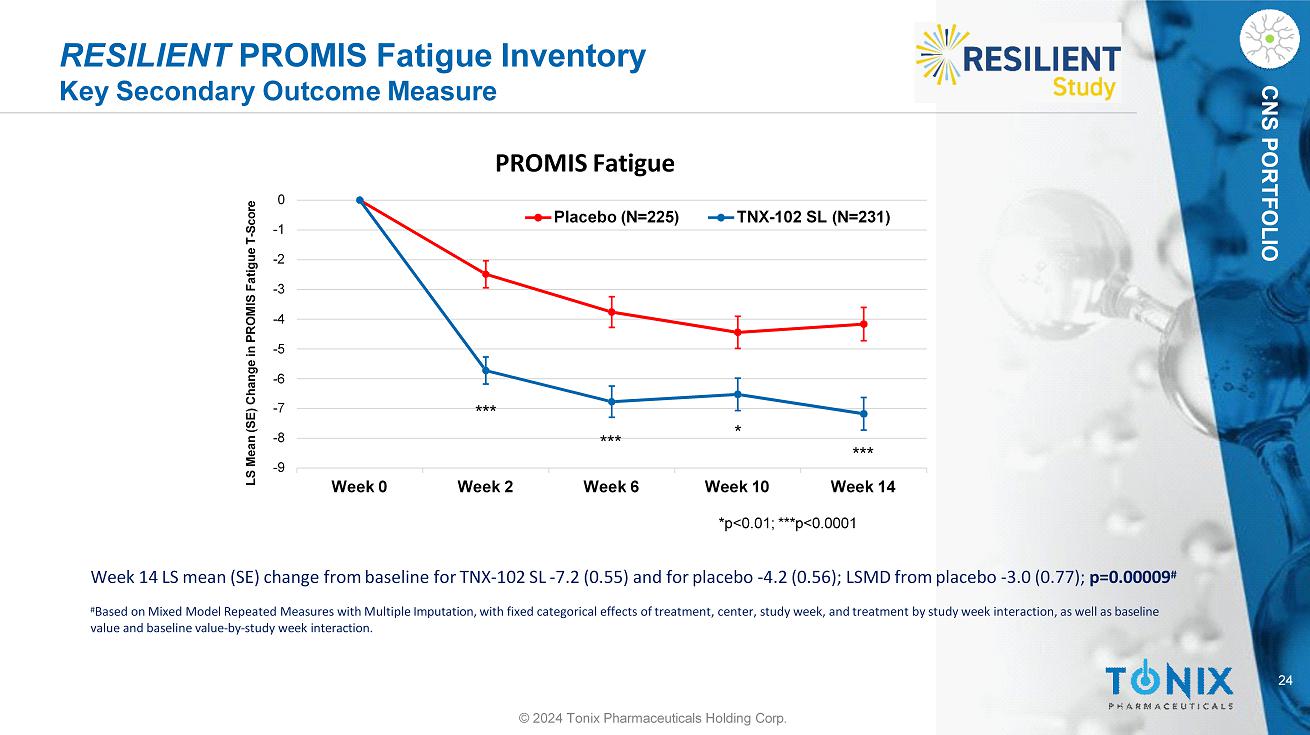
CNS PORTFOLIO RESILIENT PROMIS Fatigue Inventory Key Secondary Outcome Measure Week 14 LS mean (SE) change from baseline for TNX - 102 SL - 7.2 (0.55) and for placebo - 4.2 (0.56); LSMD from placebo - 3.0 (0.77); p=0.00009 # # Based on Mixed Model Repeated Measures with Multiple Imputation, with fixed categorical effects of treatment, center, study week, and treatment by study week interaction, as well as baseline value and baseline value - by - study week interaction. - 9 - 8 - 7 - 2 - 3 - 4 - 5 - 6 - 1 0 Week 0 Week 2 Week 6 LS Mean (SE) Change in PROMIS Fatigue T - Score PROMIS Fatigue Placebo (N=225) TNX - 102 SL (N=231) Week 10 Week 14 *p<0.01; ***p<0.0001 * *** *** *** 24 © 2024 Tonix Pharmaceuticals Holding Corp.
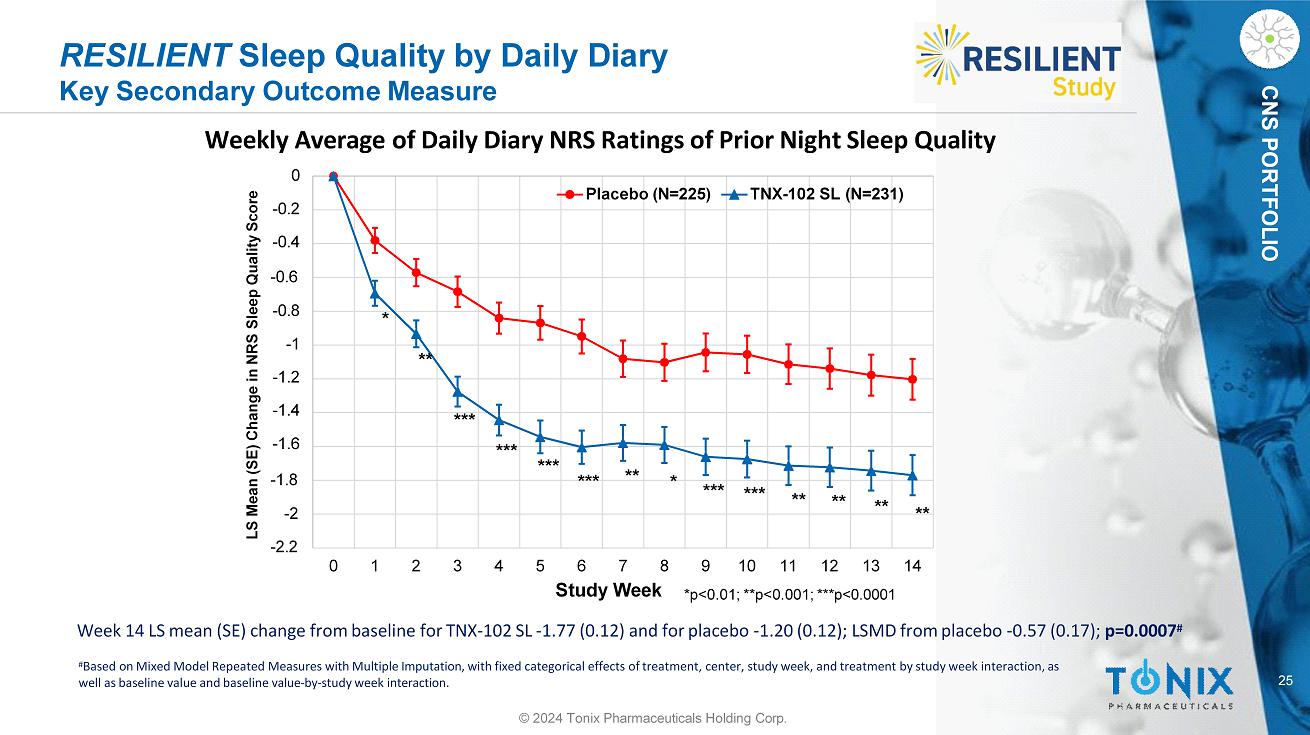
25 CNS PORTFOLIO Week 14 LS mean (SE) change from baseline for TNX - 102 SL - 1.77 (0.12) and for placebo - 1.20 (0.12); LSMD from placebo - 0.57 (0.17); p=0.0007 # RESILIENT Sleep Quality by Daily Diary Key Secondary Outcome Measure Weekly Average of Daily Diary NRS Ratings of Prior Night Sleep Quality - 2 - 1.8 - 0.8 - 1 - 1.2 - 1.4 - 1.6 - 0.6 0 - 0.2 - 0.4 - 2.2 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 LS Mean (SE) Change in NRS Sleep Quality Score Study Week Placebo (N=225) TNX - 102 SL (N=231) *p<0.01; **p<0.001; ***p<0.0001 *** * ** ** * ** *** ** ** ** *** *** *** *** # Based on Mixed Model Repeated Measures with Multiple Imputation, with fixed categorical effects of treatment, center, study week, and treatment by study week interaction, as well as baseline value and baseline value - by - study week interaction. © 2024 Tonix Pharmaceuticals Holding Corp.
26 CNS PORTFOLIO RESILIENT Summary of Primary Endpoint and Key Secondary Efficacy Endpoints © 2024 Tonix Pharmaceuticals Holding Corp. Fibromyalgia is a syndrome composed of symptoms • Widespread pain • Fatigue • Sleep disturbance Efficacy across symptoms of pain, fatigue and sleep • Pain (primary endpoint, daily pain diary): p - value of 0.00005 • Fatigue (PROMIS fatigue): • Sleep (PROMIS sleep disturbance): p - value of 0.00009 p - value of 0.0000001 Conclusion: TNX - 102 SL has “broad spectrum” or “syndromal activity” • Broad spectrum: across several symptoms • Syndromal: improves the syndrome (most of the symptoms) • Potential for a broad - spectrum drug to reduce the use of multiple drugs or “polypharmacy”
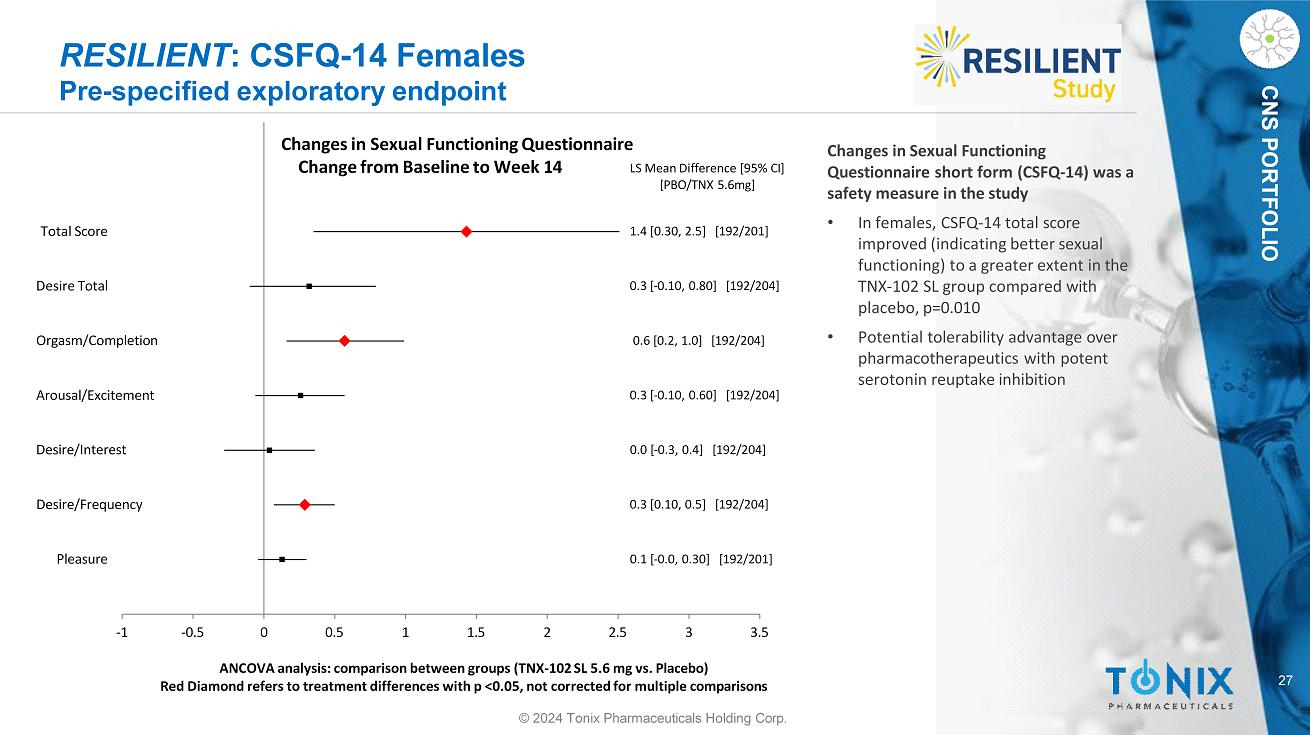
27 CNS PORTFOLIO RESILIENT : CSFQ - 14 Females Pre - specified exploratory endpoint Pleasure Desire/Frequency Desire/Interest Arousal/Excitement Orgasm/Completion Desire Total Total Score 0.1 [ - 0.0, 0.30] [192/201] 0.3 [0.10, 0.5] [192/204] 0.0 [ - 0.3, 0.4] [192/204] 0.3 [ - 0.10, 0.60] [192/204] 0.6 [0.2, 1.0] [192/204] 0.3 [ - 0.10, 0.80] [192/204] 1.4 [0.30, 2.5] [192/201] LS Mean Difference [95% CI] [PBO/TNX 5.6mg] - 1 - 0.5 0 0.5 1 1.5 2 2.5 3 3.5 ANCOVA analysis: comparison between groups (TNX - 102 SL 5.6 mg vs. Placebo) Red Diamond refers to treatment differences with p <0.05, not corrected for multiple comparisons Changes in Sexual Functioning Questionnaire Change from Baseline to Week 14 Changes in Sexual Functioning Questionnaire short form (CSFQ - 14) was a safety measure in the study • In females, CSFQ - 14 total score improved (indicating better sexual functioning) to a greater extent in the TNX - 102 SL group compared with placebo, p=0.010 • Potential tolerability advantage over pharmacotherapeutics with potent serotonin reuptake inhibition © 2024 Tonix Pharmaceuticals Holding Corp.

28 CNS PORTFOLIO RESILIENT : FIQR Individual Items 1 Affective Symptoms, Sensory Sensitivity, Cognition, and Energy Pre - specified exploratory endpoint Selected Fibromyalgia Impact Questionnaire - Revised Symptoms Domain Item Scores Pre - specified exploratory endpoints Effect Size P - value^ 95% Confidence Interval # Week 14 LS Mean (SE) Difference from Placebo # FIQ - R Item Please rate your level of... (past 7 days) 0.35 <0.001 - 1.2, - 0.6 - 0.8 (0.21) Depression 0.30 0.001 - 1.2, - 0.3 - 0.8 (0.24) Anxiety 0.22 0.020 - 1.0, - 0.1 - 0.6 (0.24) Sensitivity to…* 0.31 0.001 - 1.2, - 0.3 - 0.8 (0.23) Memory problems 0.31 <0.001 - 1.2, - 0.3 - 0.8 (0.23) Energy *…loud noises, bright lights, odors, and cold 1 FIQR=Fibromyalgia Impact Questionnaire - Revised # Mixed model repeated measures analysis (no imputation); fixed categorical effects of treatment, site, study week, and treatment x study week interaction; fixed covariates of baseline value and baseline value x study week interaction ^ Uncorrected for multiple comparisons © 2024 Tonix Pharmaceuticals Holding Corp.
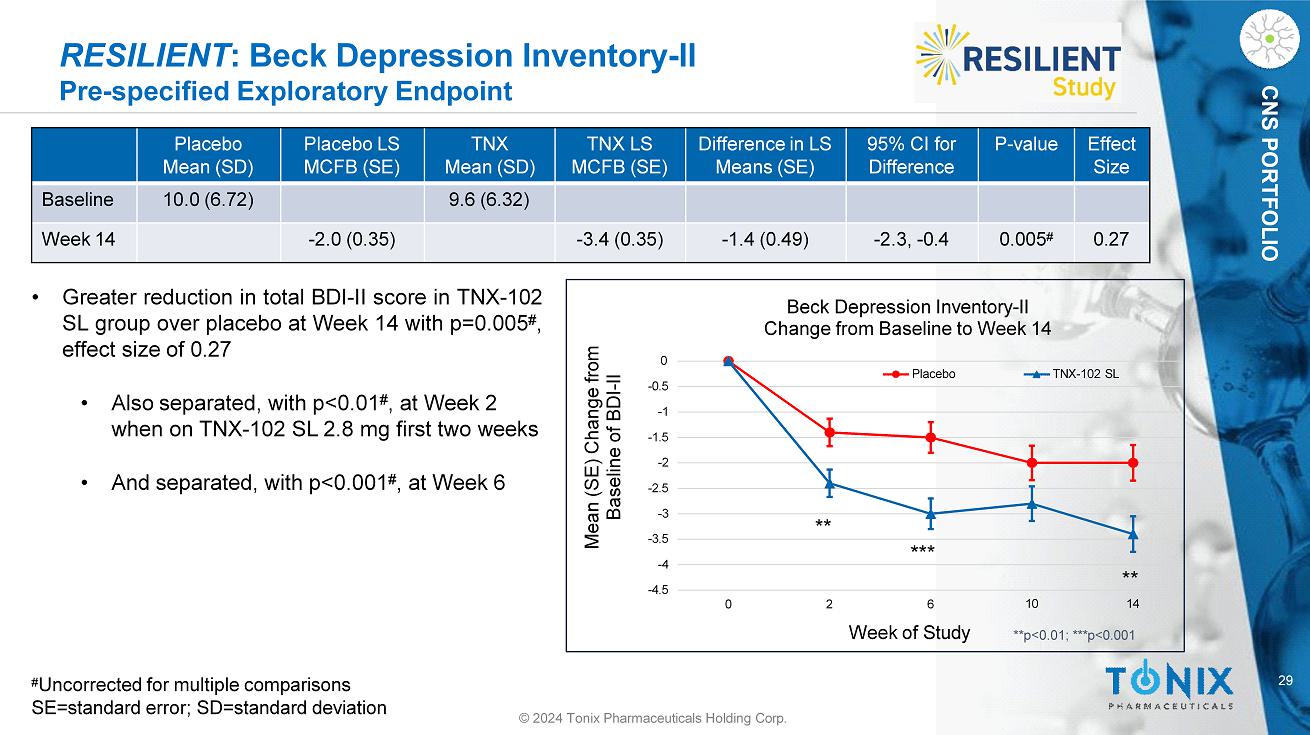
29 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO RESILIENT : Beck Depression Inventory - II Pre - specified Exploratory Endpoint Effect Size P - value 95% CI for Difference Difference in LS Means (SE) TNX LS MCFB (SE) TNX Mean (SD) Placebo LS MCFB (SE) Placebo Mean (SD) 9.6 (6.32) 10.0 (6.72) Baseline 0.27 0.005 # - 2.3, - 0.4 - 1.4 (0.49) - 3.4 (0.35) - 2.0 (0.35) Week 14 - 3.5 - 4 - 4.5 - 2 - 2.5 - 3 - 0.5 - 1 - 1.5 0 0 2 Mean (SE) Change from Baseline of BDI - II 6 Week of Study Beck Depression Inventory - II Change from Baseline to Week 14 Placebo TNX - 102 SL ** *** ** 10 14 **p<0.01; ***p<0.001 • Greater reduction in total BDI - II score in TNX - 102 SL group over placebo at Week 14 with p= 0 . 005 # , effect size of 0 . 27 • Also separated, with p<0.01 # , at Week 2 when on TNX - 102 SL 2.8 mg first two weeks • And separated, with p<0.001 # , at Week 6 # Uncorrected for multiple comparisons SE=standard error; SD=standard deviation

30 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO RESILIENT : Summary of Baseline Depression BDI - II and FIQR Item • While the rate of current MDE diagnosis was ~2% of the ITT, ~25% ITT had experienced a lifetime MDE, and ~47% reported past 6 month depression on FM Dx* • Also, about 25% of the ITT enrolled on concomitant antidepressant or buspirone • By end of treatment (Week 14), there was a greater reduction in depression severity by total BDI - II score in TNX - 102 SL group compared with placebo (p=0.005) ‒ And greater reduction in FIQR items for depression (p<0.001), anxiety (p=0.001), and sensory sensitivity (p=0.020) in the TNX - 102 SL group compared with placebo ‒ The FIQR memory item, a measure of cognitive impairment in FM, was more improved in the TNX - 102 SL group than placebo (p=0.001) ‒ The FIQR energy item, another indicator of less fatigue in FM, was also more improved in the TNX - 102 SL group than placebo (p<0.001) • Cohen’s d effect sizes were between 0.27 and 0.35 for all Week 14 outcomes above except sensory sensitivity *Wolfe F, et al. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin Arthritis Rheum. 2016; 46(3):319 - 329. Abbreviations: BDI - II, Beck Depression Inventory - II; Dx, diagnosis; FIQR, Fibromyalgia Impact Questionnaire - Revised; FM, fibromyalgia; ITT, Intention - to - Treat
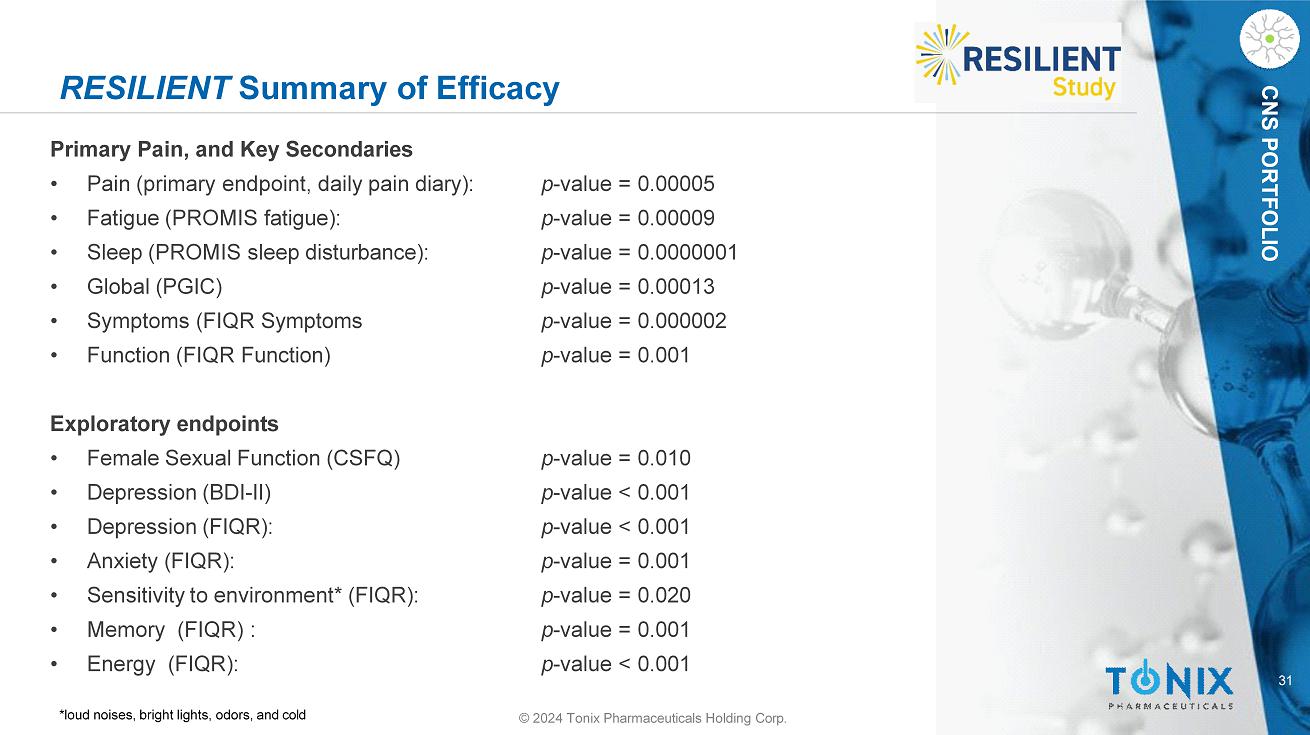
31 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO RESILIENT Summary of Efficacy Primary Pain, and Key Secondaries • Pain (primary endpoint, daily pain diary): • Fatigue (PROMIS fatigue): • Sleep (PROMIS sleep disturbance): • Global (PGIC) • Symptoms (FIQR Symptoms • Function (FIQR Function) p - value = 0.00005 p - value = 0.00009 p - value = 0.0000001 p - value = 0.00013 p - value = 0.000002 p - value = 0.001 Exploratory endpoints • Female Sexual Function (CSFQ) • Depression (BDI - II) • Depression (FIQR): • Anxiety (FIQR): • Sensitivity to environment* (FIQR): • Memory (FIQR) : • Energy (FIQR): p - value = 0.010 p - value < 0.001 p - value < 0.001 p - value = 0.001 p - value = 0.020 p - value = 0.001 p - value < 0.001 *loud noises, bright lights, odors, and cold
CNS PORTFOLIO RESILIENT Summary of Efficacy 32 © 2024 Tonix Pharmaceuticals Holding Corp. Conclusion: TNX - 102 SL has “broad spectrum” or “syndromal activity” • Broad spectrum: across several symptoms • Syndromal: improves the syndrome (most of the symptoms) • Potential for a broad - spectrum drug to reduce the use of multiple drugs or “polypharmac y”

RESILIENT Study Safety Findings 33 © 2024 Tonix Pharmaceuticals Holding Corp.
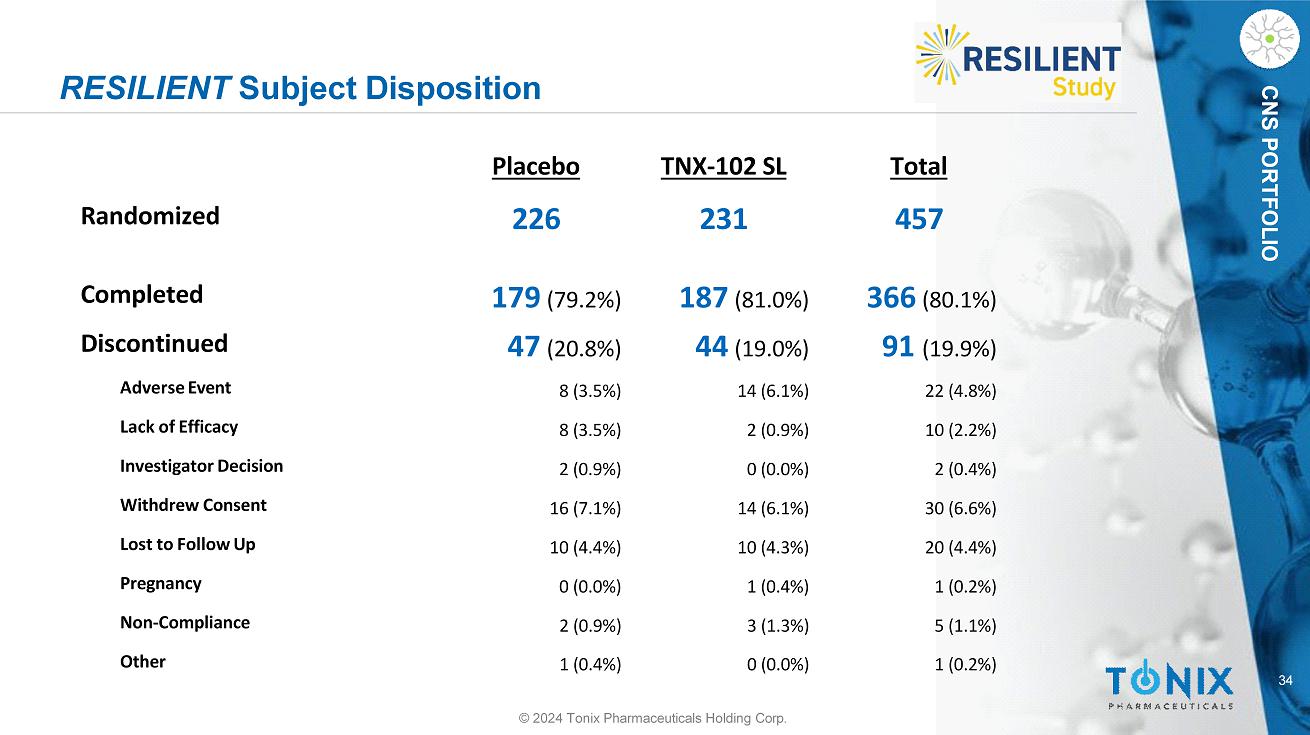
CNS PORTFOLIO RESILIENT Subject Disposition 34 © 2024 Tonix Pharmaceuticals Holding Corp. Total TNX - 102 SL Placebo 457 231 226 Randomized 366 (80.1%) 187 (81.0%) 179 (79.2%) Completed 91 (19.9%) 44 (19.0%) 47 (20.8%) Discontinued 22 (4.8%) 14 (6.1%) 8 (3.5%) Adverse Event 10 (2.2%) 2 (0.9%) 8 (3.5%) Lack of Efficacy 2 (0.4%) 0 (0.0%) 2 (0.9%) Investigator Decision 30 (6.6%) 14 (6.1%) 16 (7.1%) Withdrew Consent 20 (4.4%) 10 (4.3%) 10 (4.4%) Lost to Follow Up 1 (0.2%) 1 (0.4%) 0 (0.0%) Pregnancy 5 (1.1%) 3 (1.3%) 2 (0.9%) Non - Compliance 1 (0.2%) 0 (0.0%) 1 (0.4%) Other
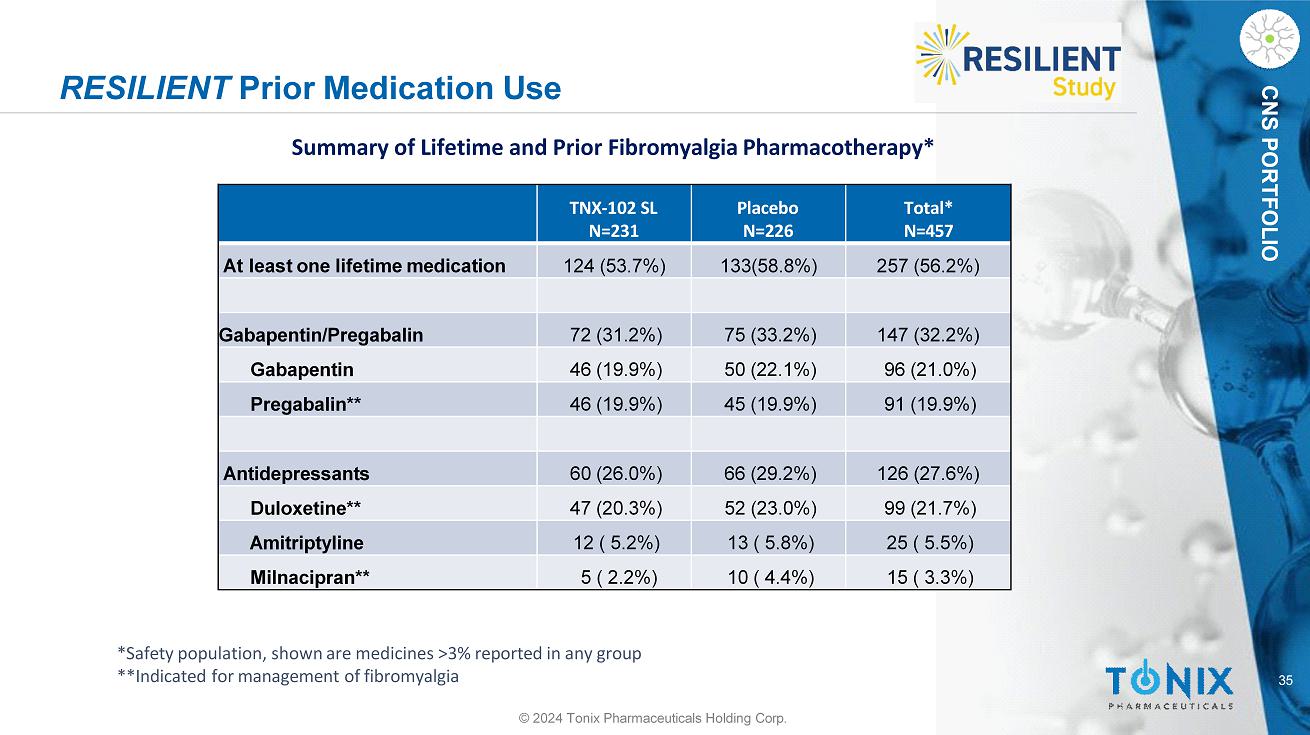
CNS PORTFOLIO RESILIENT Prior Medication Use **Indicated for management of fibromyalgia 35 © 2024 Tonix Pharmaceuticals Holding Corp. Total* N=457 Placebo N=226 TNX - 102 SL N=231 257 (56.2%) 133(58.8%) 124 (53.7%) At least one lifetime medication 147 (32.2%) 75 (33.2%) 72 (31.2%) Gabapentin/Pregabalin 96 (21.0%) 50 (22.1%) 46 (19.9%) Gabapentin 91 (19.9%) 45 (19.9%) 46 (19.9%) Pregabalin** 126 (27.6%) 66 (29.2%) 60 (26.0%) Antidepressants 99 (21.7%) 52 (23.0%) 47 (20.3%) Duloxetine** 25 ( 5.5%) 13 ( 5.8%) 12 ( 5.2%) Amitriptyline 15 ( 3.3%) 10 ( 4.4%) 5 ( 2.2%) Milnacipran** Summary of Lifetime and Prior Fibromyalgia Pharmacotherapy* *Safety population, shown are medicines >3% reported in any group
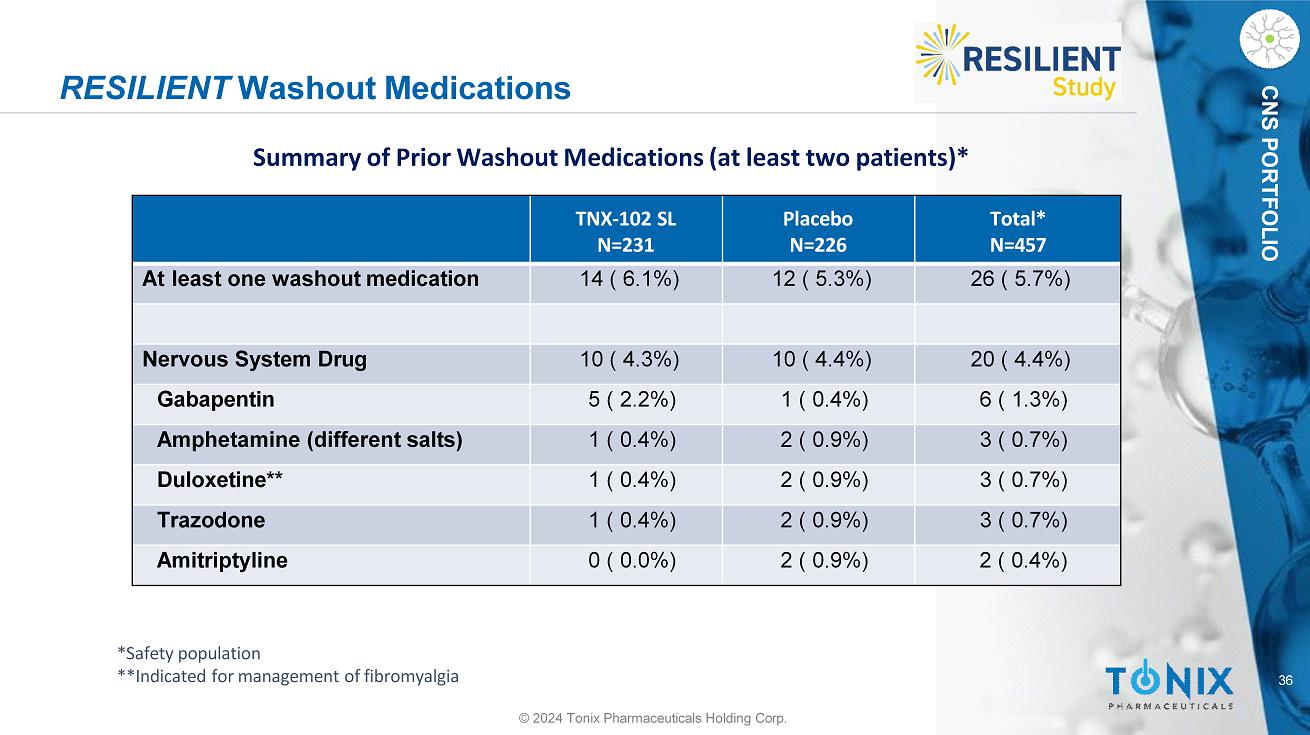
CNS PORTFOLIO RESILIENT Washout Medications **Indicated for management of fibromyalgia 36 © 2024 Tonix Pharmaceuticals Holding Corp. Total* N=457 Placebo N=226 TNX - 102 SL N=231 26 ( 5.7%) 12 ( 5.3%) 14 ( 6.1%) At least one washout medication 20 ( 4.4%) 10 ( 4.4%) 10 ( 4.3%) Nervous System Drug 6 ( 1.3%) 1 ( 0.4%) 5 ( 2.2%) Gabapentin 3 ( 0.7%) 2 ( 0.9%) 1 ( 0.4%) Amphetamine (different salts) 3 ( 0.7%) 2 ( 0.9%) 1 ( 0.4%) Duloxetine** 3 ( 0.7%) 2 ( 0.9%) 1 ( 0.4%) Trazodone 2 ( 0.4%) 2 ( 0.9%) 0 ( 0.0%) Amitriptyline Summary of Prior Washout Medications (at least two patients)* *Safety population
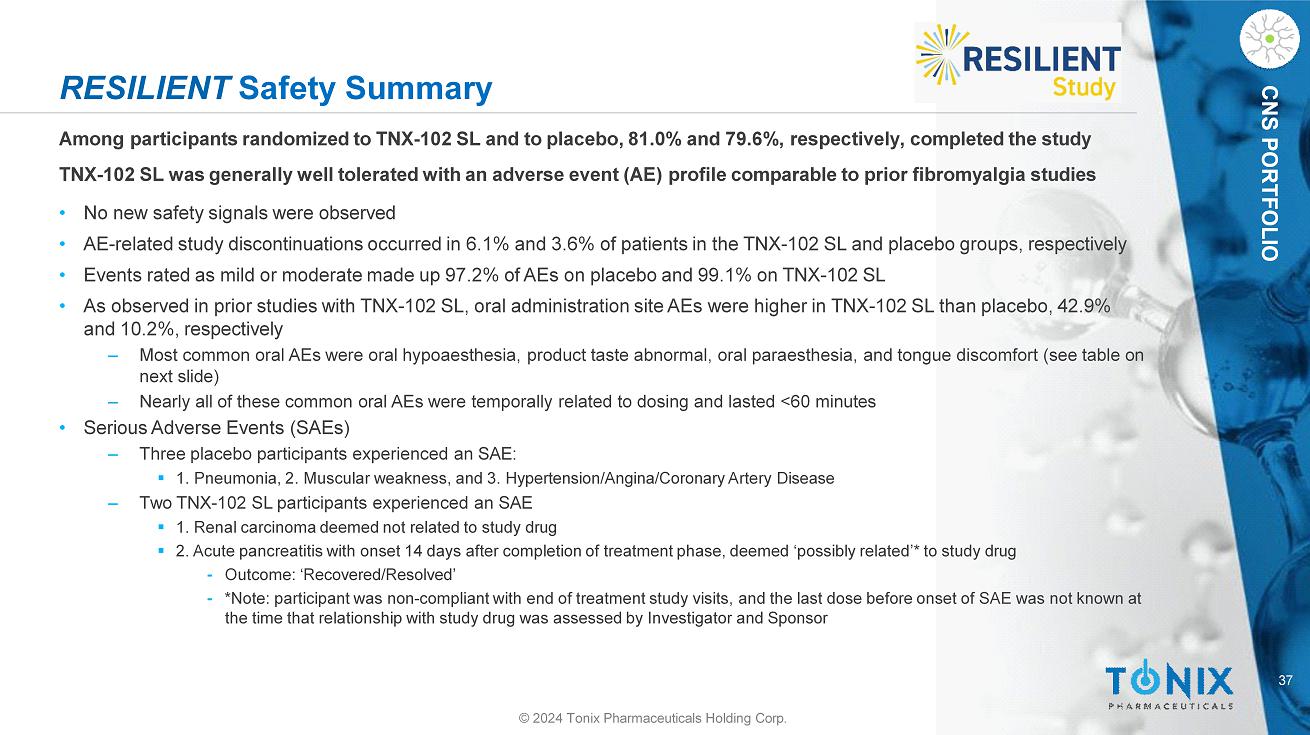
CNS PORTFOLIO RESILIENT Safety Summary Among participants randomized to TNX - 102 SL and to placebo, 81.0% and 79.6%, respectively, completed the study TNX - 102 SL was generally well tolerated with an adverse event (AE) profile comparable to prior fibromyalgia studies • No new safety signals were observed • AE - related study discontinuations occurred in 6.1% and 3.6% of patients in the TNX - 102 SL and placebo groups, respectively • Events rated as mild or moderate made up 97.2% of AEs on placebo and 99.1% on TNX - 102 SL • As observed in prior studies with TNX - 102 SL, oral administration site AEs were higher in TNX - 102 SL than placebo, 42.9% and 10.2%, respectively ‒ Most common oral AEs were oral hypoaesthesia, product taste abnormal, oral paraesthesia, and tongue discomfort (see table on next slide) ‒ Nearly all of these common oral AEs were temporally related to dosing and lasted <60 minutes • Serious Adverse Events (SAEs) ‒ Three placebo participants experienced an SAE: ▪ 1. Pneumonia, 2. Muscular weakness, and 3. Hypertension/Angina/Coronary Artery Disease ‒ Two TNX - 102 SL participants experienced an SAE ▪ 1. Renal carcinoma deemed not related to study drug ▪ 2. Acute pancreatitis with onset 14 days after completion of treatment phase, deemed ‘possibly related’* to study drug ⁃ Outcome: ‘Recovered/Resolved’ ⁃ *Note: participant was non - compliant with end of treatment study visits, and the last dose before onset of SAE was not known at the time that relationship with study drug was assessed by Investigator and Sponsor 37 © 2024 Tonix Pharmaceuticals Holding Corp.

38 CNS PORTFOLIO RESILIENT Safety © 2024 Tonix Pharmaceuticals Holding Corp. Total* N=457 Placebo N=226 TNX - 102 SL N=231 System Organ Class Preferred Term Systemic Adverse Events 17 (3.7%) 7 (3.1%) 10 (4.3%) COVID - 19 10 (2.2%) 3 (1.3%) 7 (3.0%) Somnolence 11 (2.4%) 4 (1.8%) 7 (3.0%) Headache Oral Cavity Adverse Events 56 (12.3%) 1 (0.4%) 55 (23.8%) Hypoaesthesia oral 29 (6.3%) 2 (0.9%) 27 (11.7%) Product taste abnormal 18 (3.9%) 2 (0.9%) 16 (6.9%) Paraesthesia oral 16 (3.5%) 0 (0.0%) 16 (6.9%) Tongue discomfort Treatment - Emergent Adverse Events (TEAEs) at Rate of ≥ 3% in Either Treatment Group
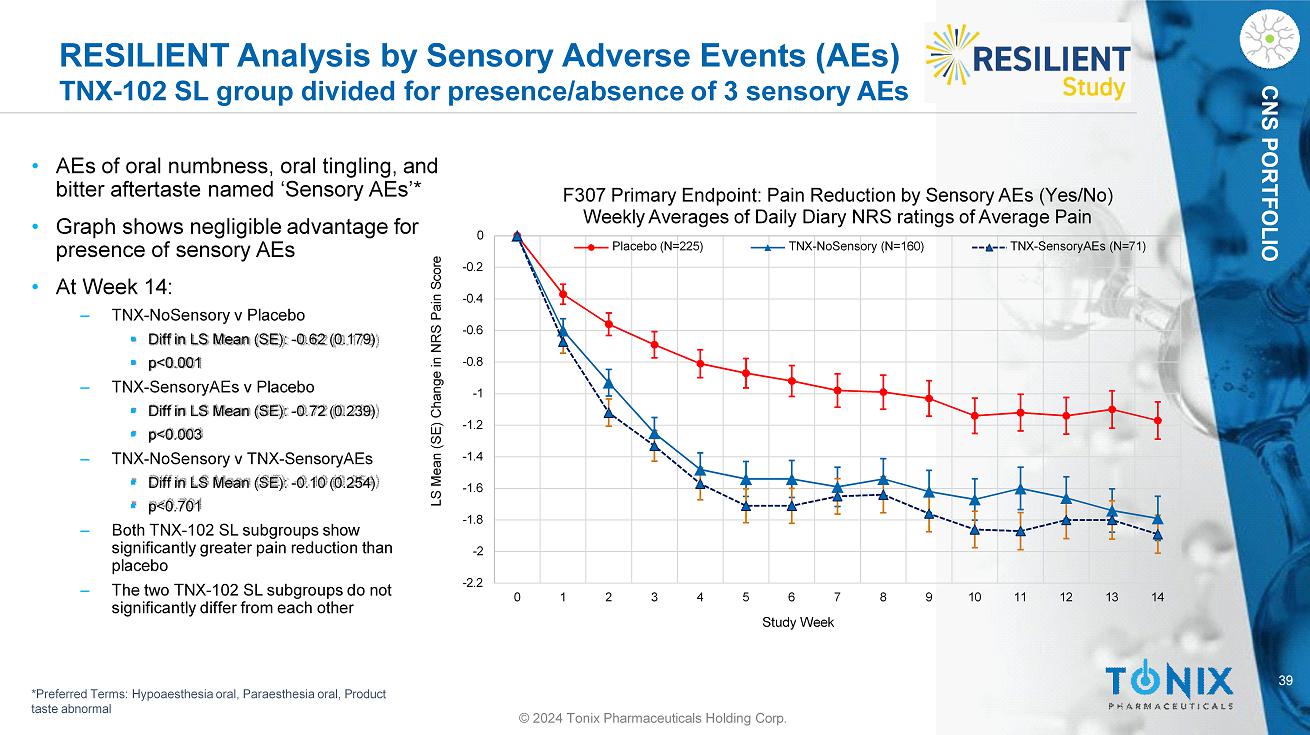
39 CNS PORTFOLIO RESILIENT Analysis by Sensory Adverse Events (AEs) TNX - 102 SL group divided for presence/absence of 3 sensory AEs • AEs of oral numbness, oral tingling, and bitter aftertaste named ‘Sensory AEs’* • Graph shows negligible advantage for presence of sensory AEs • At Week 14: ‒ TNX - NoSensory v Placebo ▪ Diff in LS Mean (SE): - 0.62 (0.179) ▪ p<0.001 ‒ TNX - SensoryAEs v Placebo ▪ Diff in LS Mean (SE): - 0.72 (0.239) ▪ p<0.003 ‒ TNX - NoSensory v TNX - SensoryAEs ▪ Diff in LS Mean (SE): - 0.10 (0.254) ▪ p<0.701 ‒ Both TNX - 102 SL subgroups show significantly greater pain reduction than placebo ‒ The two TNX - 102 SL subgroups do not significantly differ from each other - 2.2 - 2 - 1.8 - 1.6 - 1.4 - 1.2 - 1 - 0.8 - 0.6 - 0.4 - 0.2 0 0 1 2 3 4 5 8 9 10 11 12 13 14 LS Mean (SE) Change in NRS Pain Score 6 7 Study Week F307 Primary Endpoint: Pain Reduction by Sensory AEs (Yes/No) Weekly Averages of Daily Diary NRS ratings of Average Pain Placebo (N=225) TNX - NoSensory (N=160) TNX - SensoryAEs (N=71) *Preferred Terms: Hypoaesthesia oral, Paraesthesia oral, Product taste abnormal © 2024 Tonix Pharmaceuticals Holding Corp.
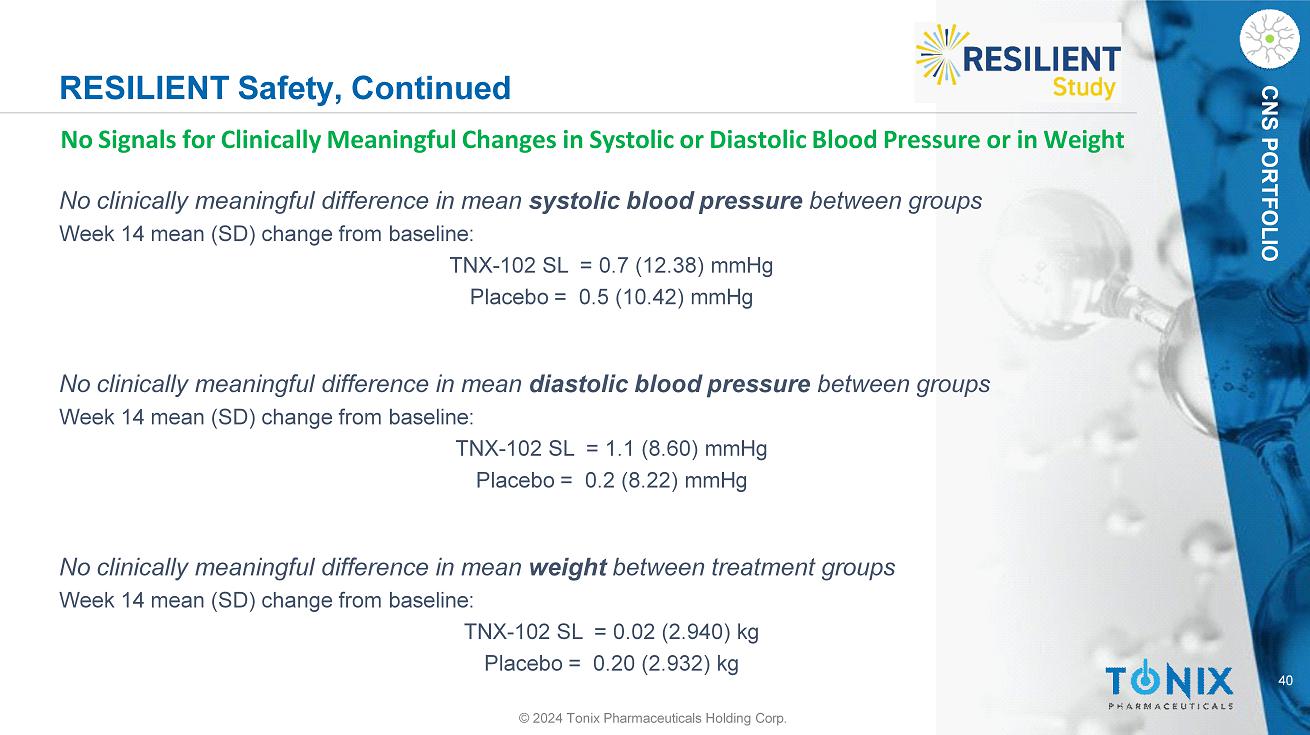
40 CNS PORTFOLIO RESILIENT Safety, Continued © 2024 Tonix Pharmaceuticals Holding Corp. No Signals for Clinically Meaningful Changes in Systolic or Diastolic Blood Pressure or in Weight No clinically meaningful difference in mean systolic blood pressure between groups Week 14 mean (SD) change from baseline: TNX - 102 SL = 0.7 (12.38) mmHg Placebo = 0.5 (10.42) mmHg No clinically meaningful difference in mean diastolic blood pressure between groups Week 14 mean (SD) change from baseline: TNX - 102 SL = 1.1 (8.60) mmHg Placebo = 0.2 (8.22) mmHg No clinically meaningful difference in mean weight between treatment groups Week 14 mean (SD) change from baseline: TNX - 102 SL = 0.02 (2.940) kg Placebo = 0.20 (2.932) kg

CNS PORTFOLIO RESILIENT Systolic blood pressure Safety Measure and vertical lines begin and end at the 5 th and 95 th percentiles. 41 © 2024 Tonix Pharmaceuticals Holding Corp. No clinically meaningful difference in mean systolic blood pressure between groups Week 14 mean (SD) change from baseline: TNX - 102 SL = 0.7 (12.38) mmHg Placebo = 0.5 (10.42) mmHg Horizontal lines are the mean for each group; boxes are the 25 th and 75 th percentiles;
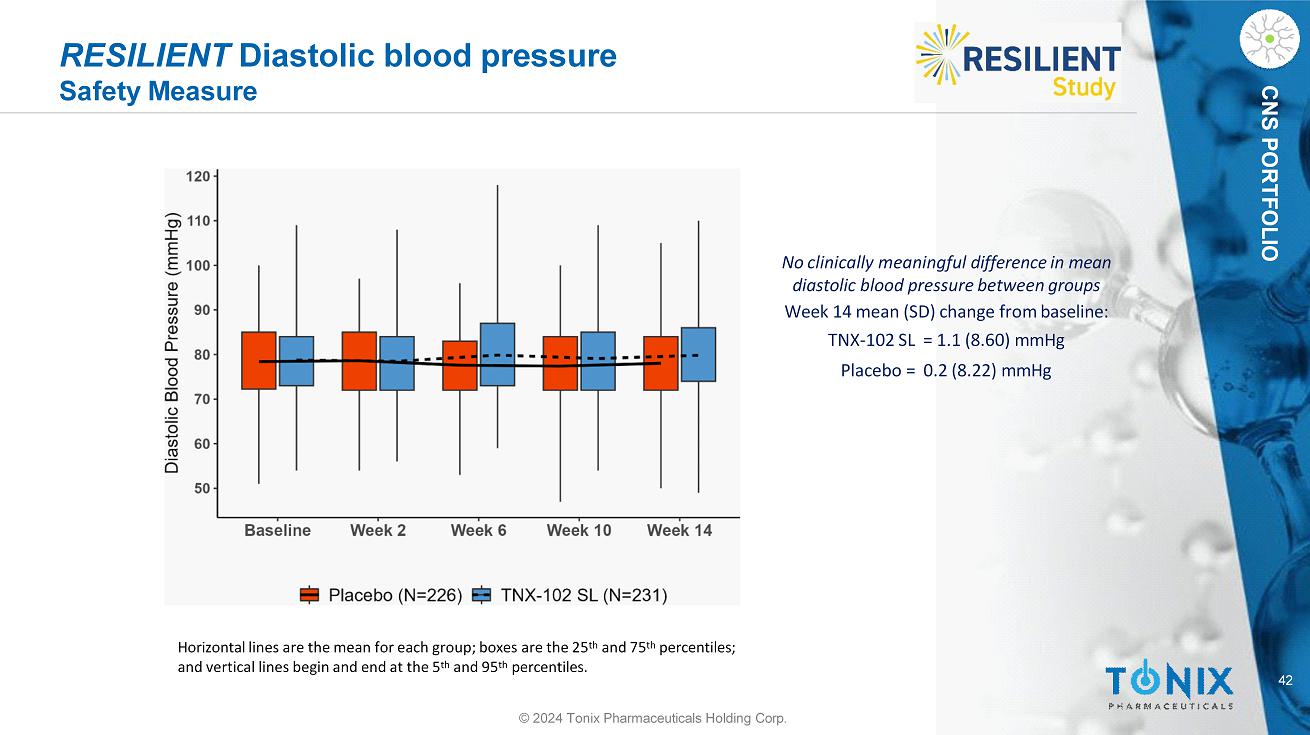
CNS PORTFOLIO RESILIENT Diastolic blood pressure Safety Measure 42 © 2024 Tonix Pharmaceuticals Holding Corp. No clinically meaningful difference in mean diastolic blood pressure between groups Week 14 mean (SD) change from baseline: TNX - 102 SL = 1.1 (8.60) mmHg Placebo = 0.2 (8.22) mmHg Horizontal lines are the mean for each group; boxes are the 25 th and 75 th percentiles; and vertical lines begin and end at the 5 th and 95 th percentiles.
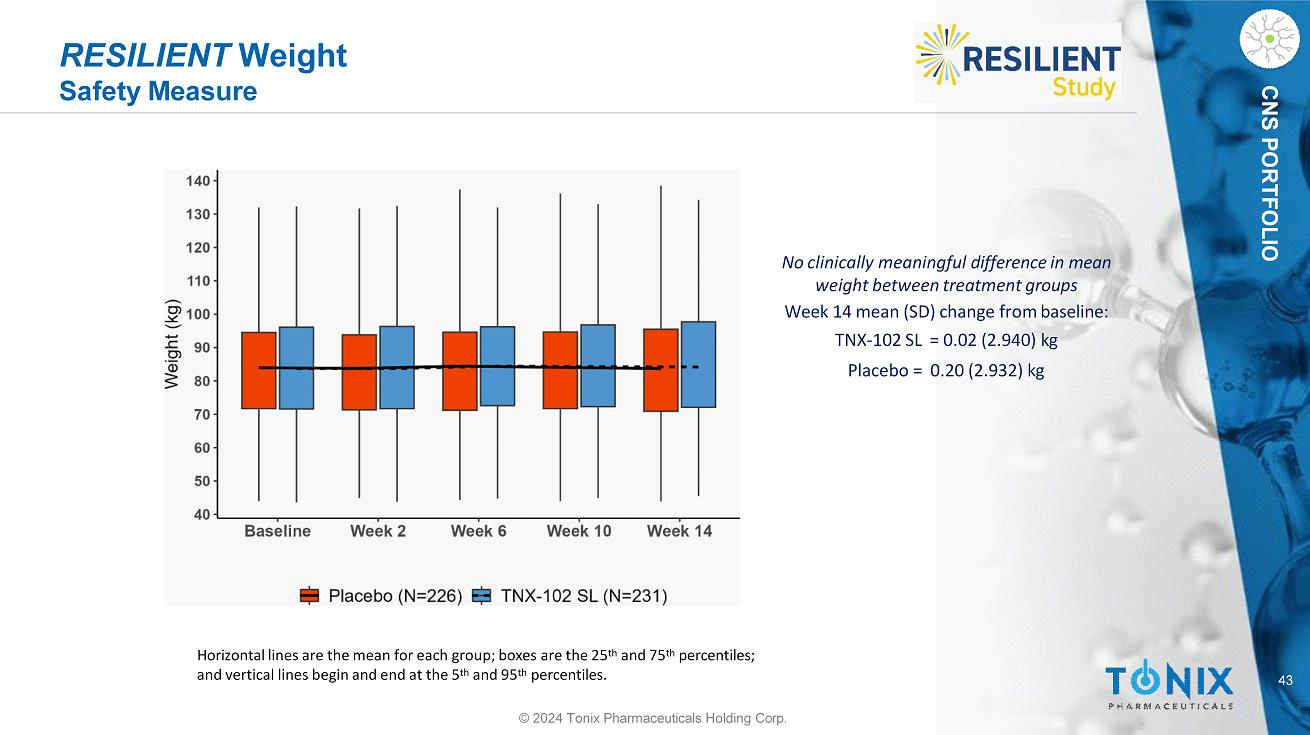
CNS PORTFOLIO RESILIENT Weight Safety Measure and vertical lines begin and end at the 5 th and 95 th percentiles. 43 © 2024 Tonix Pharmaceuticals Holding Corp. No clinically meaningful difference in mean weight between treatment groups Week 14 mean (SD) change from baseline: TNX - 102 SL = 0.02 (2.940) kg Placebo = 0.20 (2.932) kg Horizontal lines are the mean for each group; boxes are the 25 th and 75 th percentiles;

Fibromyalgia Market Characteristics 44 © 2024 Tonix Pharmaceuticals Holding Corp.

45 CNS PORTFOLIO Fibromyalgia: Market Characteristics © 2024 Tonix Pharmaceuticals Holding Corp. Prevalence • One of the more common chronic pain disorders (2 - 4% of US Population) 1 Diagnosed population • Large population but underdiagnosed 2 relative to prevalence rate • Majority receive drug treatment 3 Treatment Pattern • Polypharmacy the norm - average 2.6 drugs/patient 3 • Rotation through therapy common: average ~5 drugs/year 3 • Estimated that >22 million prescriptions are issued for the treatment of fibromyalgia (on - and off - label usage) each year 4,5 Unmet Need • Majority of patients do not respond or cannot tolerate therapy 6 1 American College of Rheumatology ( www.ACRPatientInfo.org accessed May 7, 2019) – prevalence rate of 2 - 4% for U.S. adult population (~250 million) 2 Vincent et al., 2013; diagnosed prevalence rate was 1.1% of adult population or 50% of the prevalent population 3 Robinson, et al., 2012; 85% received drug treatment 4 Vincent et al, Arthritis Care Res 2013;65:786 5 Product sales derived from IMS MIDAS; IMS NDTI used to factor usage for fibromyalgia;data accessedApril 2015. 6 Market research by Frost & Sullivan, commissioned by Tonix, 2011
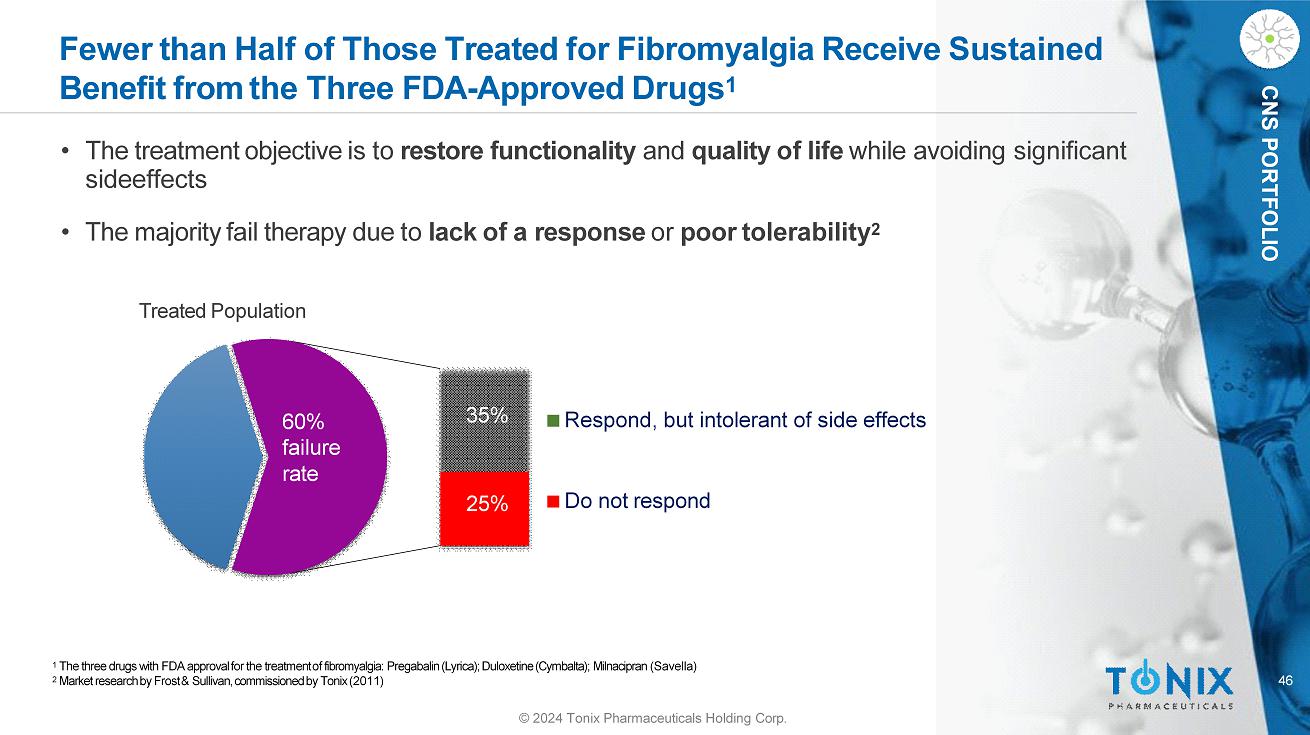
46 CNS PORTFOLIO Fewer than Half of Those Treated for Fibromyalgia Receive Sustained Benefit from the Three FDA - Approved Drugs 1 Respond, but intolerant of side effects Do not respond © 2024 Tonix Pharmaceuticals Holding Corp. 25% 35% 60% failure rate 1 The three drugs with FDA approval for the treatment of fibromyalgia: Pregabalin (Lyrica); Duloxetine (Cymbalta); Milnacipran (Savella) 2 Market research by Frost & Sullivan, commissioned by Tonix (2011) • The treatment objective is to restore functionality and quality of life while avoiding significant sideeffects • The majority fail therapy due to lack of a response or poor tolerability 2 Treated Population

47 CNS PORTFOLIO Current FDA - Approved Fibromyalgia Drugs 1 © 2024 Tonix Pharmaceuticals Holding Corp. 1 The three drugs with FDA approval for the management of fibromyalgia are Pregabalin (Lyrica); Duloxetine (Cymbalta); Milnacipran (Savella) 2 Leung MTY, et al. JAMA Netw Open. 2024;7(11):e2444488. doi: 10.1001/jamanetworkopen.2024.44488. PMID: 39535796; PMCID: PMC11561685. Cymbalta® (duloxetine) - Lilly Savella® (milnacipran) - AbbVie Lyrica® (pregabalin) - Pfizer Drug SNRI Gabapentinoid Class YES YES Pain Reduction Fibromyalgia Activity - YES Sleep Improvement YES - Fatigue Reduction - YES Fatigue increase Tolerability Issues YES - Sleep problems - YES Weight gain YES - Blood Pressure increase YES - Sexual impairment YES - GI issues - YES Hip Fractures 2 - YES DEA Scheduled Improvement in fibromyalgia pain was primary endpoint for approval • No current product addresses pain, poor sleep and fatigue • Tolerability issues limit long term use for many patients
48 CNS PORTFOLIO Large Need for New Fibromyalgia Therapies that Provide Symptom Improvement with Better Tolerability 4 Berger A, Dukes E, Martin S, Edelsberg J, Oster G, Int J ClinPract, 2007; 61(9):1498 – 1508. © 2024 Tonix Pharmaceuticals Holding Corp. • Currently - approved medications may have side effects that limit long - term use 1 • Many patients skip doses or discontinue altogether within months of treatment initiation • Medication - related side effects may be similar to fibromyalgia symptoms • High rates of discontinuation, switching andaugmentation • Attempt to treat multiple symptoms and/or avoid intolerable side effects • Average of 2 - 3 medications usedsimultaneously 2 • The typical patient has tried six differentmedications 3 • Substantial off - label use of narcotic painkillers and prescription sleep aids 3 • Among those diagnosed, more than one - third have used prescription opioids as a means of treatment 4 • TNX - 102 SL is a non - opioid, centrally - acting analgesic that could provide a new therapeutic option for fibromyalgia patients 1 Nuesch et al, Ann Rheum Dis 2013;72:955 - 62. 2 Robinson RL et al, Pain Medicine 2012;13:1366. 3 Patient Trends: Fibromyalgia”, Decision Resources,2011.

49 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 102 SL Showed Activity on Pain, Sleep and Fatigue and was Generally Well Tolerated in the RESILIENT Study 1 1 Flexeril® and Amrix® are oral formulations of cyclobenzaprine indicated for short term (2 - 3 weeks) treatment of muscle spasm 2 Four receptors are: serotonergic - 5 - HT2A, adrenergic - α1, histaminergic - H1, and muscarinic - M1 cholinergic receptors 3 TNX - 102 SL was generally well tolerated with an adverse event profile comparable to prior studies and no new safety signals were observed. In both pivotal studies, the most common treatment - emergent adverse event was tongue or mouth numbness at the administration site, which was temporally related to dosing, self - limited, never rated as severe, and rarely led to study discontinuation (one participant in each study). TNX - 102 SL Drug Muscle spasm 1 Initial Indication of active ingredient Tricyclic Class Antagonist at 4 post - synaptic receptors 2 Mechanism + Pain Fibromyalgia Activity + Sleep + Fatigue - Sleep Tolerability Issues - Fatigue + Oral administration site reaction 3
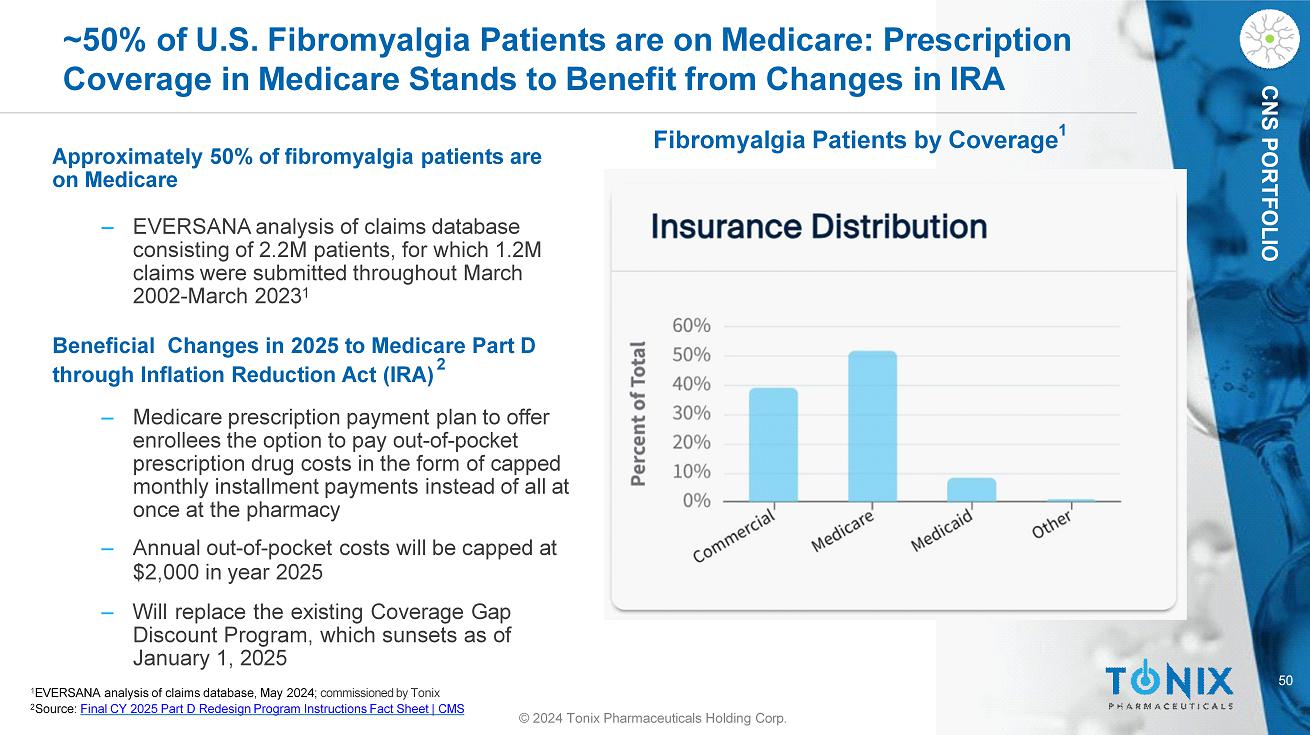
50 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO ~50% of U.S. Fibromyalgia Patients are on Medicare: Prescription Coverage in Medicare Stands to Benefit from Changes in IRA Approximately 50% of fibromyalgia patients are on Medicare ‒ EVERSANA analysis of claims database consisting of 2.2M patients, for which 1.2M claims were submitted throughout March 2002 - March 2023 1 Beneficial Changes in 2025 to Medicare Part D through Inflation Reduction Act (IRA) 2 ‒ Medicare prescription payment plan to offer enrollees the option to pay out - of - pocket prescription drug costs in the form of capped monthly installment payments instead of all at once at the pharmacy ‒ Annual out - of - pocket costs will be capped at $2,000 in year 2025 ‒ Will replace the existing Coverage Gap Discount Program, which sunsets as of January 1 , 2025 Fibromyalgia Patients by Coverage 1 1 EVERSANA analysis of claims database, May 2024; commissioned by Tonix 2 Source: Final CY 2025 Part D Redesign Program Instructions Fact Sheet | CMS
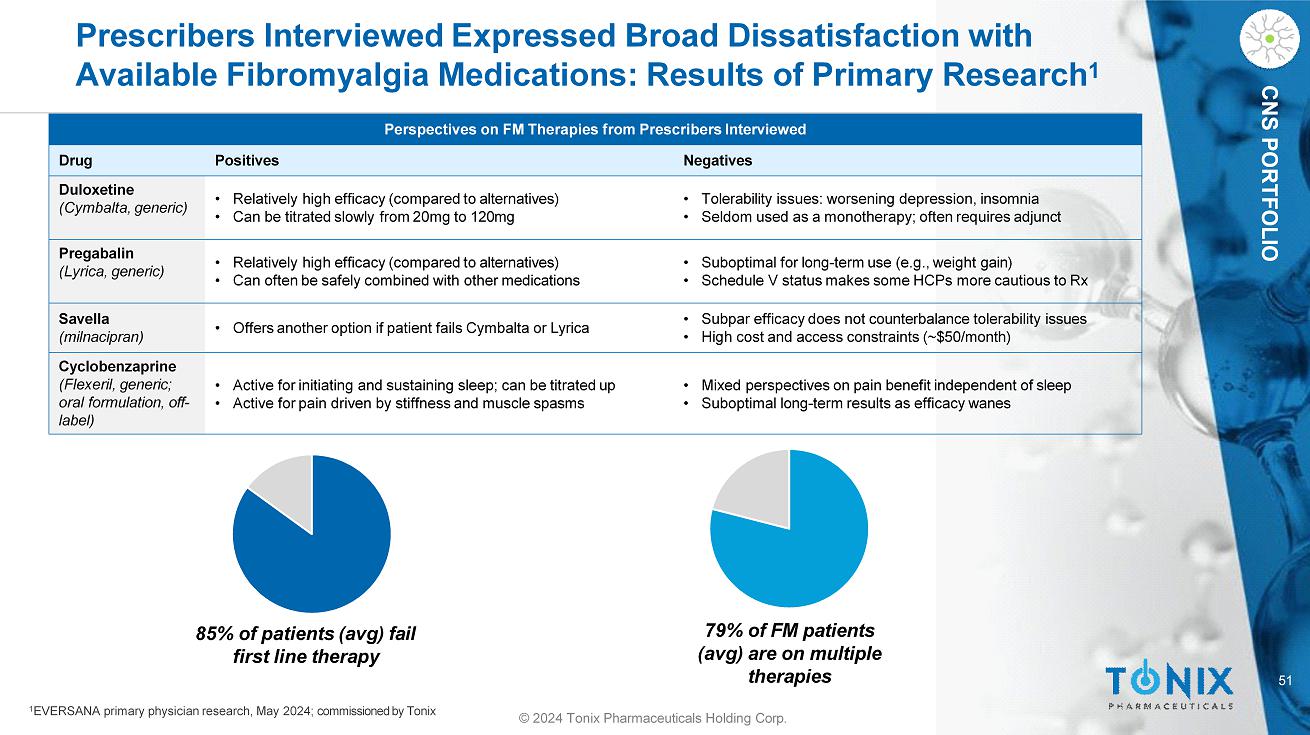
51 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Prescribers Interviewed Expressed Broad Dissatisfaction with Available Fibromyalgia Medications: Results of Primary Research 1 Perspectives on FM Therapies from Prescribers Interviewed Negatives Positives Drug • Tolerability issues: worsening depression, insomnia • Seldom used as a monotherapy; often requires adjunct • Relatively high efficacy (compared to alternatives) • Can be titrated slowly from 20mg to 120mg Duloxetine (Cymbalta, generic) • Suboptimal for long - term use (e.g., weight gain) • Schedule V status makes some HCPs more cautious to Rx • Relatively high efficacy (compared to alternatives) • Can often be safely combined with other medications Pregabalin (Lyrica, generic) • Subpar efficacy does not counterbalance tolerability issues • High cost and access constraints (~$50/month) • Offers another option if patient fails Cymbalta or Lyrica Savella (milnacipran) • Mixed perspectives on pain benefit independent of sleep • Suboptimal long - term results as efficacy wanes • Active for initiating and sustaining sleep; can be titrated up • Active for pain driven by stiffness and muscle spasms Cyclobenzaprine (Flexeril, generic; oral formulation, off - label) 85% of patients (avg) fail first line therapy 79% of FM patients (avg) are on multiple therapies 1 EVERSANA primary physician research, May 2024; commissioned by Tonix
52 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Opportunity for a New, Unique Fibromyalgia Treatment Option: Results of Primary Research from EVERSANA 1,2 • Prescribers indicate a very high unmet need in FM (ranked ≥4.0 on a 5 - point scale) • Prescribers report there is no standard of care in FM , employ an individualized approach based on symptomology • No new treatments approved since 2009 • Prescribers report minimal promotional activities by any pharmaceutical company • Highly concentrated prescriber base with 50% of patients treated by ~16k physicians FM Landscape • Physicians reacted positively to the efficacy and safety profile of TNX - 102 SL (based on Phase 3 Study results) • Median interest = 4.0 on a 5 - point scale • Driving attributes included strong efficacy, safety and tolerability • Unique & differentiating efficacy features included improvements in sleep and fatigue Physician Primary Market Research • Physicians indicated intended use in 40% of their FM patients • Majority of respondents indicated TNX - 102 SL would be their first choice, if accessible • Physicians surveyed indicated they are well - equipped to deal with access restrictions including prior authorizations and step - edits Anticipated Use 1 EVERSANA primary physician research, May 2024; commissioned by Tonix 2 EVERSANA analysis of claims database, May 2024; commissioned by Tonix

53 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Many Fibromyalgia Patients are Prescribed Off - Label Drugs; ~53% FM Patients Prescribed Opioids Off - Label 1,2 1.04% 6.03% 11.74% 12.96% 13.23% 17.58% 19.45% 20.47% 25.50% 0% 5% 10% 25% 20% 15% 30% 50% 45% 40% 35% Milnacipran Amitriptyline Meloxicam Pregabalin Tramadol Cyclobenzaprine Oxycodone Hydrocodone Duloxetine HCl Gabapentin % FMPatients (after index 3 date) 1 2022 - 2023 2 EVERSANA analysis of claims database, May 2024; commissioned by Tonix 3 Index date refers to date when ICD10 code was entered into database Anti - convulsant SNRI Opiate Tricyclic antidepressant NSAID Muscle relaxant FDA Approved Off - Label Off - Label FDA Approved Off - Label Opioid Off - Label Off - Label Opioid Off - Label Opioid FDA Approved 22.62% Off - Label

54 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Potential for Tonix to Launch and Market TNX - 102 SL Decline in personal promotion (“Detailing”) of prescription drugs • The pandemic accelerated transition to non - personal promotion o Omnichannel is more important and more sophisticated • Tele - sales • Digital • Direct mail • Growth in need to support patients with payers to seek reimbursement Fibromyalgia experts are a subset of Rheumatologists • New prescriptions for fibromyalgia drugs originate in a subset of doctors o Refills may be written by general practitioners Channels for distribution of prescription drugs are evolving • Growth of specialty pharmacies who distribute products by mail

55 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Planning for TNX - 102 SL Launch and Marketing Several companies that successfully developed CNS drugs have launched them • Big Pharma wants the commercial launch de - risked before acquisition ( e.g. , Nurtec®) To prepare for the launch of TNX - 102 SL, Tonix acquired two marketed Rx drugs: Zembrace® and Tosymra® Exit FDA Approval Indication Product Mkt Cap 1 Company 8/2022 Depression Auvelity® $3.5 B AXSM Axsome Sold to PFE for $12 B in Oct 2022 2/2020 Migraine Nurtec® $3.0 B BHVN Biohaven 12/2019 Schizophrenia Caplyta® $7.2 B ITCI IntraCellular 1/2019 Seizures Oxtella - XR® $1.4 B SUPN Supernus 4/2017 Tardive Dyskinesia Ingrezza® $13.6 B NBIX Neurocrine 4/2016 Parkinson’s psychosis Nuplazid® $2.5 B ACAD Acadia 1 Accessed June 7, 2024

56 © 2024 Tonix Pharmaceuticals Holding Corp. Milestones: Recently Completed and Upcoming TNX - 102 SL for the Management of Fibromyalgia Milestones x □ 4 th Quarter 2023 □ x 2 nd Quarter 2024 Statistically significant topline results of Phase 3 RESILIENT study – 2 nd statistically significant Phase 2 trial Type B CMC and clinical pre - NDA meetings with FDA FDA Fast Track Designation granted by FDA x □ 3 rd Quarter 2024 Submitted NDA to FDA for TNX - 102 SL for fibromyalgia in October 2024 □ x October 2024 FDA decision expected on NDA acceptance for review □ December 2024 FDA decision expected on NDA approval (“PDUFA*” Date) for standard review □ August 2025 *PDUFA = Prescription Drug User Fee Act

57 © 2024 Tonix Pharmaceuticals Holding Corp. About Cyclobenzaprine and TNX - 102 SL

58 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Cyclobenzaprine Long - Term Utilization • Flexeril® approved in 1977 by Merck for the treatment of muscle spasm • 10 mg T.I.D. for acute use (2 - 3 weeks) • Original NDA included “ 8 long term safety studies in which patients with various neurologic disorders received cyclobenzaprine up to 80 mg per day for 1 month up to 3 years .” 1 • 6 published studies in fibromyalgia 2 - 8 • N=246, placebo controlled, 4 - 24 week treatment period • Generally well tolerated, no new or unexpected AEs • Extensive safety record in humans for over 30 years • Widely used in the U.S., ~20 million prescriptions and ~ 1 billion tablets dispensed per year 9 • Chronic cyclobenzaprine use is common ( ~12% of users) 9 • Post - marketing surveillance program 1 • 7,607 patients included 297 patients treated with 10 mgs for > 30 days • Incidence of most common AEs was much lower than in controlled studies 1 1999 Merck OTC AdCom Briefing Package 2 Bennett RM, et al. Arthritis Rheum 1988. 31:1535 – 42. 3 Quimby LG, et al . J Rheumatol Suppl, 1989 Nov ; 19 : 140 – 3 . 4 Reynolds WJ, et al. J Rheumatol. 1991 . 18 : 452 – 4 . 5 Santandrea S, et al . J Int Med Res. 1993 . 21 : 74 – 80 . 6 Cantini F, et al . Minerva Med. 1994 . 85 : 97 – 100 . 7 Carette S, et al. Arthritis Rheum. 1994 . 37 : 32 – 40 . 8 Tofferi JK, et al . Arthritis Rheum. 2004 . 51 : 9 – 13 . 1 9 IMS report 2011 of cyclobenzaprine use in 2009 – Data on File
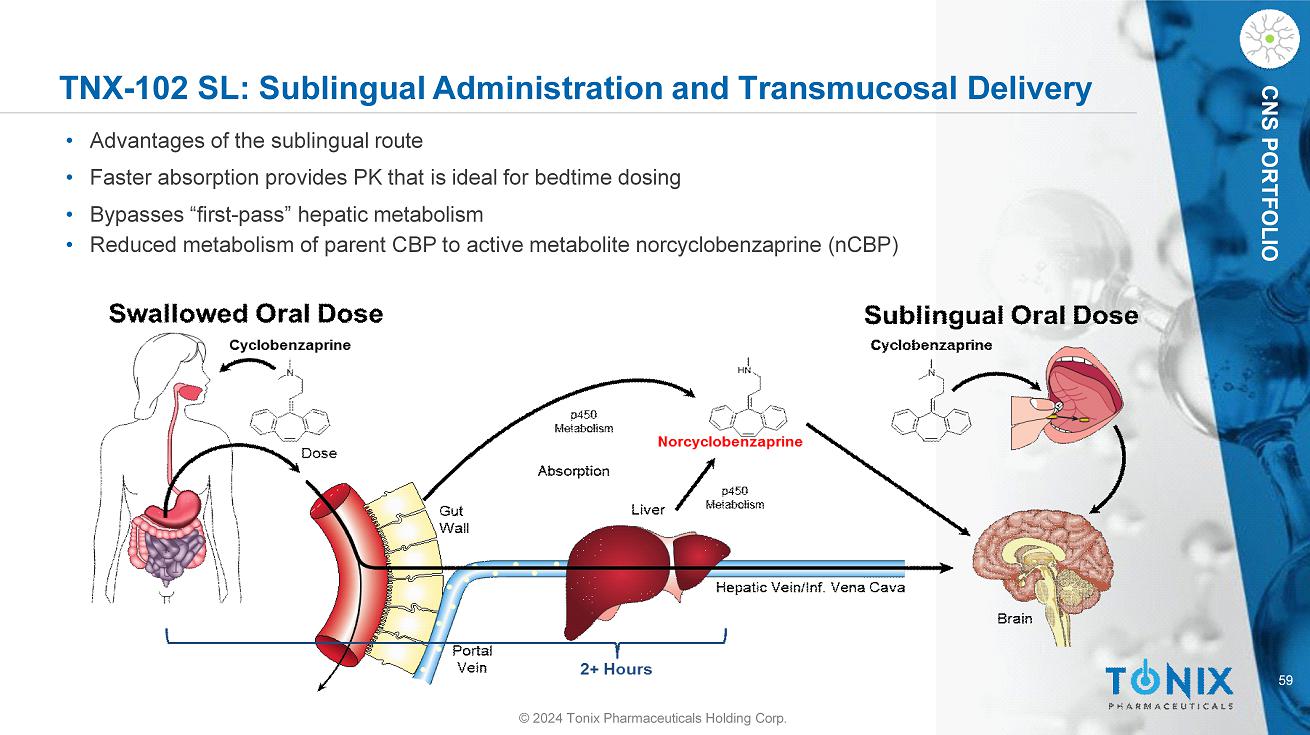
CNS PORTFOLIO TNX - 102 SL: Sublingual Administration and Transmucosal Delivery • Advantages of the sublingual route • Faster absorption provides PK that is ideal for bedtime dosing • Bypasses “first - pass” hepatic metabolism • Reduced metabolism of parent CBP to active metabolite norcyclobenzaprine (nCBP) 59 © 2024 Tonix Pharmaceuticals Holding Corp.
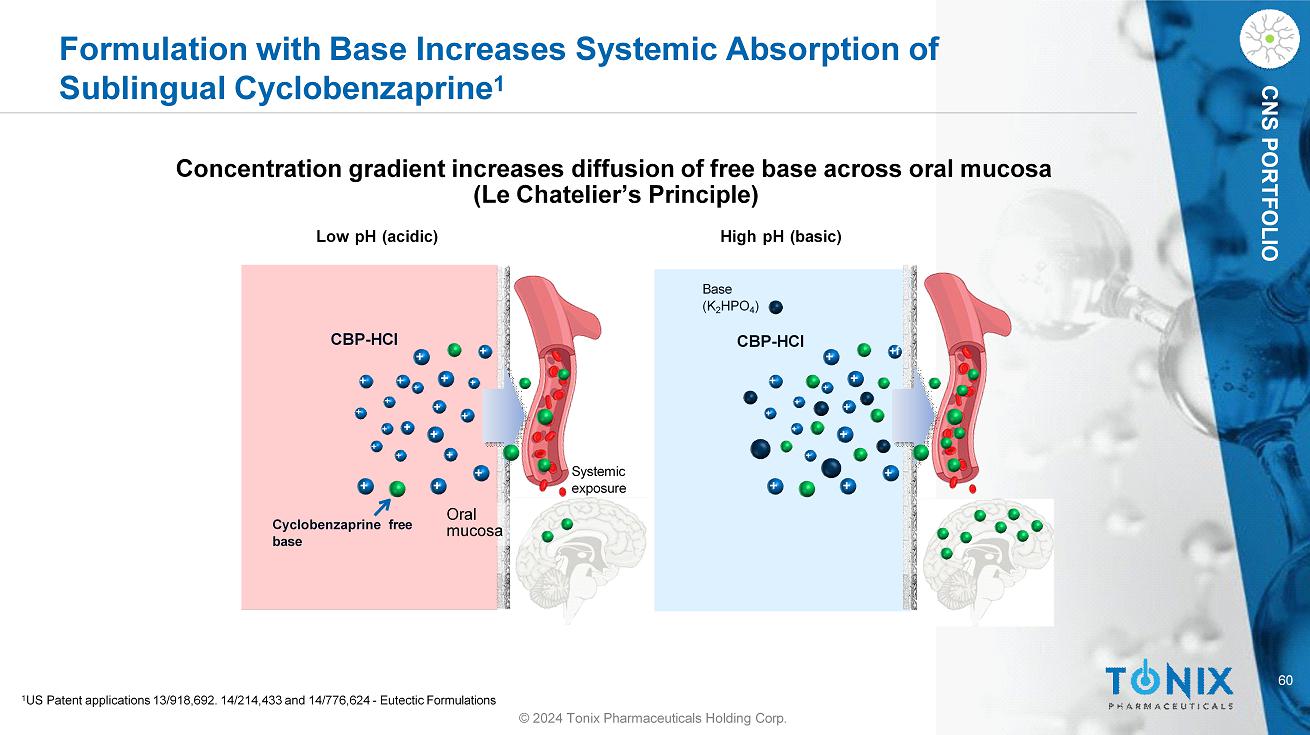
CNS PORTFOLIO Formulation with Base Increases Systemic Absorption of Sublingual Cyclobenzaprine 1 CBP - HCl Cyclobenzaprine free base Oral mucosa CBP - HCl Concentration gradient increases diffusion of free base across oral mucosa (Le Chatelier’s Principle) Low pH (acidic) High pH (basic) Systemic exposure Base (K 2 HPO 4 ) + + + + + + + + + + + + + + + + + + + + + + + + + + +f + + + + + + + 60 1 US Patent applications 13/918,692. 14/214,433 and 14/776,624 - Eutectic Formulations © 2024 Tonix Pharmaceuticals Holding Corp.
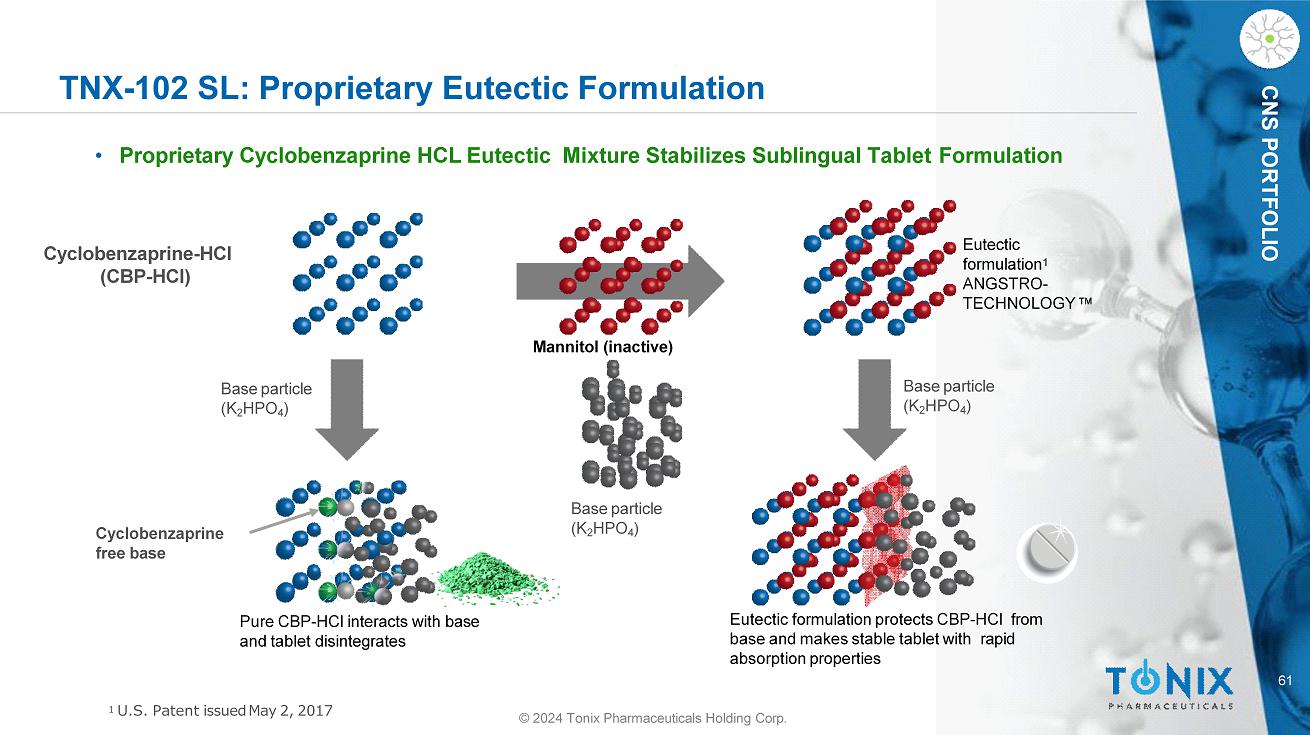
61 © 2024 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 102 SL: Proprietary Eutectic Formulation • Proprietary Cyclobenzaprine HCL Eutectic Mixture Stabilizes Sublingual Tablet Formulation Base particle (K 2 HPO 4 ) Base particle (K 2 HPO 4 ) Base particle (K 2 HPO 4 ) Cyclobenzaprine - HCl (CBP - HCl) Eutectic formulation protects CBP - HCl from base and makes stable tablet with rapid absorption properties Pure CBP - HCl interacts with base and tablet disintegrates Cyclobenzaprine free base Eutectic formulation 1 ANGSTRO - TECHNOLOGY Mannitol (inactive) 1 U.S. Patent issued May 2, 2017
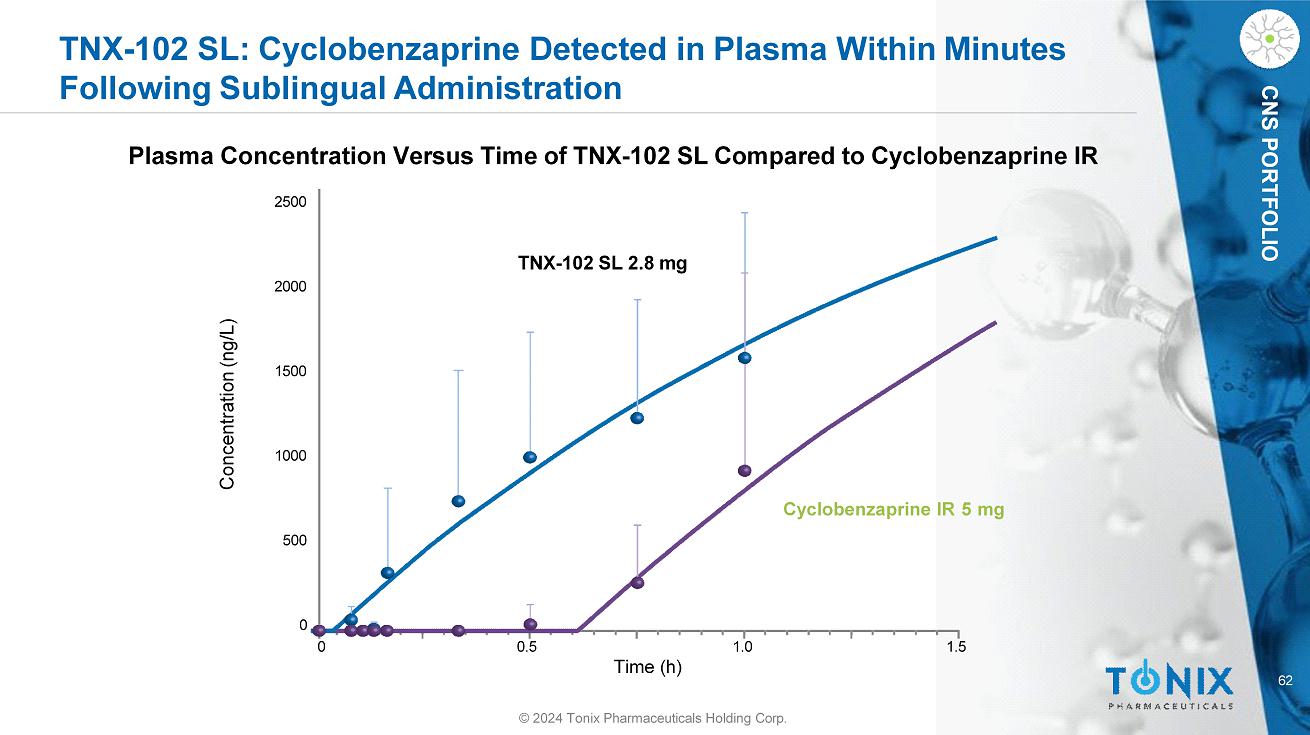
62 CNS PORTFOLIO TNX - 102 SL: Cyclobenzaprine Detected in Plasma Within Minutes Following Sublingual Administration © 2024 Tonix Pharmaceuticals Holding Corp. Plasma Concentration Versus Time of TNX - 102 SL Compared to Cyclobenzaprine IR 0 0.5 1.0 1.5 Concentration (ng/L) 2500 1500 1000 500 2000 0 TNX - 102 SL 2.8 mg Cyclobenzaprine IR 5 mg Time (h)
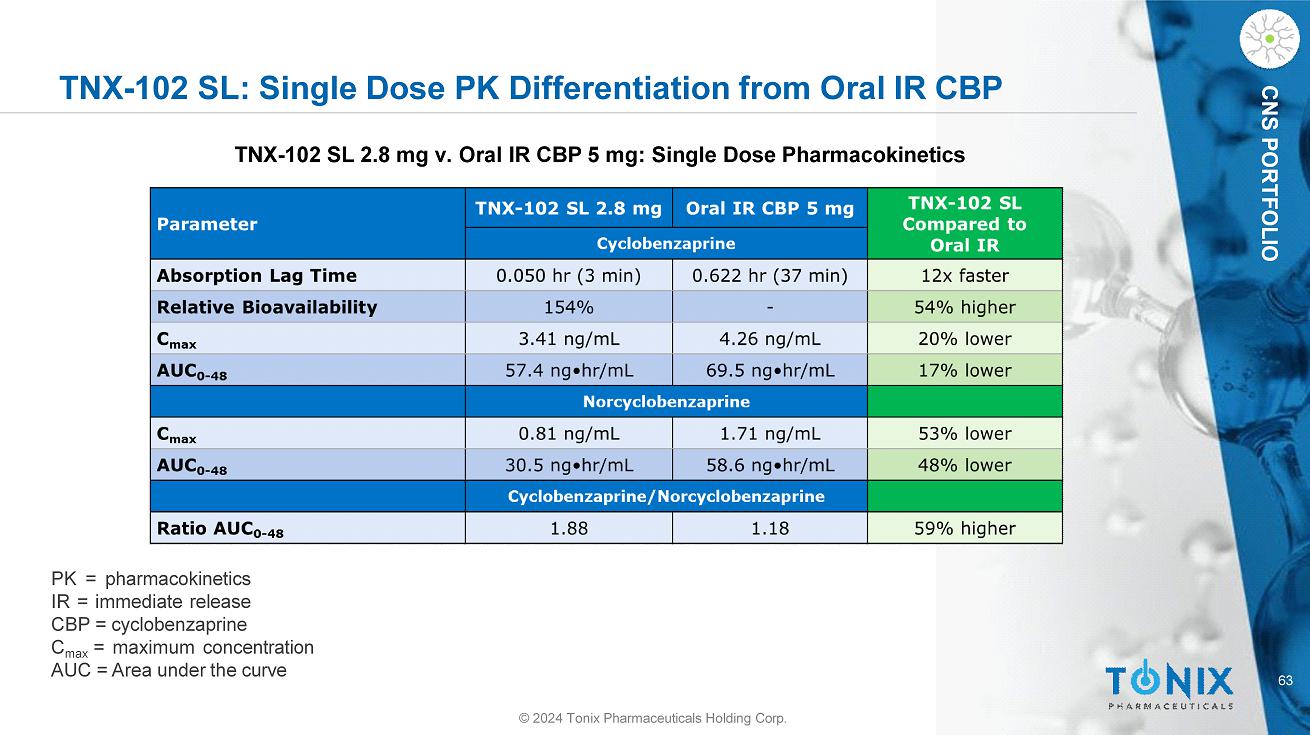
63 CNS PORTFOLIO TNX - 102 SL: Single Dose PK Differentiation from Oral IR CBP © 2024 Tonix Pharmaceuticals Holding Corp. TNX - 102 SL 2.8 mg v. Oral IR CBP 5 mg: Single Dose Pharmacokinetics PK = pharmacokinetics IR = immediate release CBP = cyclobenzaprine C max = maximum concentration AUC = Area under the curve
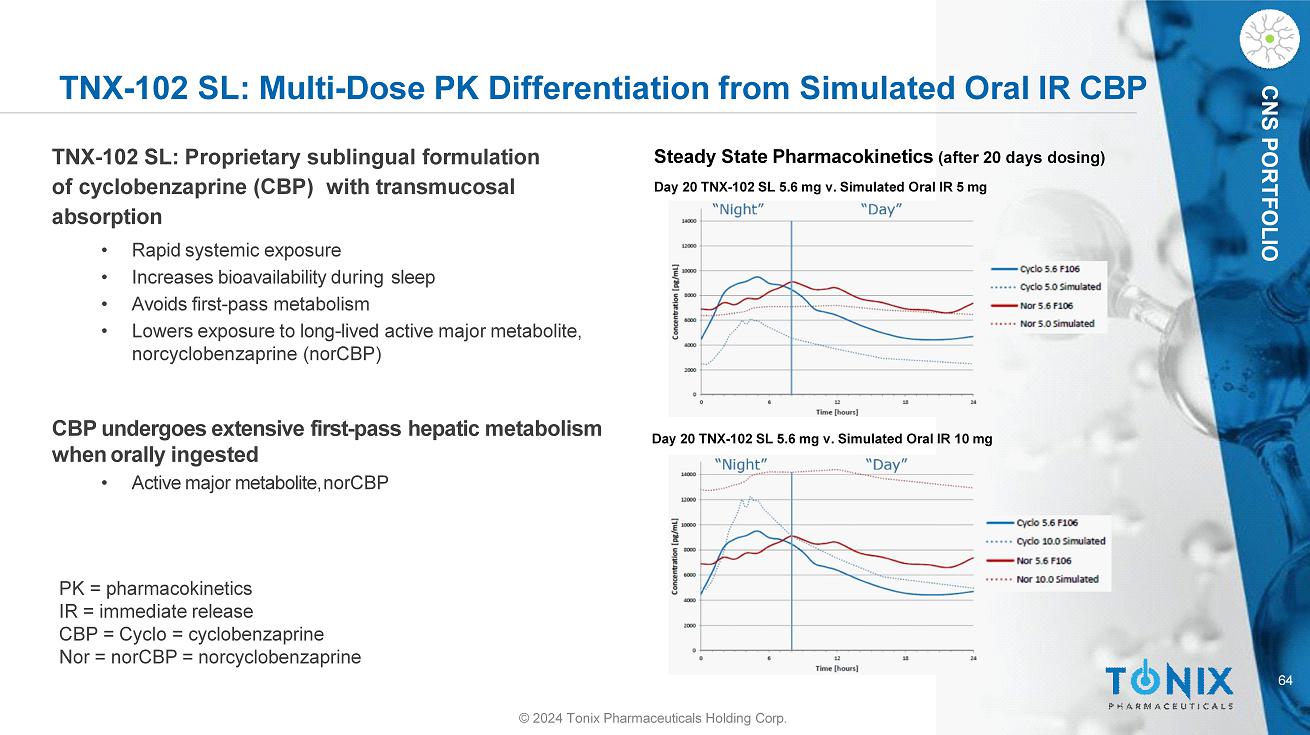
CNS PORTFOLIO TNX - 102 SL: Multi - Dose PK Differentiation from Simulated Oral IR CBP 64 © 2024 Tonix Pharmaceuticals Holding Corp. Day 20 TNX - 102 SL 5.6 mg v. Simulated Oral IR 10 mg Steady State Pharmacokinetics (after 20 days dosing) Day 20 TNX - 102 SL 5.6 mg v. Simulated Oral IR 5 mg PK = pharmacokinetics IR = immediate release CBP = Cyclo = cyclobenzaprine Nor = norCBP = norcyclobenzaprine TNX - 102 SL: Proprietary sublingual formulation of cyclobenzaprine (CBP) with transmucosal absorption • Rapid systemic exposure • Increases bioavailability during sleep • Avoids first - pass metabolism • Lowers exposure to long - lived active major metabolite, norcyclobenzaprine (norCBP) CBP undergoes extensive first - pass hepatic metabolism when orally ingested • Active major metabolite, norCBP
CNS PORTFOLIO Multi - Functional Mechanism Involves Antagonism at Four Neuronal Receptors 65 © 2024 Tonix Pharmaceuticals Holding Corp. Active ingredient, cyclobenzaprine, interacts with four receptors • Antagonist at 5 - HT 2A receptors • Similar activity to trazodone and Nuplazid ® (pimivanserin) • Antagonist at α 1 - adrenergic receptor • Similar activity to Prazosin ® (prazosin) • Antagonist at histamine H 1 receptors • Similar activity to Benadryl ® (diphenhydramine) and hydroxyzine • Antagonist at muscarinic M 1 receptors • Similar activity to Benadryl ® (diphenhydramine), Prozac® (fluoxetine), Paxil® (paroxetine), Zyprexa (olanzapine) and Seroquel® (quetiapine).
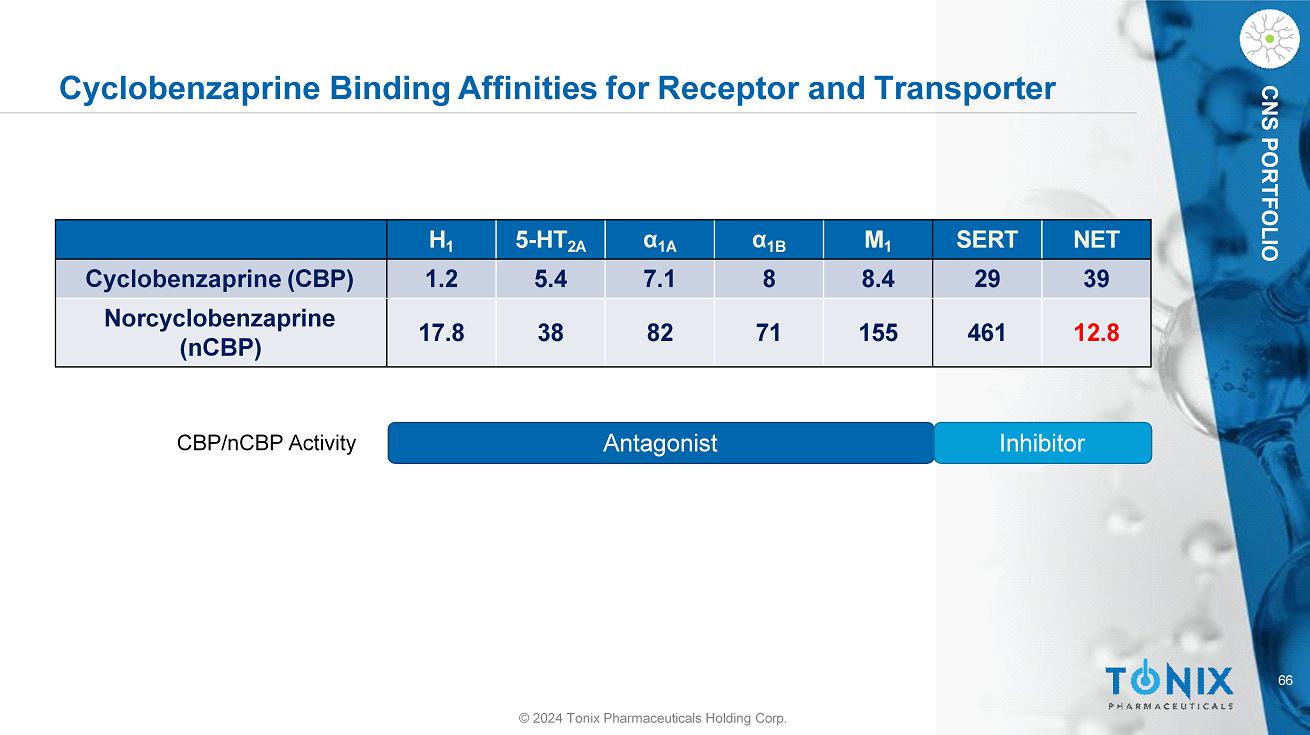
CNS PORTFOLIO Cyclobenzaprine Binding Affinities for Receptor and Transporter NET SERT M 1 α 1B α 1A 5 - HT 2A H 1 39 29 8.4 8 7.1 5.4 1.2 Cyclobenzaprine (CBP) 12.8 461 155 71 82 38 17.8 Norcyclobenzaprine (nCBP) Antagonist Inhibitor 66 © 2024 Tonix Pharmaceuticals Holding Corp. CBP/nCBP Activity

CNS PORTFOLIO Cyclobenzaprine Effects on Nerve Cell Signaling Untreated Effects of TNX - 102 SL SNARI = Serotonin and Norepinephrine receptor Antagonist and Reuptake Inhibitor Cyclobenzaprine is a multi - functional drug – SNARI • inhibits serotonin and norepinephrine reuptake • blocks serotonin 5 - HT 2A and norepinephrine α1 receptors 67 © 2024 Tonix Pharmaceuticals Holding Corp.
CNS PORTFOLIO TNX - 102 SL: No Recognized Abuse Potential in Clinical Studies 68 © 2024 Tonix Pharmaceuticals Holding Corp. Active ingredient is cyclobenzaprine, which is structurally related to tricyclic antidepressants • Cyclobenzaprine interacts with receptors that regulate sleep quality: 5 - HT2A, α1 - adrenergic and histamine H1 receptors • Cyclobenzaprine does NOT interact with the same receptors as traditional hypnotic sleep drugs, benzodiazepines or non - benzodiazepines that are associated with retrograde amnesia • Cyclobenzaprine - containing product was approved 40 years ago and current labeling (May 2016) indicates no abuse or dependence concern TNX - 102 SL NDA can be filed without drug abuse and dependency assessment studies • April 2017 meeting minutes from the March 2017 FDA meeting
69 CNS PORTFOLIO TNX - 102 SL: Sublingual Formulation is Designed for Bedtime Administration © 2024 Tonix Pharmaceuticals Holding Corp. TNX - 102 SL: Proprietary sublingual formulation of cyclobenzaprine (CBP) with transmucosal absorption • Innovation by design with patent protected CBP/mannitol eutectic • Rapid systemic exposure • Increases bioavailability during sleep • Avoids first - pass metabolism • Lowers exposure to long - lived active major metabolite, norcyclobenzaprine (norCBP) CBP undergoes extensive first - pass hepatic metabolism when orally ingested • Active major metabolite, norCBP1 • Long half - life (~72 hours) • Less selective for target receptors (5 - HT2A, α1 - adrenergic, histamine H1) • More selective for norepinephrine transporter and muscarinic M1 Multi - functional activity suggests potential for other indications • TNX - 102 SL was developed for the management of fibromyalgia (Phase 3) • Sleep quality is a problem in other conditions
70 CNS PORTFOLIO TNX - 102 SL: Patents and Patent Applications © 2024 Tonix Pharmaceuticals Holding Corp. • U.S. Composition:* • A 75:25 cyclobenzaprine HCl - mannitol eutectic (dependent claims add a basifying agent). • 5 US Patents (Expire November 2034) • 1 Pending US Application (Would expire November 2034) • A composition of a cyclobenzaprine HCl and a basifying agent suitable for sublingual absorption. • 1 Pending US Application (Would expire June 2033) • U.S. Methods of Use* (Specific Indications): • Fibromyalgia • Pain, Sleep Disturbance, Fatigue • 1 Pending US Application (Would expire December 2041) • Early Onset Response • 1 Pending US Provisional Application (Would expire December 2044) • Depressive Symptoms • 1 Pending US Application (Would expire March 2032) • Sexual Dysfunction • 1 Pending US Application (Would expire October 2041) • PASC • 1 Pending US Application (Would expire June 2043) • PTSD • 1 US Patent (Expires November 2030) • Agitation (Dementia) • 1 US Patent (Expires December 2038) • 1 Pending US Application (Would expire December 2038) • Alcohol Use Disorder • 1 Pending US Application (Would expire November 2041) • Foreign Filings • Corresponding foreign patents have been filed and some have issued: • Composition (25 patents, 3 allowed applications, 16 pending applications) • Methods of Use (9 patents, 54 pending applications) *US Patents: Issued: US Patent Nos. 9,636,408; 9,956,188; 10,117,936; 10,864,175; 11,839,594; 9,918,948; 11,826,321. Pending: US Patent Application Nos. 13/918,692; 18/385,468; 13/412,571; 18/265,525; 63/612,352; 18/382,262; 18/037,815; 17/226,058; 18/212,500.

71 TNX - 102 SL for Other Indications In Development: Acute Stress Disorder © 2024 Tonix Pharmaceuticals Holding Corp.

72 CNS PORTFOLIO © 2024 Tonix Pharmaceuticals Holding Corp. Acute Stress Reaction (ASR)/ Acute Stress Disorder (ASD) ASR/ASD are acute stress conditions resulting from trauma which can affect both civilian and military populations. Large unmet need: • According to National Center for PTSD, about 60% of men and 50% of women in the US are exposed least one traumatic experience in their lives 1 • In the US alone, one - third of emergency department visits (40 - 50 million patients per year) are for evaluation after trauma exposures 2 Current standard of care: • No medications are currently available at or near the point of care to treat patients suffering from acute traumatic events and support long - term health 1 National Center for PTSD. How Common is PTSD in Adults? https://www.ptsd.va.gov/understand/common/common_adults.asp 2 Wisco et al. J Clin Psychiatry . 2014.75(12):1338 - 46

73 CNS PORTFOLIO TNX - 102 SL for ASR/ASD: Program Status © 2024 Tonix Pharmaceuticals Holding Corp. Status: Expect to start investigator - initiated Phase 2 in 1Q 2025 Investigator - initiated Phase 2 Trial Funded by DoD grant to University of North Carolina (UNC) • UNC Institute for Trauma Recovery awarded a $3M grant from the Department of Defense (DoD) • OASIS trial will build upon infrastructure developed through the UNC - led, $40M AURORA initiative ‒ AURORA study is a major national research initiative to improve the understanding, prevention, and recovery of individuals who have experienced a traumatic event ‒ Supported in part by funding from the National Institutes of Health (NIH) and the health care arm of Google’s parent company Alphabet • Opportunity to investigate the correlation between motor vehicle collisions and the emergence of ASD and PTSD • Supported by multiple clinical trials: • Phase 2 trial in military - related PTSD (AtEase or NCT02277704) • Phase 3 trial in military - related PTSD (HONOR or NCT03062540) • Phase 3 trial in primarily civilian PTSD (RECOVERY or NCT03841773) • In each of these studies, early and sustained improvements in sleep were associated with TNX - 102 SL treatment by the PROMIS sleep disturbance (SD) scale and the Clinician Administered PTSD Scale (CAPS - 5) “sleep disturbance” item. Together these studies provide preliminary evidence that TNX - 102 SL is generally well - tolerated and may promote recovery from PTSD via a pharmacodynamic facilitation of sleep - dependent emotional memory processing

74 CNS PORTFOLIO TNX - 102 SL for ASR/ASD: Phase 2 OASIS Study Design General study characteristics: • Randomized, double - blind, placebo - controlled study in Acute Stress Reaction (ASR) / Acute Stress Disorder (ASD) • The proposed O ptimizing A cute S tress reaction Interventions with TNX - 102 S L (OASIS) trial will examine the safety and efficacy of TNX - 102 SL to reduce adverse posttraumatic neuropsychiatric sequelae among patients presenting to the emergency department after a motor vehicle collision (MVC) • The trial will enroll approximately 180 individuals who acutely experienced trauma at study sites across the US • Participants will be randomized in the emergency department to receive a two - week course of either TNX - 102 SL or placebo • Investigator - initiated IND Objective: • Investigate the potential of Tonix’s TNX - 102 SL (cyclobenzaprine HCl sublingual tablets) to reduce the frequency and severity of the adverse effects of traumatic exposure, including acute stress reaction (ASR), acute stress disorder (ASD), and posttraumatic stress disorder (PTSD). • ASR refers to the body’s immediate response to trauma, whereas ASD is the short - term effects of trauma (within 1 month), and PTSD is the long - term effects of trauma (beyond 1 month) * First dose of TNX - 102 SL 5.6 mg versus placebo taken in the emergency department, and then daily at bedtime to finish 2 weeks of treatment A Phase 2 Study to Evaluate the Efficacy and Safety of TNX - 102 SL Taken Daily in Patients With ASR/ ASD (OASIS) • Primary outcome measure: Acute Stress Disorder Scale (ASDS) assessed at 7 and 21 days post MVC • Posttraumatic stress symptom severity assessed at 6 and 12 weeks post MVC using the PTSD Checklist for DSM - 5 (PCL - 5) • Standardized survey instruments of sleep disturbances, anxiety and depression symptoms, general physical and mental health, and clinical global improvement also employed • Detailed and brief neurocognitive assessments are performed from baseline to 12 weeks after MVC at specific timepoints throughout study participation period Placebo once - daily at bedtime 2 weeks TNX - 102 SL once - daily at bedtime 5.6 mg (2 x 2.8 mg tablets) * © 2024 Tonix Pharmaceuticals Holding Corp.

75 Fibromyalgia and Long - COVID © 2024 Tonix Pharmaceuticals Holding Corp.
76 CNS PORTFOLIO Significant Overlap between Fibromyalgia and Long - COVID © 2024 Tonix Pharmaceuticals Holding Corp. National Institutes of Health (NIH) - sponsored RECOVER study found many Long COVID patients suffer from pain at multiple sites 1 • Suggests that many Long - COVID patients may meet the diagnostic criteria for fibromyalgia Tonix has previously presented its analysis of real - world evidence from the TriNetX claims database suggesting that over 40% of Long COVID patients present with a constellation of symptoms that overlap with fibromyalgia 2,3 1 Thaweethai T, et al. JAMA . 2023 329(22):1934 - 1946. 2 Feb 22, 2023 Tonix Pharmaceuticals Press Release. URL: https://ir.tonixpharma.com/news - events/press - releases/detail/1369/tonix - pharmaceuticals - describes - emerging - research - on - the 3 September 21, 2022, Tonix Pharmaceuticals Poster at the IASP, “Retrospective observational database study of patients with Long COVID with multi - site pain, fatigue and insomnia”. URL: www.tonixpharma.com/wp - content/uploads/2022/09/Retrospective - Observational - Database - Study - of - Patients - with - Long - COVID - with - Multi - Site - Pain - Fatigue - and - Insomnia_A - Real - World - Analysis - of - Symptomatology - and - Opioid - Use.pdf
77 CNS PORTFOLIO NASEM Definition of Long - COVID © 2024 Tonix Pharmaceuticals Holding Corp. • In June 2024, the US National Academies of Sciences, Engineering and Medicine (NASEM) described fibromyalgia as a ‘ diagnosable condition’ in people suffering from Long COVID 1 • This definition provides guidance to government, healthcare professionals and industry that fibromyalgia can arise after infection with the SARS - CoV - 2 virus and can be diagnosed in Long COVID patients Fibromyalgia prevalence prior to COVID - 19 pandemic: >10M adults in the US 2 Long - COVID prevalence: 5.3% or ~14M adults in the US 3 Tonix believes that diagnosing fibromyalgia in Long COVID patients will increase the potential market for TNX - 102 SL as compared to market estimates from before the COVID - 19 pandemic 1 U.S. National Academies of Sciences, Engineering, and Medicine. 2024. A Long COVID Definition: A chronic, systemic disease state with profound consequences. Washington, DC: The National Academies Press. https://doi.org/10.17226/27768 . http://www.nationalacademies.org/long - covid - definition . 2 Vincent A, et al. Arthritis Care Res (Hoboken). 2013 65(5):786 - 92. doi: 10.1002 3 National Center for Health Statistics. U.S. Census Bureau, Household Pulse Survey, 2022 – 2024. Long COVID. Generated interactively: July 22, 2024 from https://www.cdc.gov/nchs/covid19/pulse/long - covid.htm

78 CNS PORTFOLIO NASEM Language Highlighted by Senate Labor - HHS Appropriations Subcommittee (August 1, 2024) • Senate Appropriations Committee report on FY25 Labor, Health, and Human Services Appropriations Act includes language on Long COVID, fibromyalgia and nociplastic syndromes 1 1 FY25 Labor - HHS Senate Report, p145 - 146. Accessed Aug 7, 2024: https://www.appropriations.senate.gov/download/fy25 - lhhs - senate - report © 2024 Tonix Pharmaceuticals Holding Corp. “Long COVID Treatments. — The Committee remains concerned about the economic and overall health impact that Long COVID inflicts on the Nation. It is currently estimated that between 6 percent and 19 percent of those infected with SARS – CoV – 2 go on to develop Long COVID, resulting in up to 20 million Americans suffering from this set of debilitating chronic symptoms. Long COVID is characterized by a wide range of symptoms including severe fatigue, non - restorative sleep, cognitive dysfunction, and widespread pain. Further, it resembles other post - acute infection syndromes [PAISs], such as fibromyalgia, myalgic encephalomyelitis/chronic fatigue syndrome [ME/CFS] and related conditions, known as 146 chronic overlapping pain conditions [COPCs] or nociplastic syndromes. While the Committee is pleased that NIH’s HEAL and RECOVER initiatives plan to target some specific symptoms of Long COVID, the Committee is concerned that NIH has not expanded the evaluation of treatments to address many common symptoms associated with Long COVID either individually or that present as syndromes which are combinations of symptoms. Furthermore, NIH’s research program has defined Long COVID narrowly, excluding many of the common symptoms plaguing Long COVID sufferers. In June 2024, NASEM released the 2024 NASEM Long COVID Definition, which encompasses extensive lists of the symptoms and diagnosable conditions that current science attributes to Long COVID. The Committee urges NIH to rebalance its research program to prioritize clinical trials in pursuit of effective treatments and to use the NASEM Long COVID definition to guide its choice of symptoms and conditions to be address by the candidate treatments. Such trials should target key symptoms and symptom complexes associated with Long COVID including widespread pain, fatigue, non - restorative sleep, brain fog, dizziness, post - exertional malaise [PEM], postural orthostatic tachycardia syndrome [POTS] and loss of taste and smell. Further, the Committee urges NIH to prioritize the support of clinical trials evaluating therapies for Long COVID including therapies that have demonstrated efficacy in treating COPCs or nociplastic syndromes that overlap with Long COVID.”

THANK YOU © 2023 Tonix Pharmaceuticals Holding Corp.

APPENDIX © 2023 Tonix Pharmaceuticals Holding Corp.
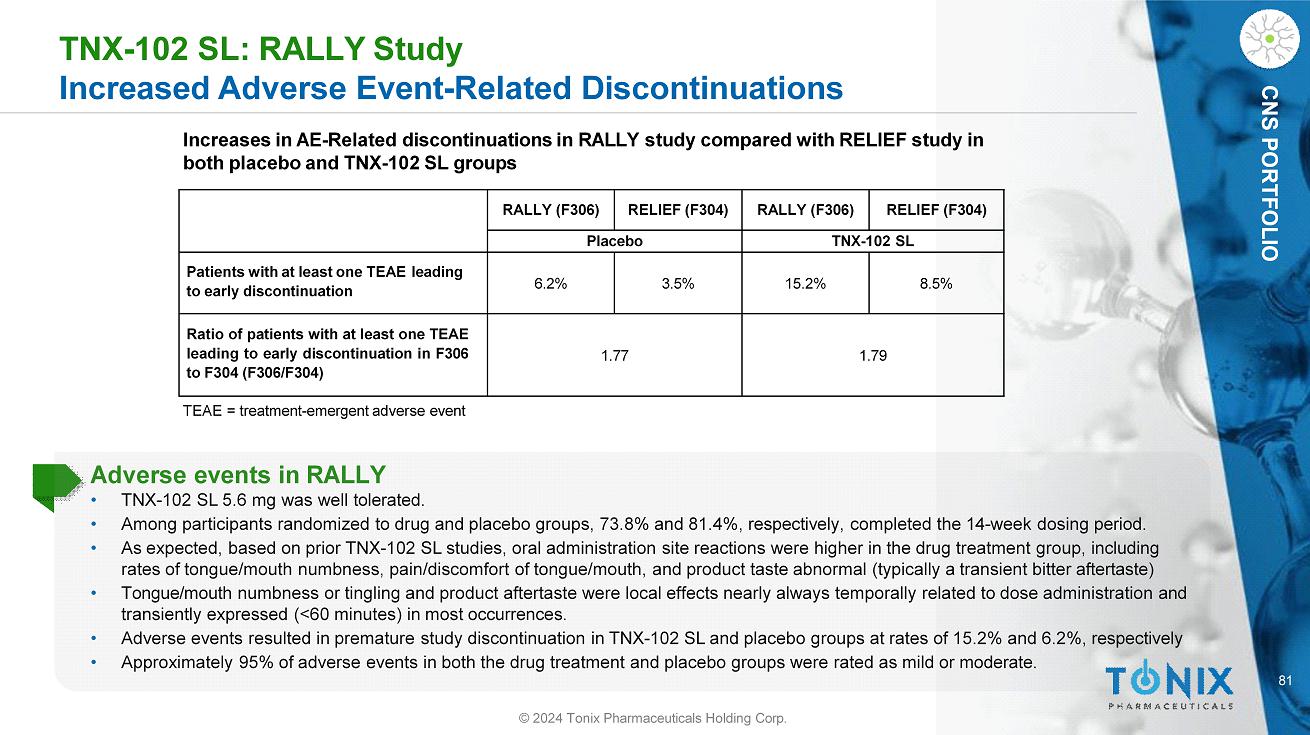
81 CNS PORTFOLIO TNX - 102 SL: RALLY Study Increased Adverse Event - Related Discontinuations TEAE = treatment - emergent adverse event Increases in AE - Related discontinuations in RALLY study compared with RELIEF study in both placebo and TNX - 102 SL groups Adverse events in RALLY • TNX - 102 SL 5.6 mg was well tolerated. • Among participants randomized to drug and placebo groups, 73.8% and 81.4%, respectively, completed the 14 - week dosing period. • As expected, based on prior TNX - 102 SL studies, oral administration site reactions were higher in the drug treatment group, including rates of tongue/mouth numbness, pain/discomfort of tongue/mouth, and product taste abnormal (typically a transient bitter aftertaste) • Tongue/mouth numbness or tingling and product aftertaste were local effects nearly always temporally related to dose administration and transiently expressed (<60 minutes) in most occurrences. • Adverse events resulted in premature study discontinuation in TNX - 102 SL and placebo groups at rates of 15.2% and 6.2%, respectively • Approximately 95% of adverse events in both the drug treatment and placebo groups were rated as mild or moderate. RELIEF (F304) RALLY (F306) RELIEF (F304) RALLY (F306) TNX - 102 SL Placebo 8.5% 15.2% 3.5% 6.2% Patients with at least one TEAE leading to early discontinuation 1.79 1.77 Ratio of patients with at least one TEAE leading to early discontinuation in F 306 to F 304 (F 306 /F 304 ) © 2024 Tonix Pharmaceuticals Holding Corp.
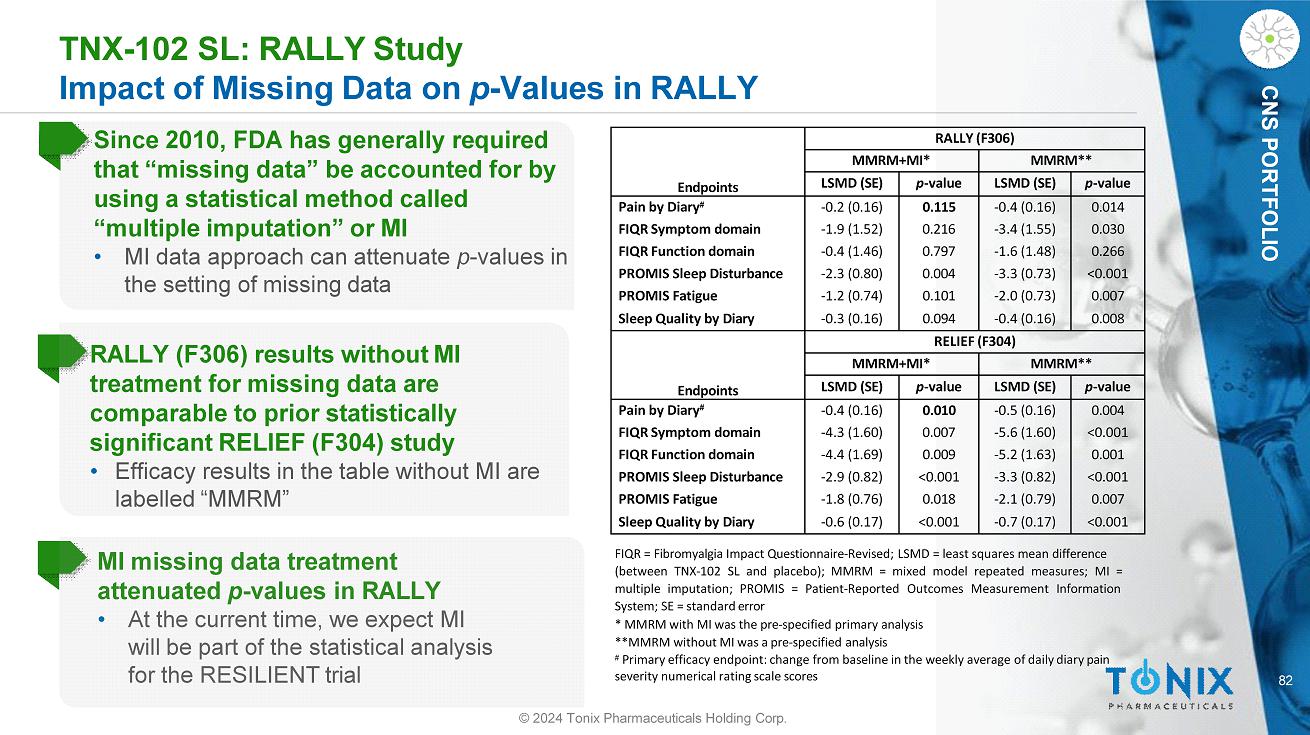
82 CNS PORTFOLIO TNX - 102 SL: RALLY Study Impact of Missing Data on p - Values in RALLY MI missing data treatment attenuated p - values in RALLY • At the current time, we expect MI will be part of the statistical analysis for the RESILIENT trial FIQR = Fibromyalgia Impact Questionnaire - Revised; LSMD = least squares mean difference (between TNX - 102 SL and placebo); MMRM = mixed model repeated measures; MI = multiple imputation; PROMIS = Patient - Reported Outcomes Measurement Information System; SE = standard error * MMRM with MI was the pre - specified primary analysis **MMRM without MI was a pre - specified analysis # Primary efficacy endpoint: change from baseline in the weekly average of daily diary pain severity numerical rating scale scores © 2024 Tonix Pharmaceuticals Holding Corp. Since 2010, FDA has generally required that “missing data” be accounted for by using a statistical method called “multiple imputation” or MI • MI data approach can attenuate p - values in the setting of missing data RALLY (F306) results without MI treatment for missing data are comparable to prior statistically significant RELIEF (F304) study • Efficacy results in the table without MI are labelled “MMRM” RALLY (F306) Endpoints MMRM** MMRM+MI* p - value LSMD (SE) p - value LSMD (SE) 0.014 - 0.4 (0.16) 0.115 - 0.2 (0.16) Pain by Diary # 0.030 - 3.4 (1.55) 0.216 - 1.9 (1.52) FIQR Symptom domain 0.266 - 1.6 (1.48) 0.797 - 0.4 (1.46) FIQR Function domain <0.001 - 3.3 (0.73) 0.004 - 2.3 (0.80) PROMIS Sleep Disturbance 0.007 - 2.0 (0.73) 0.101 - 1.2 (0.74) PROMIS Fatigue 0.008 - 0.4 (0.16) 0.094 - 0.3 (0.16) Sleep Quality by Diary RELIEF (F304) Endpoints MMRM** MMRM+MI* p - value LSMD (SE) p - value LSMD (SE) 0.004 - 0.5 (0.16) 0.010 - 0.4 (0.16) Pain by Diary # <0.001 - 5.6 (1.60) 0.007 - 4.3 (1.60) FIQR Symptom domain 0.001 - 5.2 (1.63) 0.009 - 4.4 (1.69) FIQR Function domain <0.001 - 3.3 (0.82) <0.001 - 2.9 (0.82) PROMIS Sleep Disturbance 0.007 - 2.1 (0.79) 0.018 - 1.8 (0.76) PROMIS Fatigue <0.001 - 0.7 (0.17) <0.001 - 0.6 (0.17) Sleep Quality by Diary
83 CNS PORTFOLIO Chronic Overlapping Pain Conditions (COPC) Believed to Result from Shared Brain Processes • COPC is a set of disorders that co - aggregate; these disorders can include but are not limited to 1,2 : • Temporomandibular disorder • Fibromyalgia • Irritable bowel syndrome • Vulvodynia • CFS/ME 3 • Interstitial cystitis/painful bladder syndrome • Endometriosis • Chronic tension - type headache • Migraine headache • Chronic lower back pain 1 Maixner W, et al. J Pain . 2016;17(9 Suppl):T93 - T107. 2 Veasley C, et al. http://www.chronicpainresearch. org/public/CPRA_WhitePaper_2015 - FINAL - Digital.pdf. Published May 2015. Accessed July 26, 2021. 3 CFS/ME – chronic fatigue syndrome/myalgic encephalomyelitis © 2024 Tonix Pharmaceuticals Holding Corp. • Similar central mechanisms play significant roles in all pain conditions, even those with known peripheral contributions 1,2
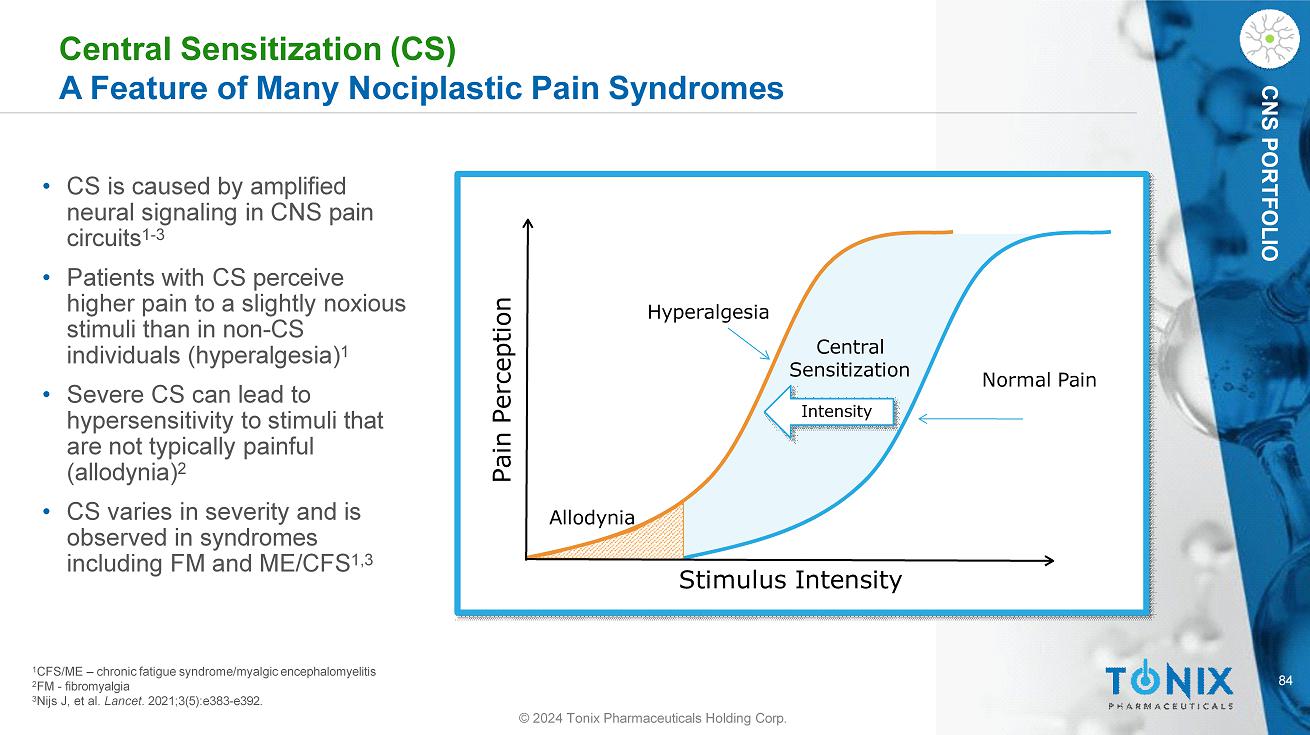
84 CNS PORTFOLIO Central Sensitization (CS) A Feature of Many Nociplastic Pain Syndromes • CS is caused by amplified neural signaling in CNS pain circuits 1 - 3 • Patients with CS perceive higher pain to a slightly noxious stimuli than in non - CS individuals (hyperalgesia) 1 • Severe CS can lead to hypersensitivity to stimuli that are not typically painful (allodynia) 2 • CS varies in severity and is observed in syndromes including FM and ME/CFS 1,3 Stimulus Intensity Pain Perception Normal Pain Hyperalgesia Allodynia Central Sensitization Intensity 3 Nijs J, et al. Lancet . 2021;3(5):e383 - e392. © 2024 Tonix Pharmaceuticals Holding Corp. 1 CFS/ME – chronic fatigue syndrome/myalgic encephalomyelitis 2 FM - fibromyalgia
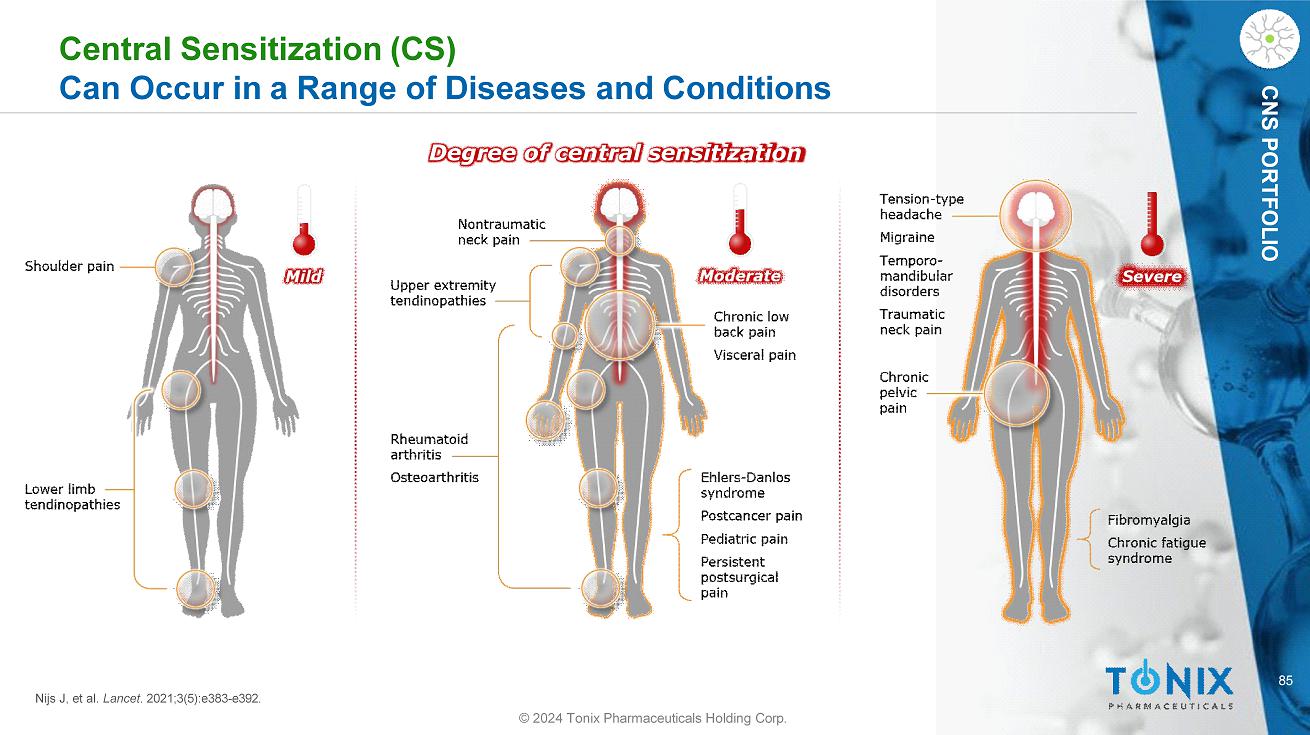
85 CNS PORTFOLIO Central Sensitization (CS) Can Occur in a Range of Diseases and Conditions Degree of central sensitization Nijs J, et al. Lancet . 2021;3(5):e383 - e392. © 2024 Tonix Pharmaceuticals Holding Corp.