
Tonix Pharmaceuticals Holding Corp 8-K
Exhibit 99.02

© 2025 Tonix Pharmaceuticals Holding Corp. Corporate Presentation February 2025 NASDAQ: TNXP PO6043 Feb 6, 2025 (Doc 1559 )

2 © 2025 Tonix Pharmaceuticals Holding Corp. Cautionary Note on Forward - Looking Statements Certain statements in this presentation regarding strategic plans, expectations and objectives for future operations or results are “forward - looking statements” as defined by the Private Securities Litigation Reform Act of 1995. These statements may be identified by the use of forward - looking words such as “anticipate,” “believe,” “forecast,” “estimate” and “intend,” among others. These forward - looking statements are based on Tonix’s current expectations and actual results could differ materially. There are a number of factors that could cause actual events to differ materially from those indicated by such forward - looking statements. These factors include, but are not limited to, the risks related to failure to obtain FDA clearances or approvals and noncompliance with FDA regulations; risks related to the failure to successfully market any of our products; risks related to the timing and progress of clinical development of our product candidates; our need for additional financing; uncertainties of patent protection and litigation; uncertainties of government or third party payor reimbursement; limited research and development efforts and dependence upon third parties; and substantial competition. As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products. The forward - looking statements in this presentation are made as of the date of this presentation, even if subsequently made available by Tonix on its website or otherwise. Tonix does not undertake an obligation to update or revise any forward - looking statement, except as required by law. Investors should read the risk factors set forth in the Annual Report on Form 10 - K for the year ended December 31, 2023, as filed with the Securities and Exchange Commission (the “SEC”) on April 1, 2024, and periodic reports and current reports filed with the SEC on or after the date thereof. All of Tonix's forward - looking statements are expressly qualified by all such risk factors and other cautionary statements.

3 © 2025 Tonix Pharmaceuticals Holding Corp. Tonix is committed to developing and marketing therapeutics to treat pain, neurologic, psychiatric and addiction conditions through our central nervous system portfolio and within other areas of high unmet need , including immunology, infectious disease, and rare disease …Transforming therapies for pain management and vaccines for public health challenges… Who We Are
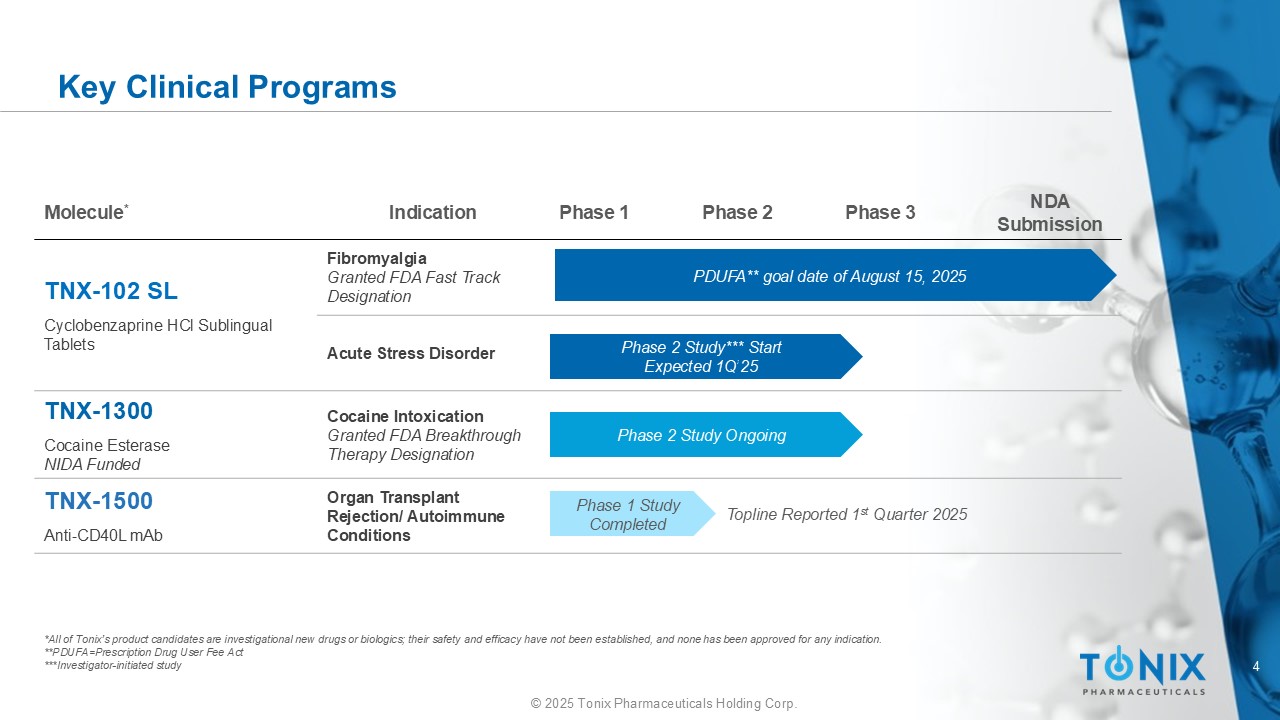
4 © 2025 Tonix Pharmaceuticals Holding Corp. NDA Submission Phase 3 Phase 2 Phase 1 Indication Molecule * Fibromyalgia Granted FDA Fast Track Designation TNX - 102 SL Cyclobenzaprine HCl Sublingual Tablets Acute Stress Disorder Cocaine Intoxication Granted FDA Breakthrough Therapy Designation TNX - 1300 Cocaine Esterase NIDA Funded Organ Transplant Rejection/ Autoimmune Conditions TNX - 1500 Anti - CD40L mAb Key Clinical Programs *All of Tonix’s product candidates are investigational new drugs or biologics; their safety and efficacy have not been established, and none ha s been approved for any indication. **PDUFA=Prescription Drug User Fee Act ***Investigator - initiated study PDUFA** goal date of August 15, 2025 Phase 2 Study Ongoing Phase 1 Study Completed Phase 2 Study*** Start Expected 1Q’25 Topline Reported 1 st Quarter 2025
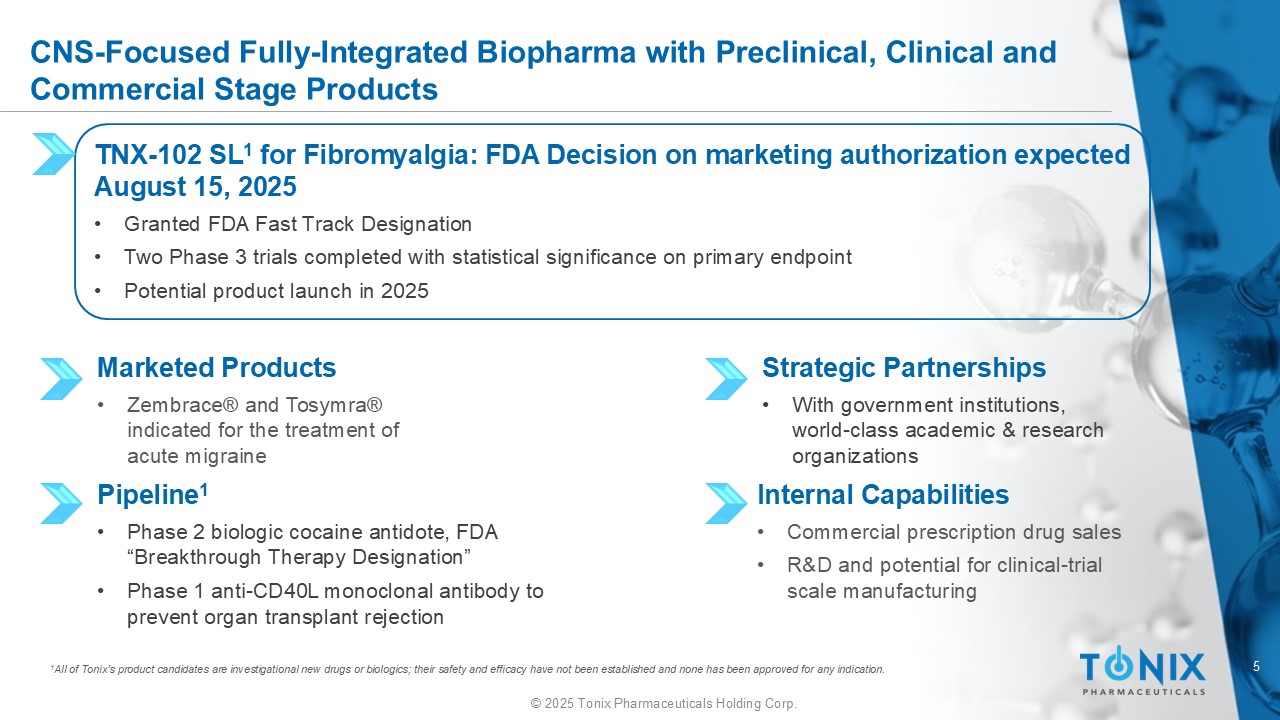
5 © 2025 Tonix Pharmaceuticals Holding Corp. CNS - Focused Fully - Integrated Biopharma with Preclinical, Clinical and Commercial Stage Products Marketed Products • Zembrace ® and Tosymra ® indicated for the treatment of acute migraine TNX - 102 SL 1 for Fibromyalgia: FDA Decision on marketing authorization expected August 15, 2025 • Granted FDA Fast Track Designation • Two Phase 3 trials completed with statistical significance on primary endpoint • Potential product launch in 2025 Internal Capabilities • Commercial prescription drug sales • R&D and potential for clinical - trial scale manufacturing Strategic Partnerships • With government institutions, world - class academic & research organizations Pipeline 1 • Phase 2 biologic cocaine antidote, FDA “Breakthrough Therapy Designation” • Phase 1 anti - CD40L monoclonal antibody to prevent organ transplant rejection 1 All of Tonix’s product candidates are investigational new drugs or biologics; their safety and efficacy have not been establi she d and none has been approved for any indication.

© 2025 Tonix Pharmaceuticals Holding Corp. CNS: KEY DEVELOPMENT CANDIDATES

7 © 2025 Tonix Pharmaceuticals Holding Corp. TNX - 102 SL* ( Cyclobenzaprine HCl Sublingual Tablets) 5.6 mg A unique, sublingual formulation of cyclobenzaprine (CBP) designed to optimize absorption and delivery *5.6 mg once - daily at bedtime, TNX - 102 SL is an investigational new drug, its efficacy and safety have not been established and it has not been approved for any indication norCBP = norCyclobenzaprine • Non - opioid analgesic – Tertiary Amine Tricyclic (TAT) • Rapid drug exposure following once - nightly sublingual administration • Reduction in persistent active metabolite norCBP with chronic dosing • Durable (14 week) reduction in fibromyalgia pain in two pivotal studies • Generally well tolerated • PDUFA goal date August 15, 2025
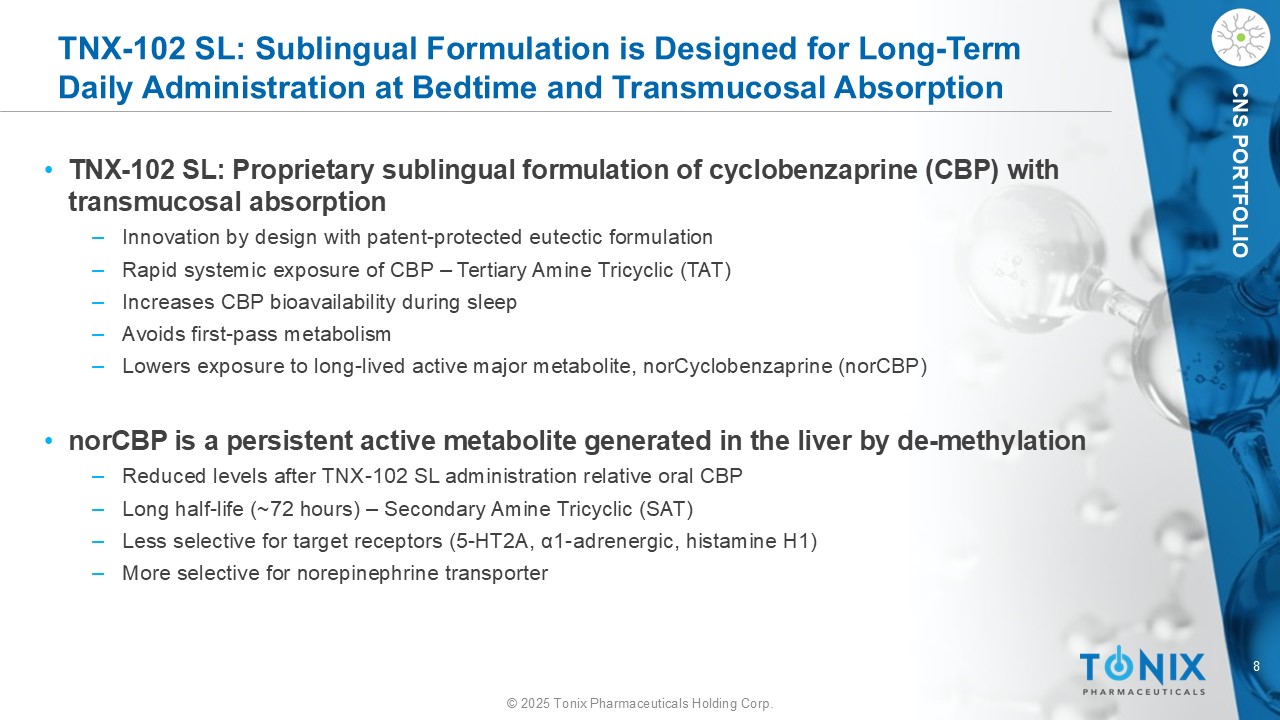
8 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 102 SL: Sublingual Formulation is Designed for Long - Term Daily Administration at Bedtime and Transmucosal Absorption • TNX - 102 SL: Proprietary sublingual formulation of cyclobenzaprine (CBP) with transmucosal absorption ‒ Innovation by design with patent - protected eutectic formulation ‒ Rapid systemic exposure of CBP – Tertiary Amine Tricyclic (TAT) ‒ Increases CBP bioavailability during sleep ‒ Avoids first - pass metabolism ‒ Lowers exposure to long - lived active major metabolite, norCyclobenzaprine ( norCBP ) • norCBP is a persistent active metabolite generated in the liver by de - methylation ‒ Reduced levels after TNX - 102 SL administration relative oral CBP ‒ Long half - life (~72 hours) – Secondary Amine Tricyclic (SAT) ‒ Less selective for target receptors (5 - HT2A, α1 - adrenergic, histamine H1) ‒ More selective for norepinephrine transporter

9 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 102 SL (5.6 mg) Fibromyalgia Pivotal Clinical Trial Results • Activity • First pivotal Phase 3 study ( RELIEF ) reported – December 2020 1 ‒ Statistically significant reduction in daily pain compared to placebo ( p = 0.010) • Second Phase 3 study ( RALLY ) missed primary endpoint – July 2021 • Confirmatory pivotal Phase 3 study ( RESILIENT ) reported – December 2023 ‒ Statistically significant reduction in daily pain compared to placebo ( p = 0.00005) • Tolerability in two pivotal trials • G enerally well tolerated with an adverse event profile comparable to prior studies and with no new safety signals observed • The most common treatment - emergent adverse event was tongue or mouth numbness at the administration site, which was temporally related to dosing, self - limited, never rated as severe, and rarely led to study discontinuation (one participant in each study) • Excluding COVID - 19, rates of systemic adverse events in each of the two studies were all below 4.0% 1 Lederman S, et al. Arthritis Care Res (Hoboken) . 2023 Nov;75(11):2359 - 2368. doi : 10.1002.
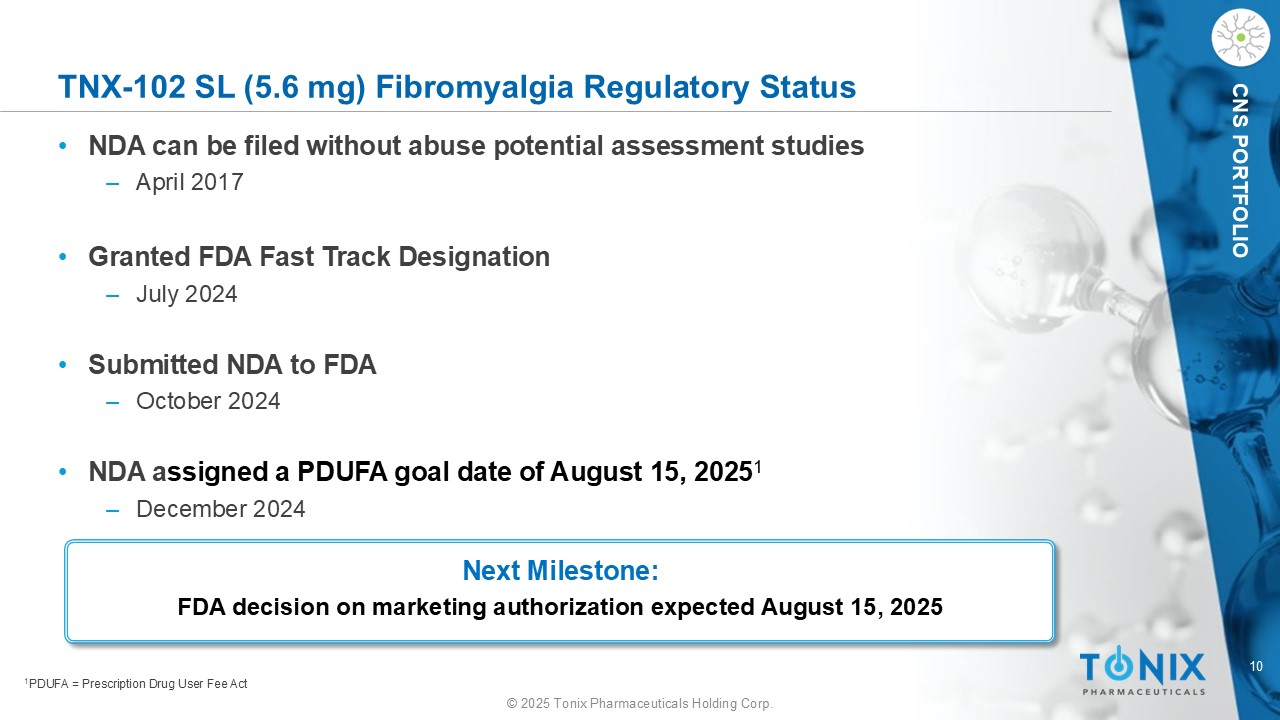
10 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 102 SL (5.6 mg) Fibromyalgia Regulatory Status • NDA can be filed without abuse potential assessment studies ‒ April 2017 • Granted FDA Fast Track Designation ‒ July 2024 • Submitted NDA to FDA ‒ October 2024 • NDA a ssigned a PDUFA goal date of August 15, 2025 1 ‒ December 2024 1 PDUFA = Prescription Drug User Fee Act Next Milestone: FDA decision on marketing authorization expected August 15, 2025
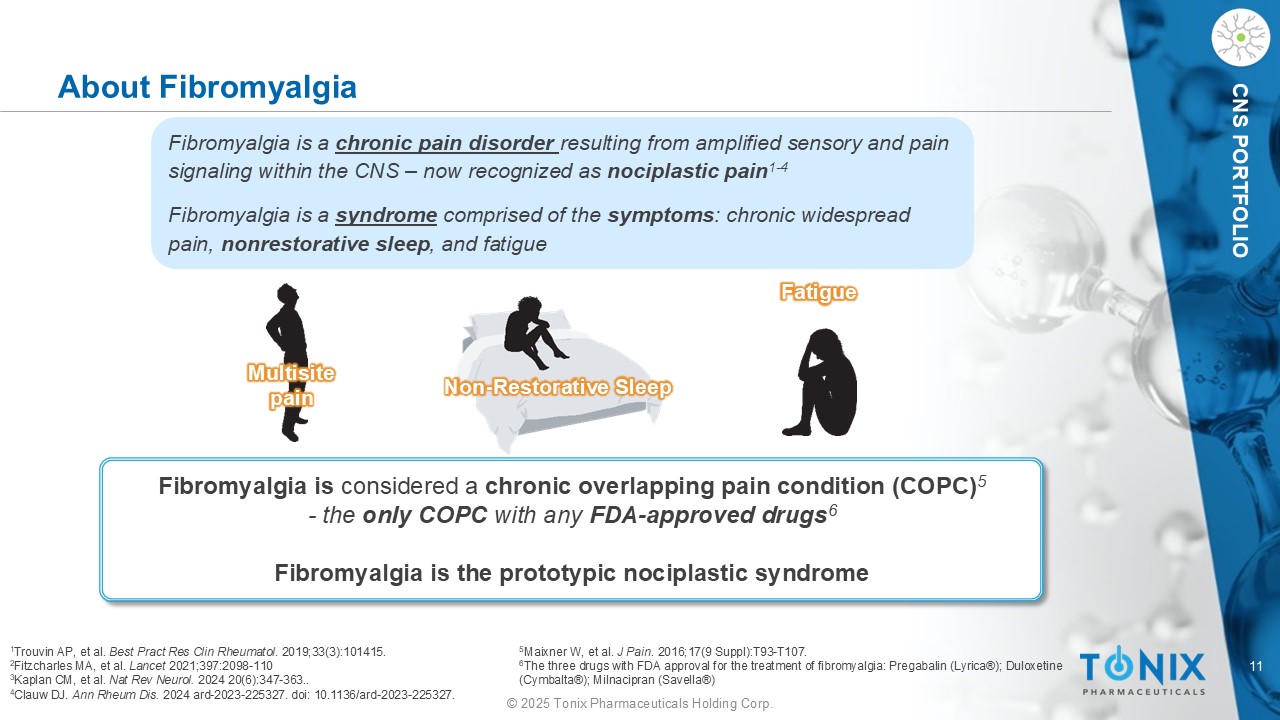
11 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO About Fibromyalgia Fibromyalgia is considered a chronic overlapping pain condition (COPC) 5 - the only COPC with any FDA - approved drugs 6 Fibromyalgia is the prototypic nociplastic syndrome Multisite pain Fatigue Non - Restorative Sleep Fibromyalgia is a chronic pain disorder resulting from amplified sensory and pain signaling within the CNS – now recognized as nociplastic pain 1 - 4 Fibromyalgia is a syndrome comprised of the symptoms : chronic widespread pain, nonrestorative sleep , and fatigue 1 Trouvin AP, et al. Best Pract Res Clin Rheumatol . 2019;33(3):101415. 2 Fitzcharles MA, et al. Lancet 2021;397:2098 - 110 3 Kaplan CM, et al. Nat Rev Neurol. 2024 20(6):347 - 363.. 4 Clauw DJ. Ann Rheum Dis. 2024 ard - 2023 - 225327. doi : 10.1136/ard - 2023 - 225327. 5 Maixner W, et al. J Pain . 2016;17(9 Suppl):T93 - T107. 6 The three drugs with FDA approval for the treatment of fibromyalgia: Pregabalin (Lyrica®); Duloxetine (Cymbalta®); Milnacipran ( Savella ®)

12 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Fibromyalgia is a Large, Underserved and Dissatisfied Population • More than 10 million U.S. adults are affected – predominantly women 1,2 ‒ Debilitating and life altering condition ‒ Significant economic impact • Patients have expressed dissatisfaction, despite three FDA approved drugs 3,4 ‒ 85% of patients fail first - line therapy 5 : efficacy variability, tolerability issues especially when used long - term and lack of broad - spectrum activity ‒ Typical for patients to rotate between drugs and be on multiple drugs at the same time; 79% of FM patients are on multiple therapies 5 • ~2.7 million FM patients diagnosed and treated 6 ‒ >22 million prescriptions are issued for the treatment of fibromyalgia (on - and off - label usage) each year 7,8 • No new R x product since 2009 • The treatment objective is to restore functionality and quality of life while avoiding intolerable side effect burden 1 American College of Rheumatology ( www.ACRPatientInfo.org accessed May 7, 2019) – prevalence rate of 2 - 4% for U.S. adult population (~250 million) 2 Vincent A, et al. Arthritis Care Res (Hoboken) . 2013 65(5):786 - 92. doi : 10.1002 ; diagnosed prevalence rate was 1.1% of adult population or 50% of the prevalent population 3 Robinson RL, et al. Pain Med . 2012 13(10):1366 - 76. doi : 10.1111; 85% received drug treatment 4 The three drugs with FDA approval for the treatment of fibromyalgia: Pregabalin (Lyrica); Duloxetine (Cymbalta); Milnacipran ( Savella ) 5 EVERSANA primary physician research, May 2024; commissioned by Tonix 6 EVERSANA analysis of claims database, May 2024; commissioned by Tonix 7 Product sales derived from IMS MIDAS; IMS NDTI used to factor usage for fibromyalgia; data accessed April 2015. 8 Market research by Frost & Sullivan, commissioned by Tonix , 2011
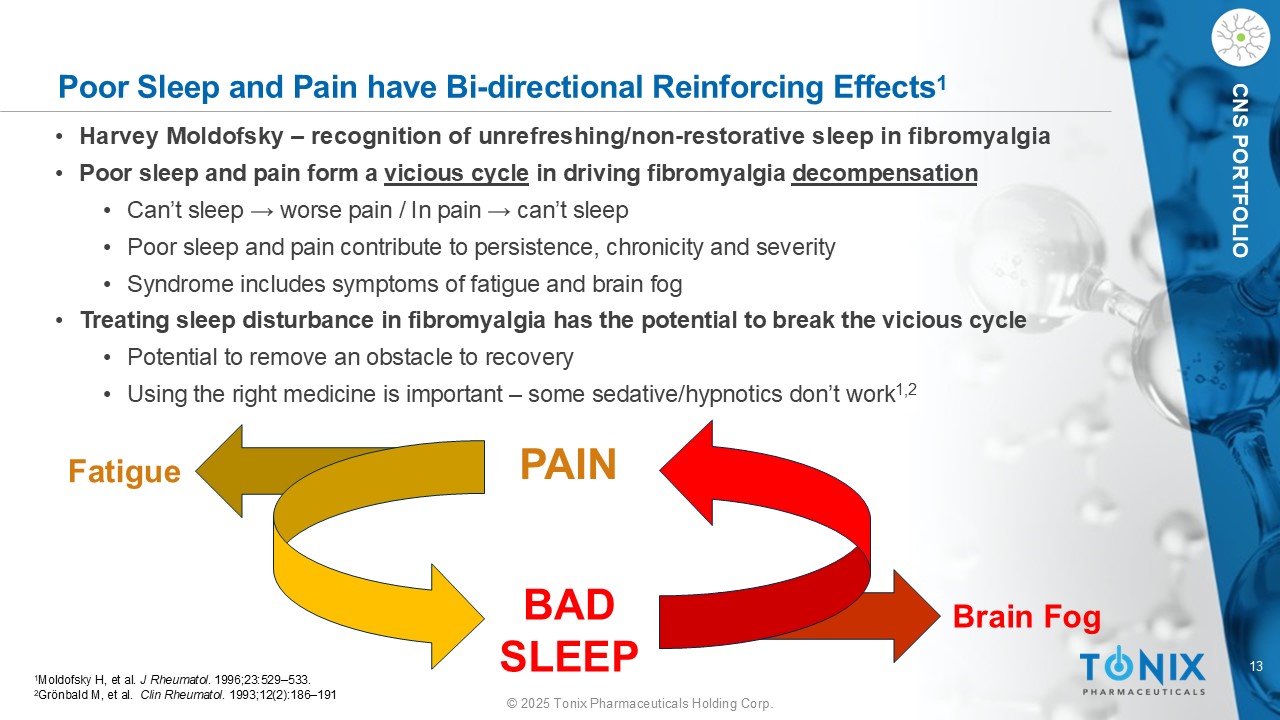
13 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Poor Sleep and Pain have Bi - directional Reinforcing Effects 1 1 Moldofsky H, et al. J Rheumatol . 1996;23:529 – 533. 2 Grönbald M, et al. Clin Rheumatol . 1993;12(2):186 – 191 • Harvey Moldofsky – recognition of unrefreshing/non - restorative sleep in fibromyalgia • Poor sleep and pain form a vicious cycle in driving fibromyalgia decompensation • Can’t sleep → worse pain / In pain → can’t sleep • Poor sleep and pain contribute to persistence, chronicity and severity • Syndrome includes symptoms of fatigue and brain fog • Treating sleep disturbance in fibromyalgia has the potential to break the vicious cycle • Potential to remove an obstacle to recovery • Using the right medicine is important – some sedative/hypnotics don’t work 1,2 PAIN BAD SLEEP Fatigue Brain Fog
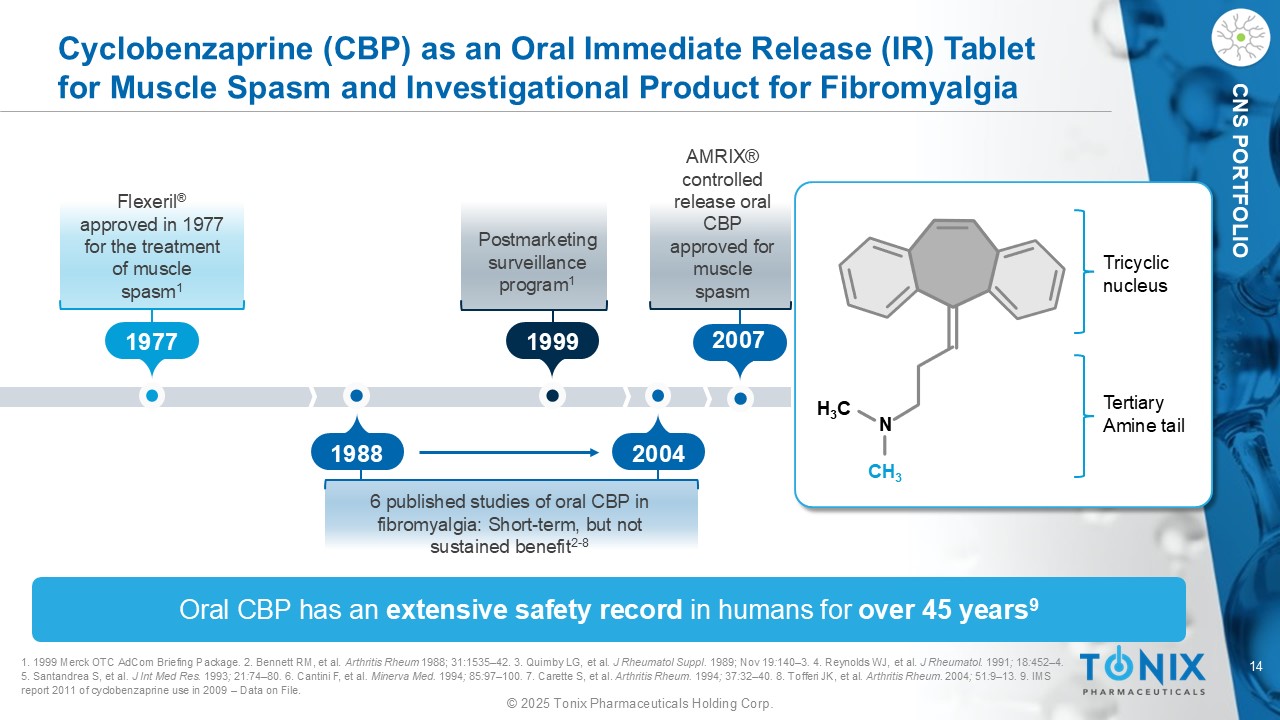
14 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Cyclobenzaprine (CBP) as an Oral Immediate Release (IR) Tablet for Muscle Spasm and Investigational Product for Fibromyalgia Oral CBP has an extensive safety record in humans for over 45 years 9 1. 1999 Merck OTC AdCom Briefing Package . 2. Bennett RM, et al. Arthritis Rheum 1988 ; 31 : 1535 – 42 . 3. Quimby LG, et al . J Rheumatol Suppl. 1989; Nov 19 : 140 – 3 . 4. Reynolds WJ, et al. J Rheumatol . 1991 ; 18 : 452 – 4 . 5. Santandrea S, et al . J Int Med Res. 1993 ; 21 : 74 – 80 . 6. Cantini F, et al . Minerva Med. 1994 ; 85 : 97 – 100 . 7. Carette S, et al. Arthritis Rheum. 1994 ; 37 : 32 – 40 . 8. Tofferi JK, et al . Arthritis Rheum. 2004 ; 51 : 9 – 13 . 9. IMS report 2011 of cyclobenzaprine use in 2009 – Data on File. 1977 1988 2004 1999 Flexeril ® approved in 1977 for the treatment of muscle spasm 1 6 published studies of oral CBP in fibromyalgia: Short - term, but not sustained benefit 2 - 8 Postmarketing surveillance program 1 H 3 C N CH 3 Tricyclic nucleus Tertiary Amine tail 2007 AMRIX® controlled release oral CBP approved for muscle spasm
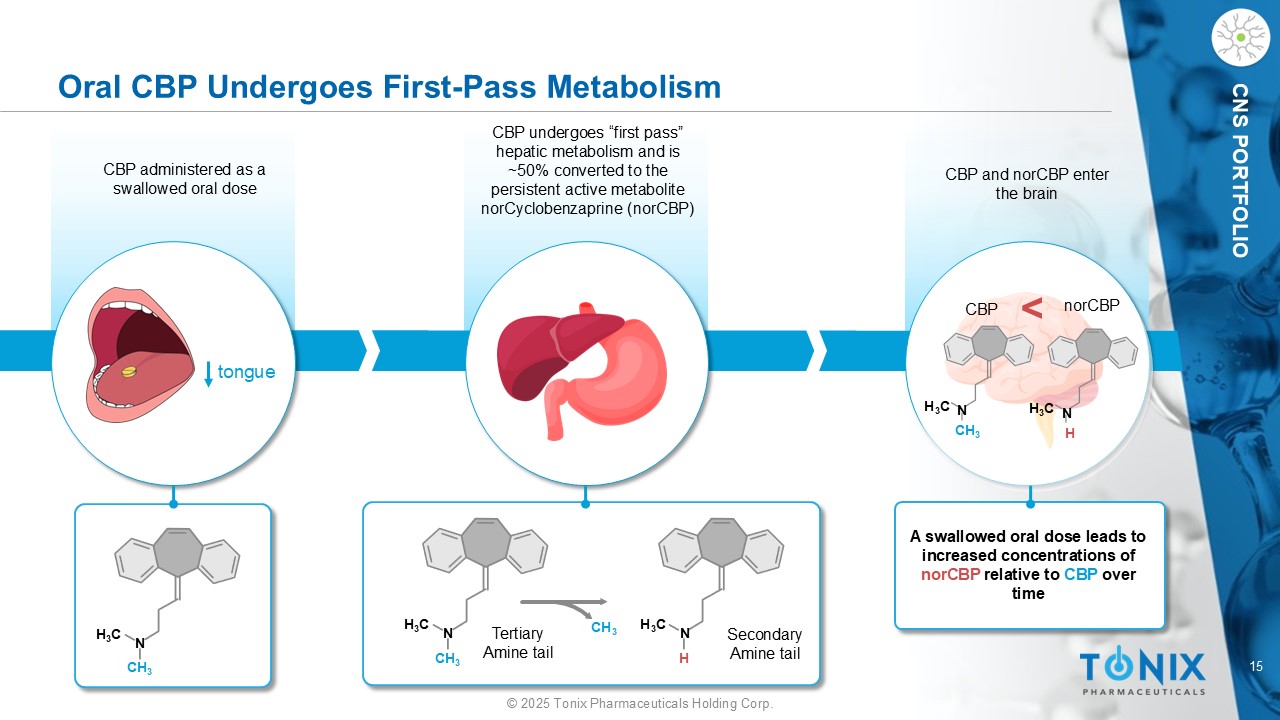
15 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO CBP and norCBP enter the brain Oral CBP Undergoes First - Pass Metabolism tongue CBP administered as a swallowed oral dose CBP undergoes “first pass” hepatic metabolism and is ~50% converted to the persistent active metabolite norCyclobenzaprine ( norCBP ) H 3 C N CH 3 H 3 C N CH 3 CH 3 H 3 C N H H 3 C N CH 3 H 3 C N H A swallowed oral dose leads to increased concentrations of norCBP relative to CBP over time < CBP norCBP Secondary Amine tail Tertiary Amine tail
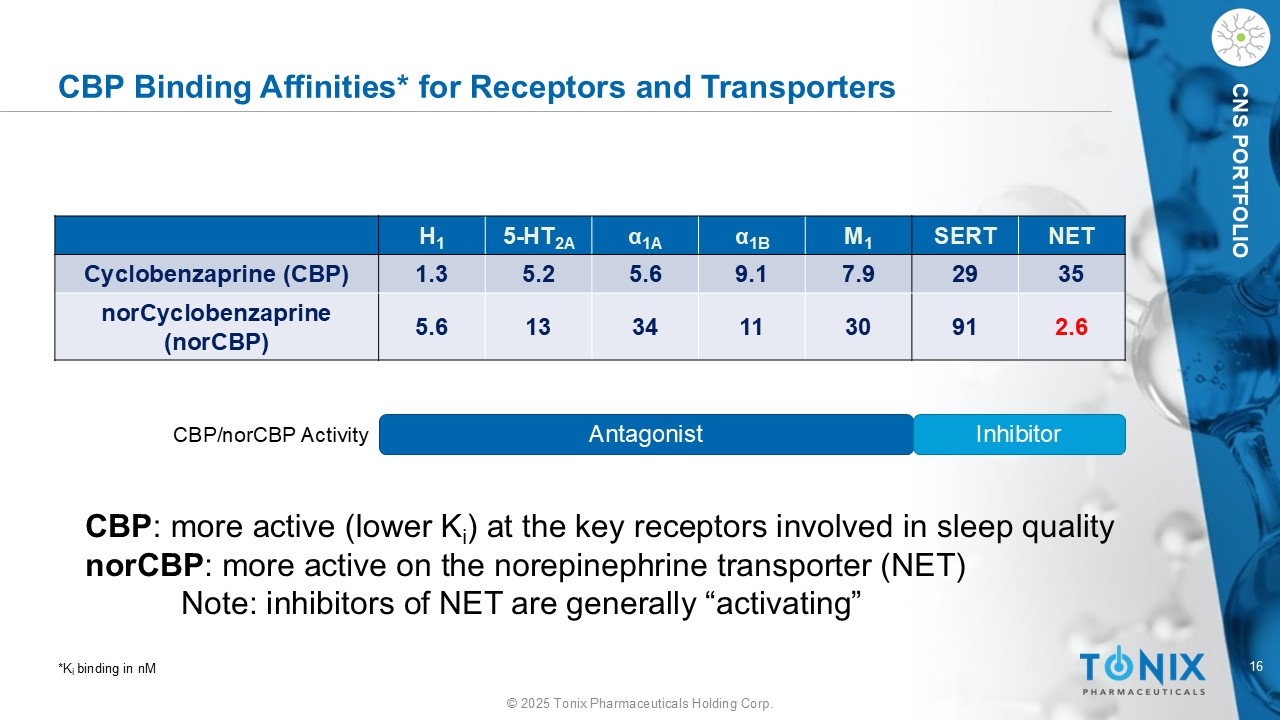
16 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO CBP Binding Affinities* for Receptors and Transporters NET SERT M 1 α 1B α 1A 5 - HT 2A H 1 35 29 7.9 9.1 5.6 5.2 1.3 Cyclobenzaprine (CBP) 2.6 91 30 11 34 13 5.6 norCyclobenzaprine ( norCBP ) Antagonist Inhibitor CBP/ norCBP Activity CBP : more active (lower K i ) at the key receptors involved in sleep quality norCBP : more active on the norepinephrine transporter (NET) Note: inhibitors of NET are generally “activating” *K i binding in nM
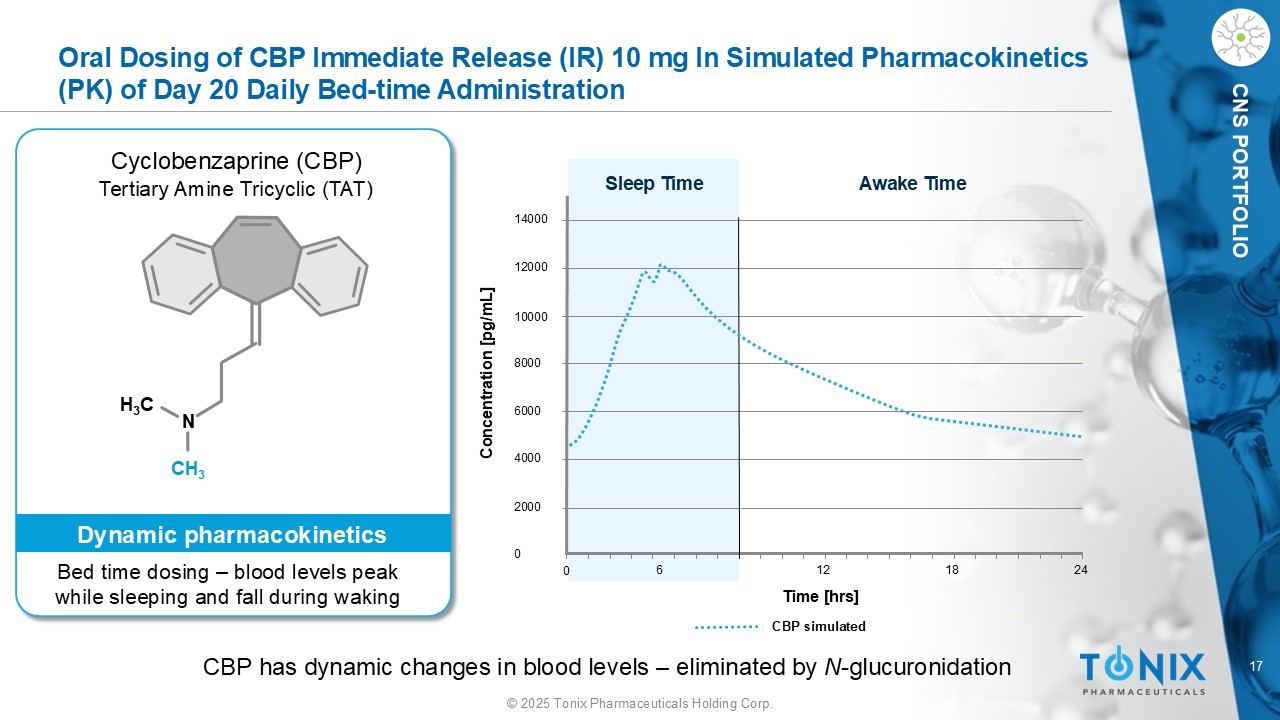
17 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Oral Dosing of CBP Immediate Release (IR) 10 mg In Simulated Pharmacokinetics (PK) of Day 20 Daily Bed - time Administration 14000 12000 10000 8000 6000 4000 2000 0 0 6 12 18 24 Sleep Time Awake Time Concentration [ pg /mL] Time [ hrs ] CBP simulated H 3 C N CH 3 Cyclobenzaprine (CBP) Tertiary Amine Tricyclic (TAT) Bed time dosing – blood levels peak while sleeping and fall during waking Dynamic pharmacokinetics CBP has dynamic changes in blood levels – eliminated by N - glucuronidation
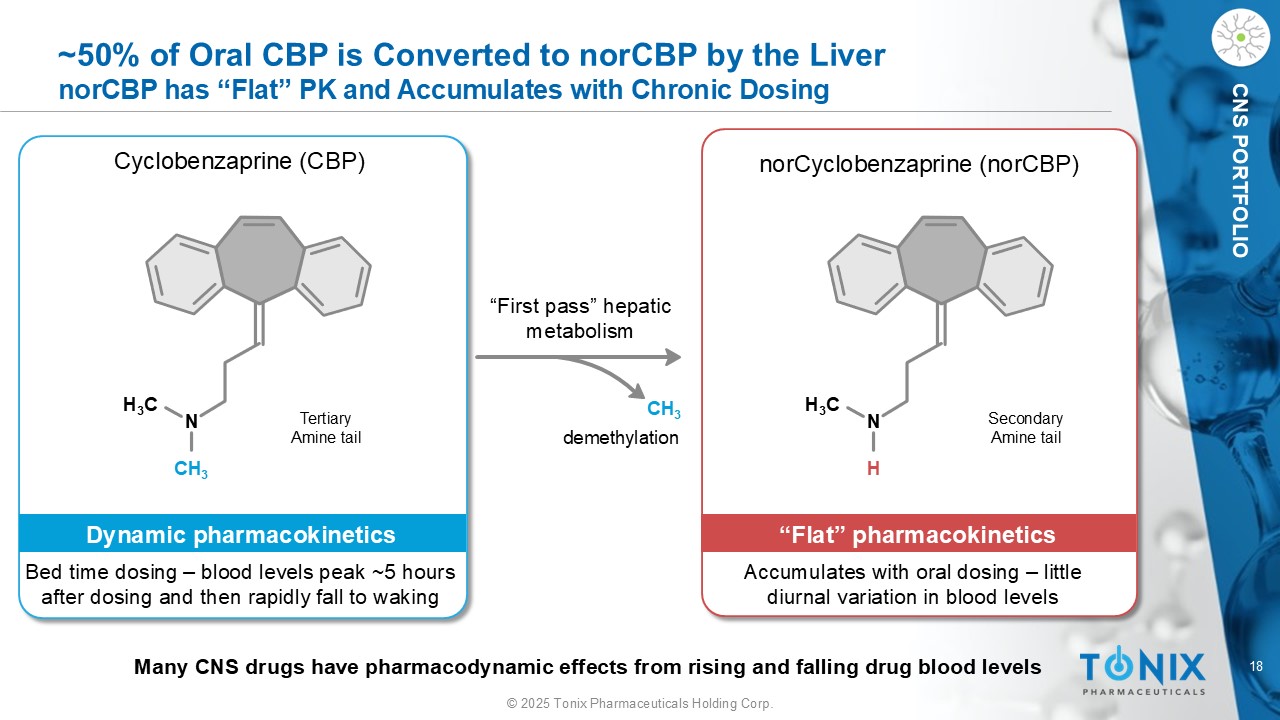
18 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO ~50% of Oral CBP is Converted to norCBP by the Liver norCBP has “Flat” PK and Accumulates with Chronic Dosing H 3 C N CH 3 Cyclobenzaprine (CBP) Bed time dosing – blood levels peak ~5 hours after dosing and then rapidly fall to waking Dynamic pharmacokinetics CH 3 H 3 C N H norCyclobenzaprine ( norCBP ) Accumulates with oral dosing – little diurnal variation in blood levels “Flat” pharmacokinetics “First pass” hepatic metabolism Many CNS drugs have pharmacodynamic effects from rising and falling drug blood levels demethylation Tertiary Amine tail Secondary Amine tail
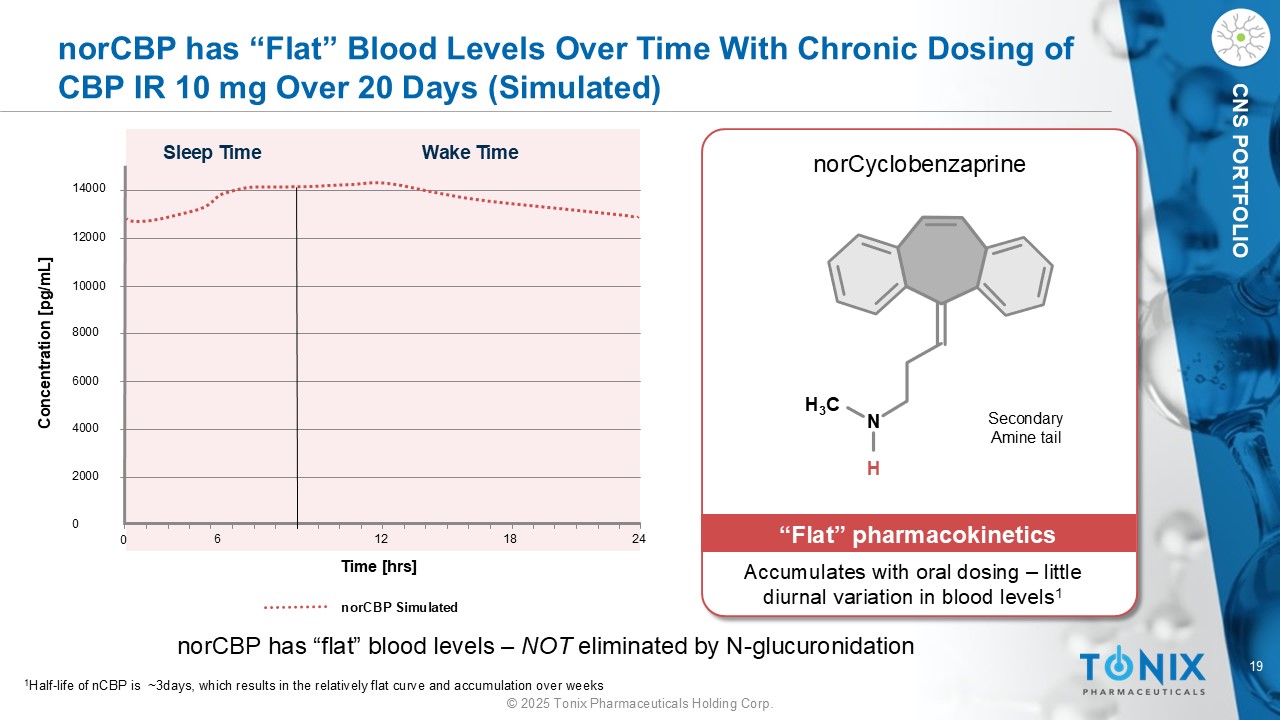
19 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO norCBP has “Flat” Blood Levels Over Time With Chronic Dosing of CBP IR 10 mg Over 20 Days (Simulated) 14000 12000 10000 8000 6000 4000 2000 0 0 6 12 18 24 Sleep Time Wake Time Concentration [ pg /mL] Time [ hrs ] n orCBP Simulated H 3 C N H norCyclobenzaprine Accumulates with oral dosing – little diurnal variation in blood levels 1 “Flat” pharmacokinetics norCBP has “flat” blood levels – NOT eliminated by N - glucuronidation 1 H alf - life of nCBP is ~3days, which results in the relatively flat curve and accumulation over weeks Secondary Amine tail
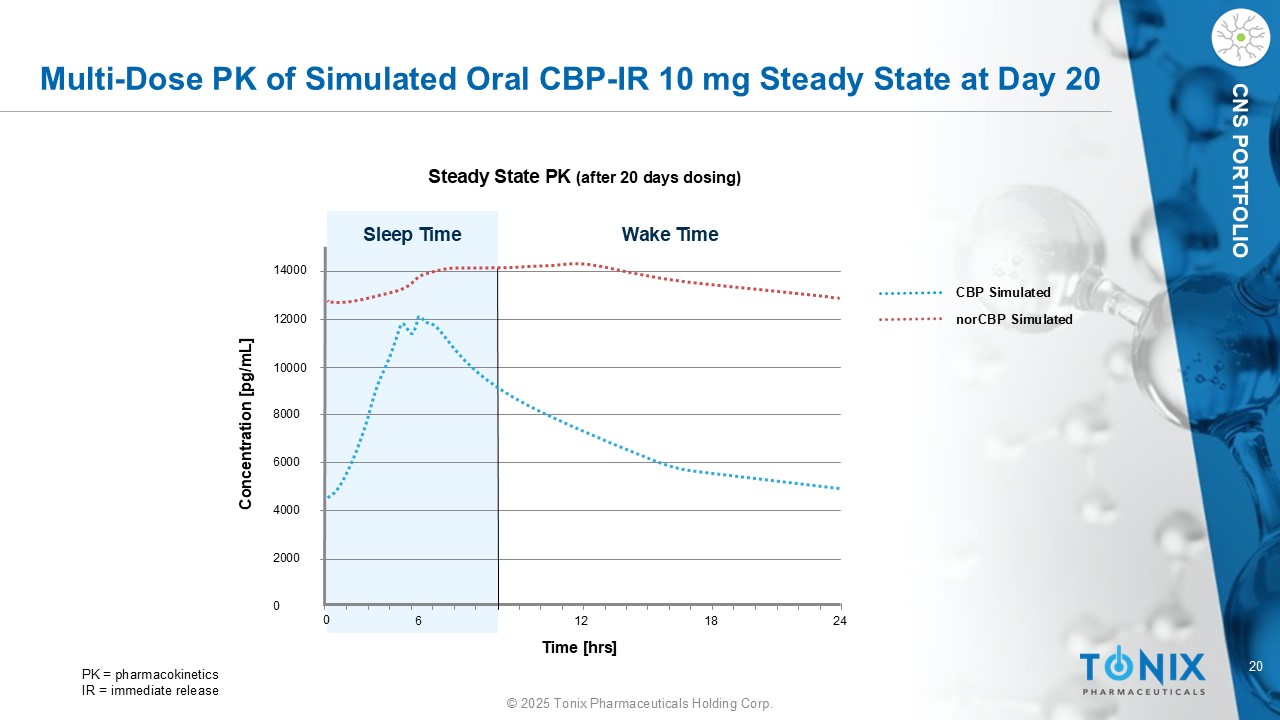
20 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Multi - Dose PK of Simulated Oral CBP - IR 10 mg Steady State at Day 20 Steady State PK (after 20 days dosing) 14000 12000 10000 8000 6000 4000 2000 0 0 6 12 18 24 Sleep Time Wake Time Concentration [ pg /mL] Time [ hrs ] CBP Simulated norCBP Simulated PK = pharmacokinetics IR = immediate release
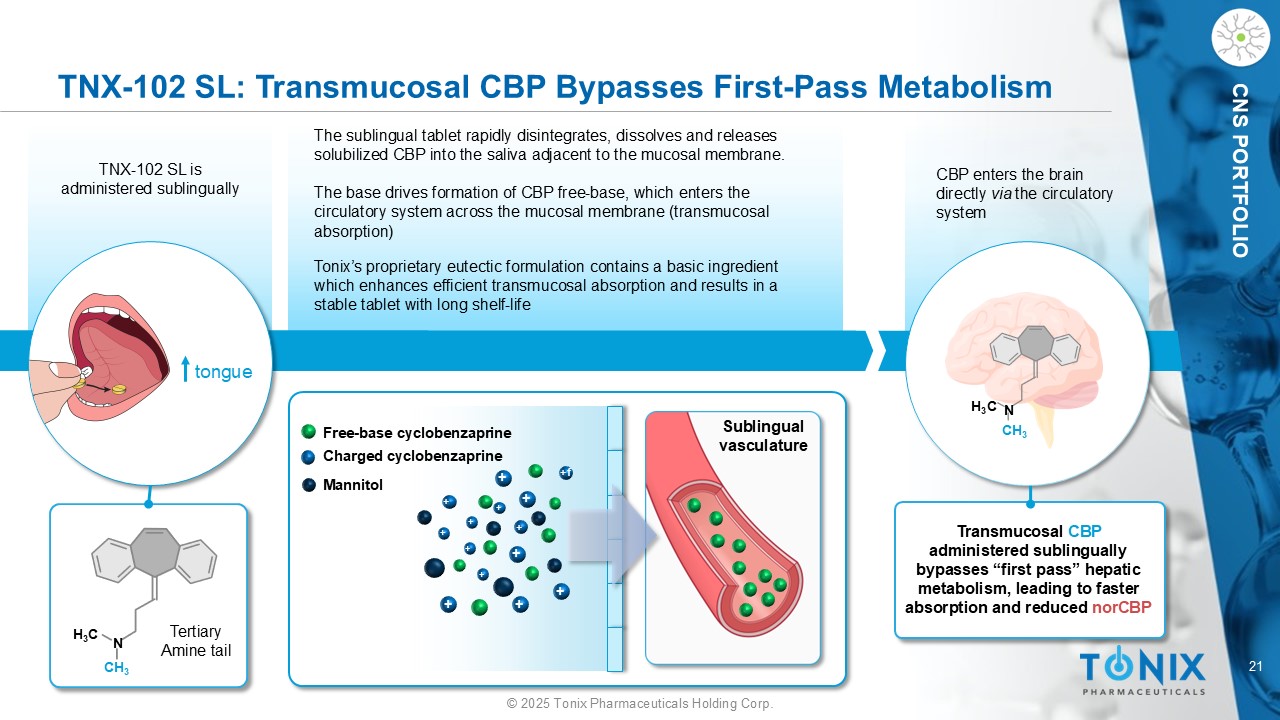
21 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO CBP enters the brain directly via the circulatory system TNX - 102 SL: Transmucosal CBP Bypasses First - Pass Metabolism tongue TNX - 102 SL is administered sublingually The sublingual tablet rapidly disintegrates, dissolves and releases solubilized CBP into the saliva adjacent to the mucosal membrane. The base drives formation of CBP free - base, which enters the circulatory system across the mucosal membrane (transmucosal absorption) H 3 C N CH 3 H 3 C N CH 3 Transmucosal CBP administered sublingually bypasses “first pass” hepatic metabolism, leading to faster absorption and reduced norCBP + + + + + + + + +f + + + + + Mannitol Free - base cyclobenzaprine Charged cyclobenzaprine Tonix’s proprietary eutectic formulation contains a basic ingredient which enhances efficient transmucosal absorption and results in a stable tablet with long shelf - life Sublingual vasculature Tertiary Amine tail
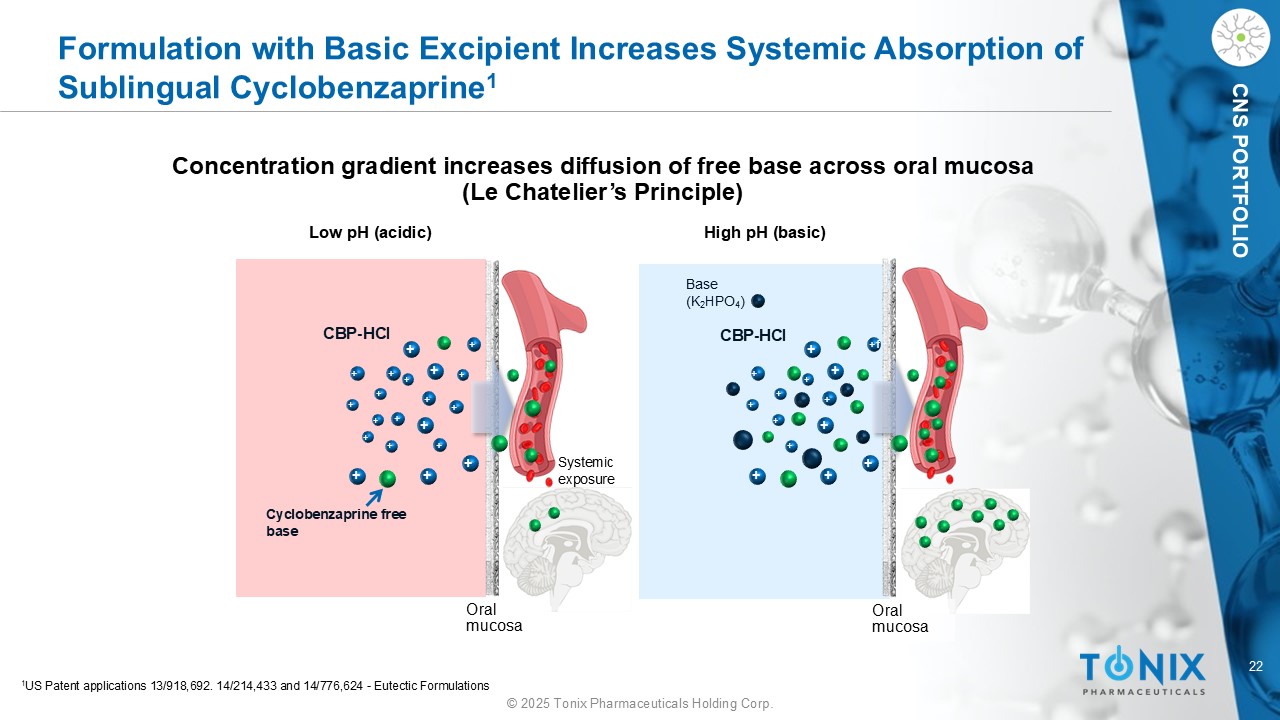
22 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Formulation with Basic Excipient Increases Systemic Absorption of Sublingual Cyclobenzaprine 1 Concentration gradient increases diffusion of free base across oral mucosa (Le Chatelier’s Principle) CBP - HCl Cyclobenzaprine free base Oral mucosa CBP - HCl Low pH (acidic) High pH (basic) Systemic exposure Base (K 2 HPO 4 ) + + + + + + + + + + + + + + + + + + + + + + + + + + +f + + + + + + + 1 US Patent applications 13/918,692. 14/214,433 and 14/776,624 - Eutectic Formulations Oral mucosa
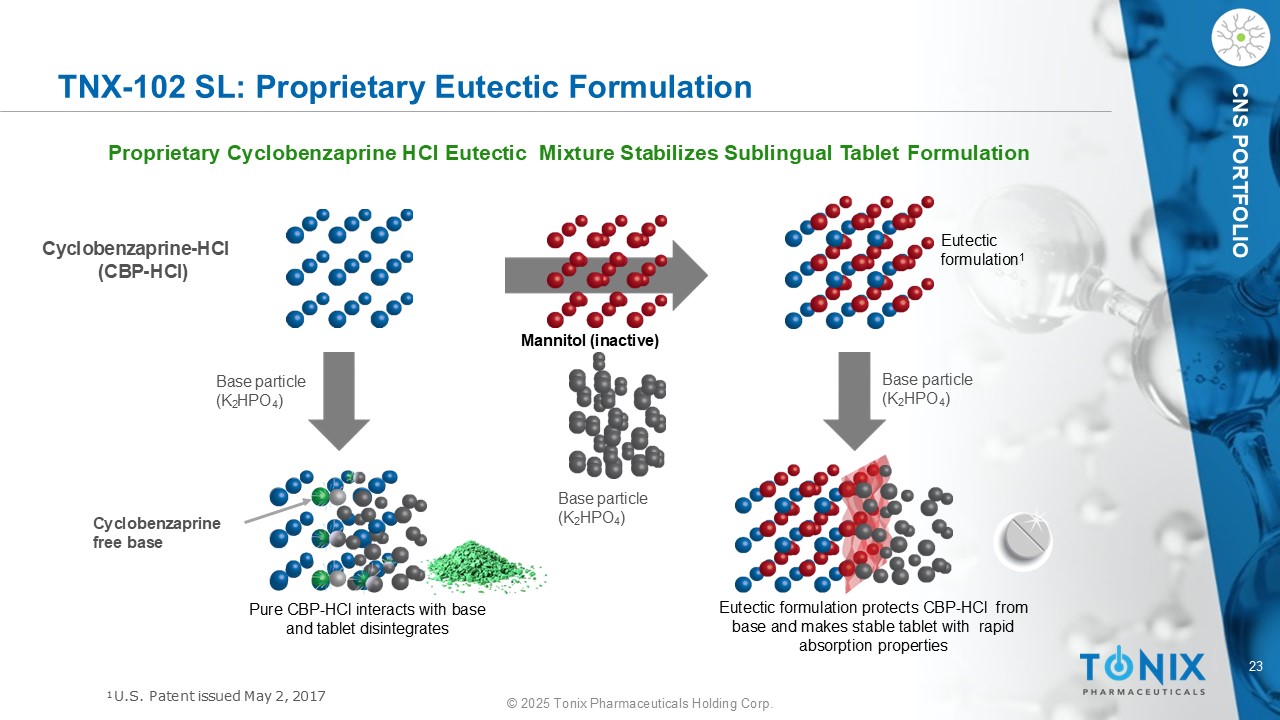
23 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 102 SL: Proprietary Eutectic Formulation Proprietary Cyclobenzaprine HCl Eutectic Mixture Stabilizes Sublingual Tablet Formulation Base particle (K 2 HPO 4 ) Base particle (K 2 HPO 4 ) Base particle (K 2 HPO 4 ) C y cl o be n z a p r i n e - HCl (CBP - HCl) Eutectic formulation protects CBP - HCl from base and makes stable tablet with rapid absorption properties Pure CBP - HCl interacts with base and tablet disintegrates Cy c l ob en zapr ine free base Eutectic formulation 1 Mannitol (inactive) 1 U.S. Patent issued May 2, 2017
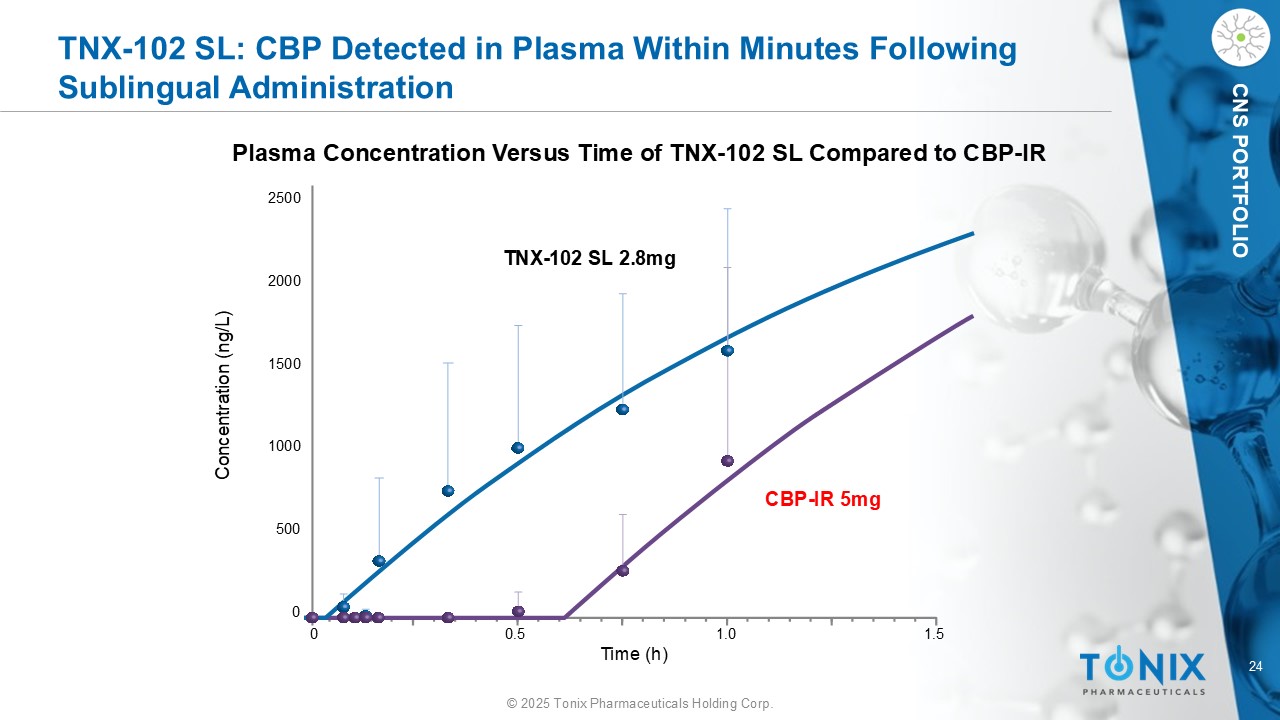
24 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 102 SL: CBP Detected in Plasma Within Minutes Following Sublingual Administration Plasma Concentration Versus Time of TNX - 102 SL Compared to CBP - IR 0 0.5 1.0 1.5 Concentration (ng/L) 2500 1500 1000 500 2000 0 TNX - 102 SL 2.8mg CBP - IR 5mg Time (h)
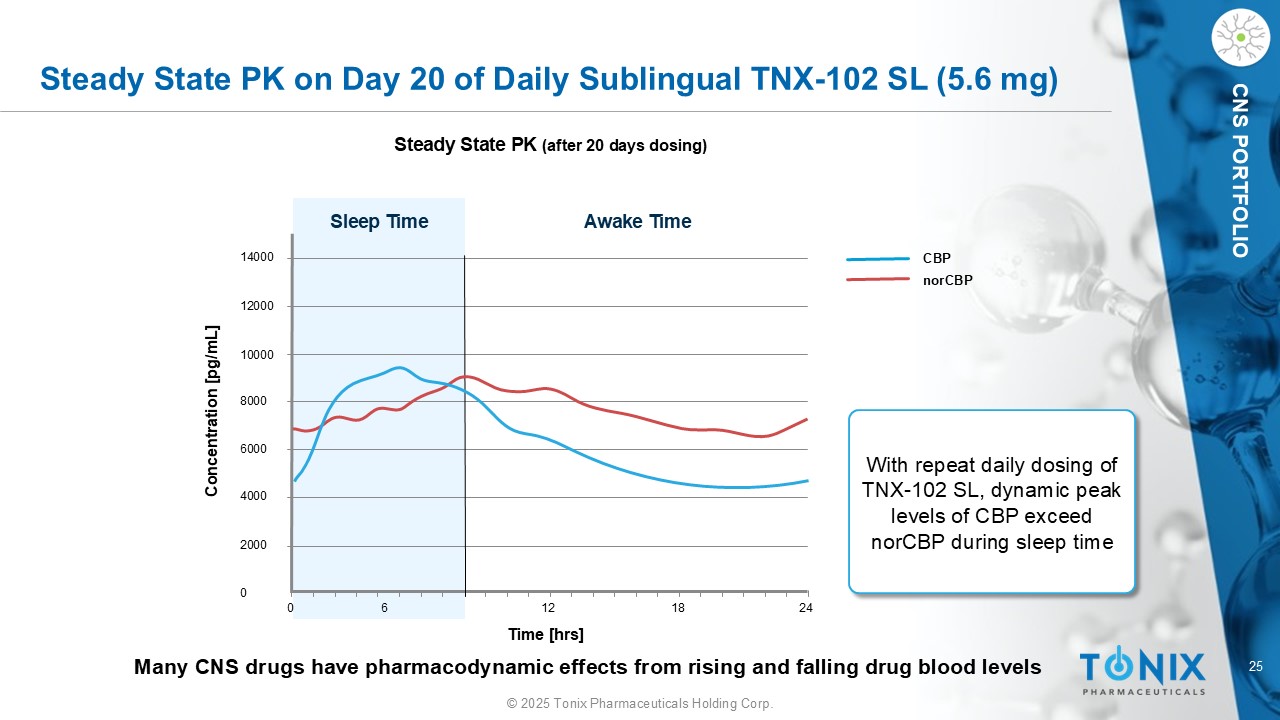
25 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Steady State PK on Day 20 of Daily Sublingual TNX - 102 SL (5.6 mg) Steady State PK (after 20 days dosing) 14000 12000 10000 8000 6000 4000 2000 0 0 6 12 18 24 Sleep Time Awake Time Concentration [ pg /mL] Time [ hrs ] CBP norCBP With repeat daily dosing of TNX - 102 SL, dynamic peak levels of CBP exceed norCBP during sleep time Many CNS drugs have pharmacodynamic effects from rising and falling drug blood levels
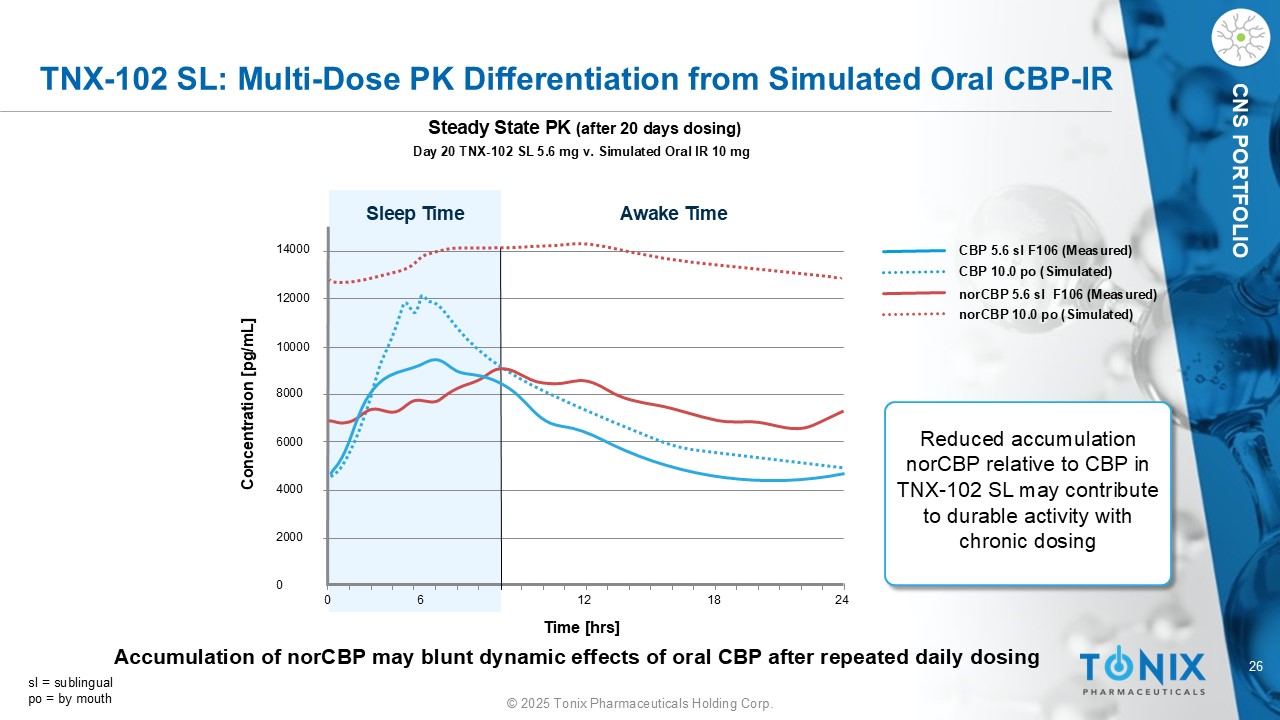
26 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 102 SL: Multi - Dose PK Differentiation from Simulated Oral CBP - IR Day 20 TNX - 102 SL 5.6 mg v. Simulated Oral IR 10 mg Steady State PK (after 20 days dosing) 14000 12000 10000 8000 6000 4000 2000 0 0 6 12 18 24 Sleep Time Awake Time Concentration [ pg /mL] Time [ hrs ] CBP 5.6 sl F106 (Measured) CBP 10.0 po (Simulated) norCBP 5.6 sl F106 (Measured) norCBP 10.0 po (Simulated) Reduced accumulation norCBP relative to CBP in TNX - 102 SL may contribute to durable activity with chronic dosing sl = sublingual po = by mouth Accumulation of norCBP may blunt dynamic effects of oral CBP after repeated daily dosing
27 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 102 SL: Sublingual Formulation is Designed for Long - Term Daily Administration at Bedtime and Transmucosal Absorption • Cyclobenzaprine (CBP) - Tertiary Amine Tricyclic (TAT) ‒ Dynamic pharmacokinetics (PK) ▪ Elimination by N - glucuronidation • Oral administration results in f irst - pass metabolism ‒ Generation of active metabolite, norCBP • NorCyclobenzaprine ( norCBP ) – Secondary Amine Tricyclic (SAT) ‒ Flat pharmacokinetics (PK) ▪ No elimination by N - glucuronidation • TNX - 102 SL delivers CBP by transmucosal absorption and is designed to bypass first - pass hepatic metabolism and lower norCBP accumulation ‒ Provides rapid absorption for bedtime dosing
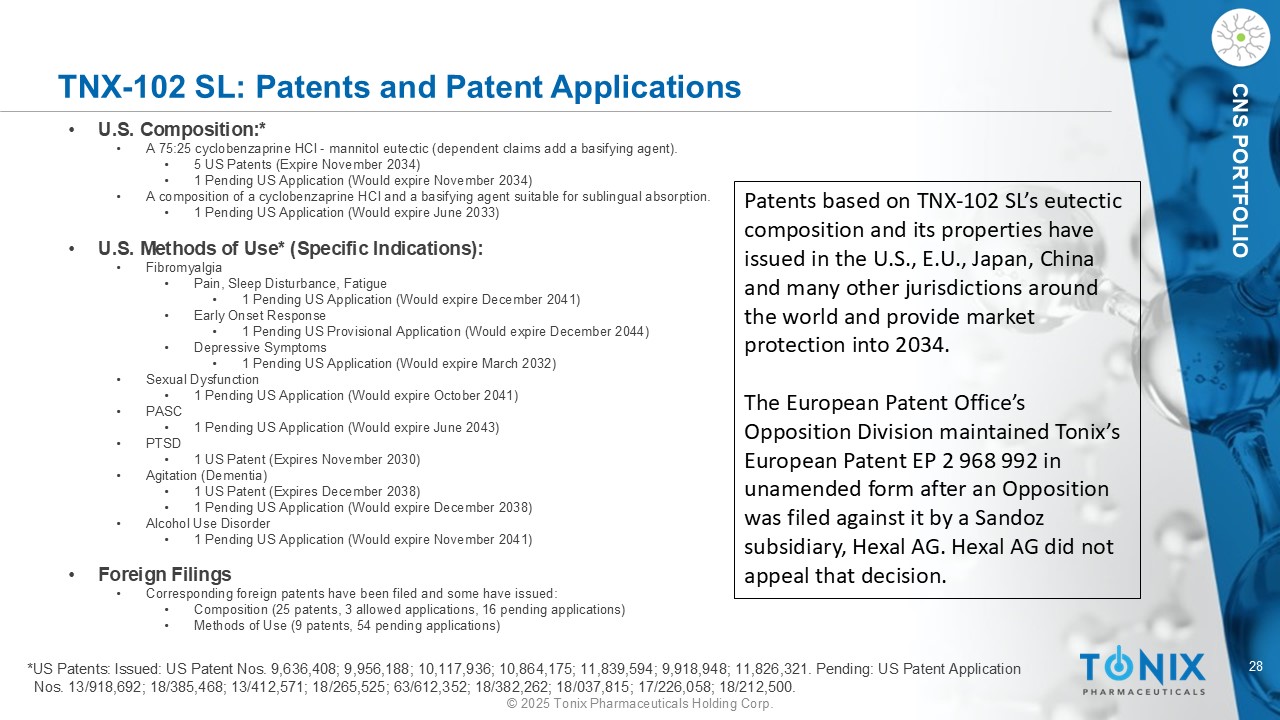
28 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 102 SL: Patents and Patent Applications • U.S. Composition:* • A 75:25 cyclobenzaprine HCl - mannitol eutectic (dependent claims add a basifying agent). • 5 US Patents (Expire November 2034) • 1 Pending US Application (Would expire November 2034) • A composition of a cyclobenzaprine HCl and a basifying agent suitable for sublingual absorption. • 1 Pending US Application (Would expire June 2033) • U.S. Methods of Use* (Specific Indications): • Fibromyalgia • Pain, Sleep Disturbance, Fatigue • 1 Pending US Application (Would expire December 2041) • Early Onset Response • 1 Pending US Provisional Application (Would expire December 2044) • Depressive Symptoms • 1 Pending US Application (Would expire March 2032) • Sexual Dysfunction • 1 Pending US Application (Would expire October 2041) • PASC • 1 Pending US Application (Would expire June 2043) • PTSD • 1 US Patent (Expires November 2030) • Agitation (Dementia) • 1 US Patent (Expires December 2038) • 1 Pending US Application (Would expire December 2038) • Alcohol Use Disorder • 1 Pending US Application (Would expire November 2041) • Foreign Filings • Corresponding foreign patents have been filed and some have issued: • Composition (25 patents, 3 allowed applications, 16 pending applications) • Methods of Use (9 patents, 54 pending applications) *US Patents: Issued: US Patent Nos. 9,636,408; 9,956,188; 10,117,936; 10,864,175; 11,839,594; 9,918,948; 11,826,321. Pending: US Patent Application Nos. 13/918,692; 18/385,468; 13/412,571; 18/265,525; 63/612,352; 18/382,262; 18/037,815; 17/226,058; 18/212,500. Patents based on TNX - 102 SL’s eutectic composition and its properties have issued in the U.S., E.U., Japan, China and many other jurisdictions around the world and provide market protection into 2034. The European Patent Office’s Opposition Division maintained Tonix’s European Patent EP 2 968 992 in unamended form after an Opposition was filed against it by a Sandoz subsidiary, Hexal AG. Hexal AG did not appeal that decision.
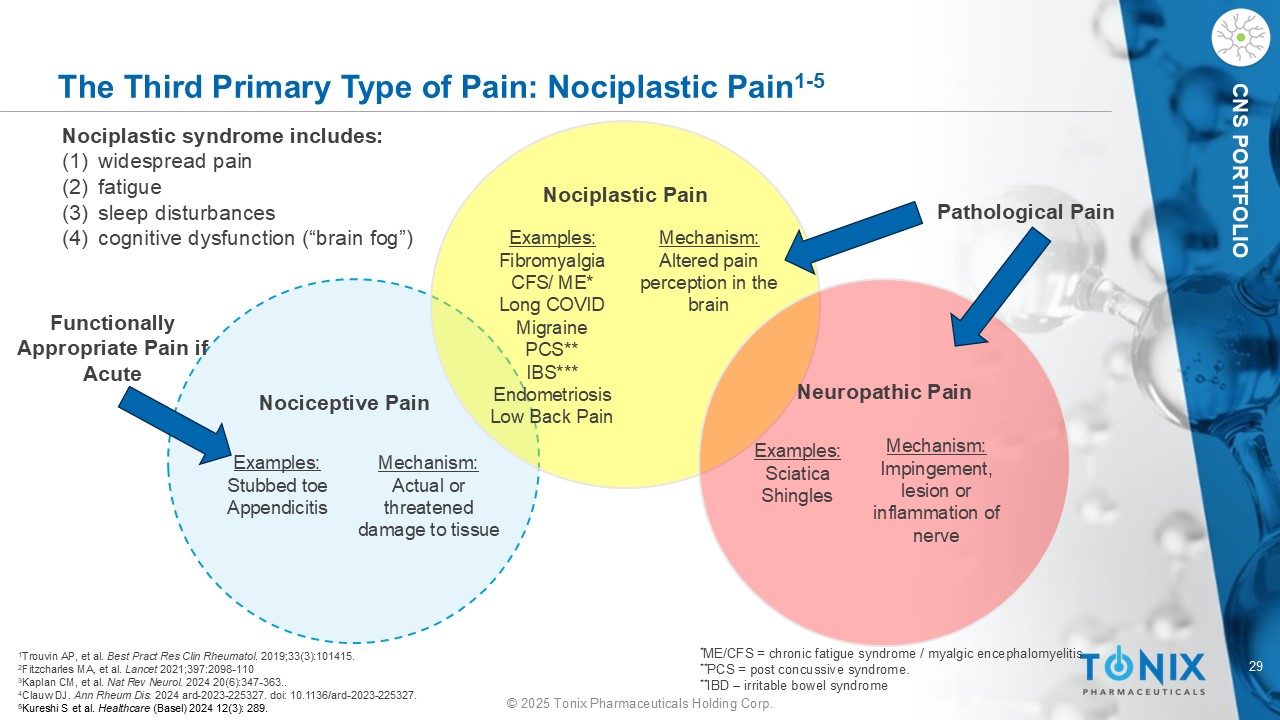
29 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO The Third Primary Type of Pain: Nociplastic Pain 1 - 5 Nociplastic syndrome includes: (1) widespread pain (2) fatigue (3) sleep disturbances (4) cognitive dysfunction (“brain fog”) Nociplastic Pain Examples: Fibromyalgia CFS / ME* Long COVID Migraine PCS** IBS*** Endometriosis Low Back Pain Examples: Stubbed toe Appendicitis Examples: Sciatica Shingles Mechanism: Altered pain perception in the brain Mechanism: Impingement, lesion or inflammation of nerve Mechanism: Actual or threatened damage to tissue Nociceptive Pain Neuropathic Pain Pathological Pain Functionally Appropriate Pain if Acute 1 Trouvin AP, et al. Best Pract Res Clin Rheumatol . 2019;33(3):101415. 2 Fitzcharles MA, et al. Lancet 2021;397:2098 - 110 3 Kaplan CM, et al. Nat Rev Neurol. 2024 20(6):347 - 363.. 4 Clauw DJ. Ann Rheum Dis. 2024 ard - 2023 - 225327. doi : 10.1136/ard - 2023 - 225327. 5 Kureshi S et al. Healthcare (Basel) 2024 12(3): 289. * ME/CFS = chronic fatigue syndrome / myalgic encephalomyelitis ** PCS = post concussive syndrome. ** IBD – irritable bowel syndrome

30 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Fibromyalgia is Believed to Result from Chronic Pain or Prior Stress Experiences Stresses that may precede or precipitate FM include : Chronic nociceptive pain • e.g. , osteoarthritis Chronic neuropathic pain • e.g. , diabetic neuropathy Infectious • e.g. , viral illness Cancer • e.g. , breast cancer Chemical • e.g., cancer chemotherapy Traumatic • e.g. , motor vehicle accident Head trauma • e.g. , post - concussive syndrome Physiologic • e.g. , disturbed sleep The pain system evolved to detect acute pain • The body’s “check engine” light Chronic pain breaks down the system that determines whether a sensory experience is painful • Chronic pain results in nociplastic syndromes • Nociplastic syndrome was formerly known as “Central and Peripheral Sensitization” Chronic Overlapping Pain Conditions (COPCs) are Nociplastic Syndromes : • Fibromyalgia • ME/CFS • Migraine • Irritable Bowel Syndrome • Endometriosis • Low Back Pain

31 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Common Chronic Conditions are a Challenge for Pharma Fibromyalgia is a common chronic disease 1 • Chronic pain syndrome that persists for years or decades No animal model is recognized for nociplastic syndromes or its component symptoms • Widespread pain • Fatigue • Sleep disturbance • Cognitive impairment Nociplastic symptoms are subjective • Humans need to report symptoms using scales Clinical trials measuring subjective symptoms are challenging • Placebo response is typically observed • Long - term therapy means requires long - term tolerability 1 The U.S. Centers for Disease Control defines chronic diseases as “conditions that last 1 year or more and require ongoing med ica l attention or limit activities of daily living or both.” www.cdc.gov/chronicdisease/about/index.htm . (accessed Jan 28, 2024)

32 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Common Chronic Conditions are a Challenge for Society The Opioid Crisis in the U.S. was driven by mistreatment of chronic pain, which was often nociplastic pain • The e pidemic of prescription pain killers was addressed by regulations which limited the availability of opioids • Many individuals who are opioid dependent have transitioned to illegal street heroin and fentanyl • Illegal drugs contribute to homelessness There is an unmet need for non - opioid analgesics that address nociplastic pain • No new drug for fibromyalgia has been approved since 2009
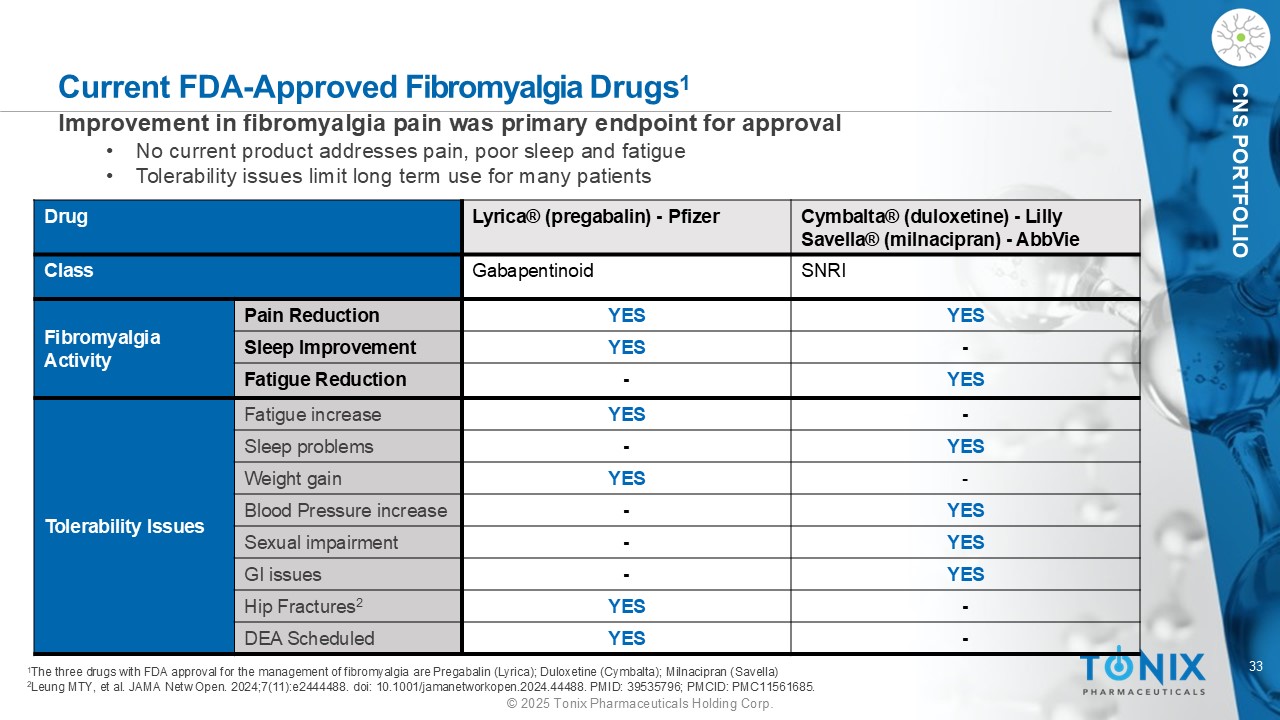
33 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Current FDA - Approved Fibromyalgia Drugs 1 1 The three drugs with FDA approval for the management of fibromyalgia are Pregabalin (Lyrica); Duloxetine (Cymbalta); Milnacipran ( Savella ) 2 Leung MTY, et al. JAMA Netw Open. 2024;7(11):e2444488. doi : 10.1001/jamanetworkopen.2024.44488. PMID: 39535796; PMCID: PMC11561685. Cymbalta® (duloxetine) - Lilly Savella ® (milnacipran) - AbbVie Lyrica® (pregabalin) - Pfizer Drug SNRI Gabapentinoid Class YES YES Pain Reduction Fibromyalgia Activity - YES Sleep Improvement YES - Fatigue Reduction - YES Fatigue increase Tolerability Issues YES - Sleep problems - YES Weight gain YES - Blood Pressure increase YES - Sexual impairment YES - GI issues - YES Hip Fractures 2 - YES DEA Scheduled Improvement in fibromyalgia pain was primary endpoint for approval • No current product addresses pain, poor sleep and fatigue • Tolerability issues limit long term use for many patients

34 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO ~50% of U.S. Fibromyalgia Patients are on Medicare: Prescription Coverage in Medicare Stands to Benefit from Changes in IRA Approximately 50% of fibromyalgia patients are on Medicare ‒ EVERSANA analysis of claims database consisting of 2.2M patients, for which 1.2M claims were submitted throughout March 2002 - March 2023 1 Beneficial Changes in 2025 to Medicare Part D through Inflation Reduction Act (IRA) 2 ‒ Medicare prescription payment plan to offer enrollees the option to pay out - of - pocket prescription drug costs in the form of capped monthly installment payments instead of all at once at the pharmacy ‒ Annual out - of - pocket costs will be capped at $2,000 in year 2025 ‒ Will replace the existing Coverage Gap Discount Program, which sunsets as of January 1, 2025 Fibromyalgia Patients by Coverage 1 1 EVERSANA analysis of claims database, May 2024; commissioned by Tonix 2 Source: Final CY 2025 Part D Redesign Program Instructions Fact Sheet | CMS
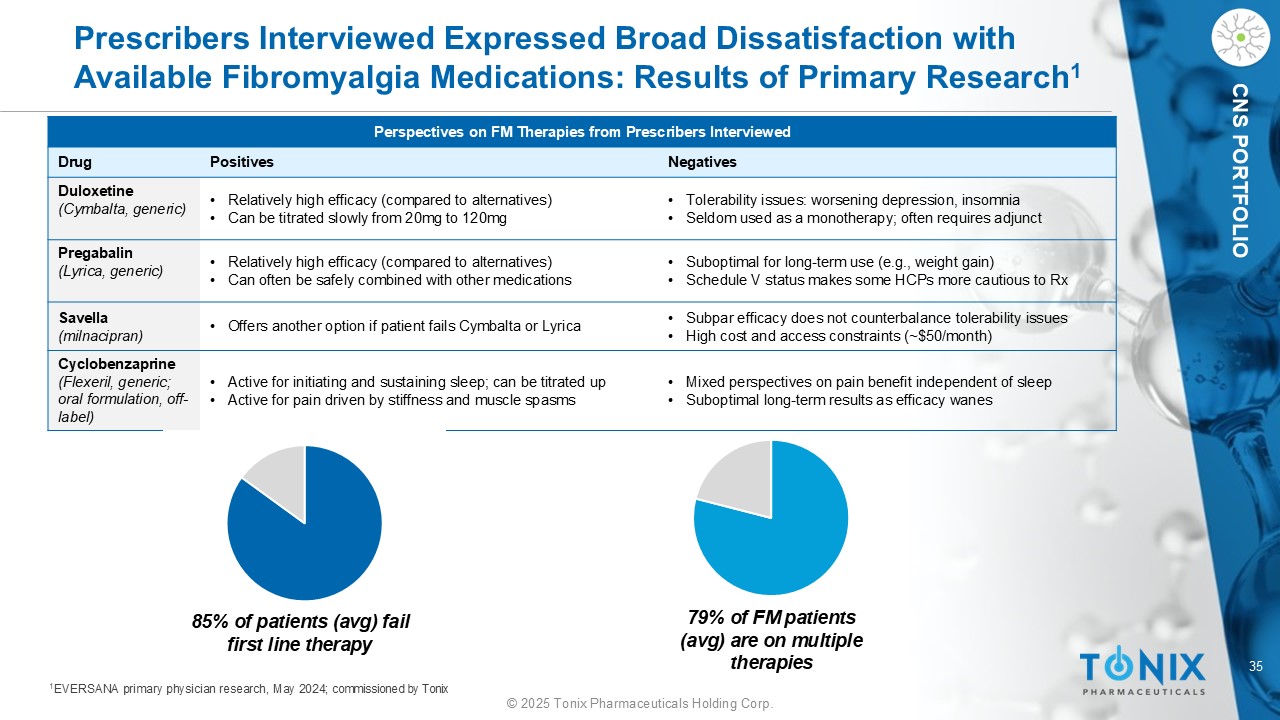
35 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Prescribers Interviewed Expressed Broad Dissatisfaction with Available Fibromyalgia Medications: Results of Primary Research 1 Perspectives on FM Therapies from Prescribers Interviewed Negatives Positives Drug • Tolerability issues: worsening depression, insomnia • Seldom used as a monotherapy; often requires adjunct • Relatively high efficacy (compared to alternatives) • Can be titrated slowly from 20mg to 120mg Duloxetine (Cymbalta, generic) • Suboptimal for long - term use (e.g., weight gain) • Schedule V status makes some HCPs more cautious to Rx • Relatively high efficacy (compared to alternatives) • Can often be safely combined with other medications Pregabalin (Lyrica, generic) • Subpar efficacy does not counterbalance tolerability issues • High cost and access constraints (~$50/month) • Offers another option if patient fails Cymbalta or Lyrica Savella (milnacipran) • Mixed perspectives on pain benefit independent of sleep • Suboptimal long - term results as efficacy wanes • Active for initiating and sustaining sleep; can be titrated up • Active for pain driven by stiffness and muscle spasms Cyclobenzaprine (Flexeril, generic; oral formulation, off - label) 85% of patients (avg) fail first line therapy 79% of FM patients (avg) are on multiple therapies 1 EVERSANA primary physician research, May 2024; commissioned by Tonix
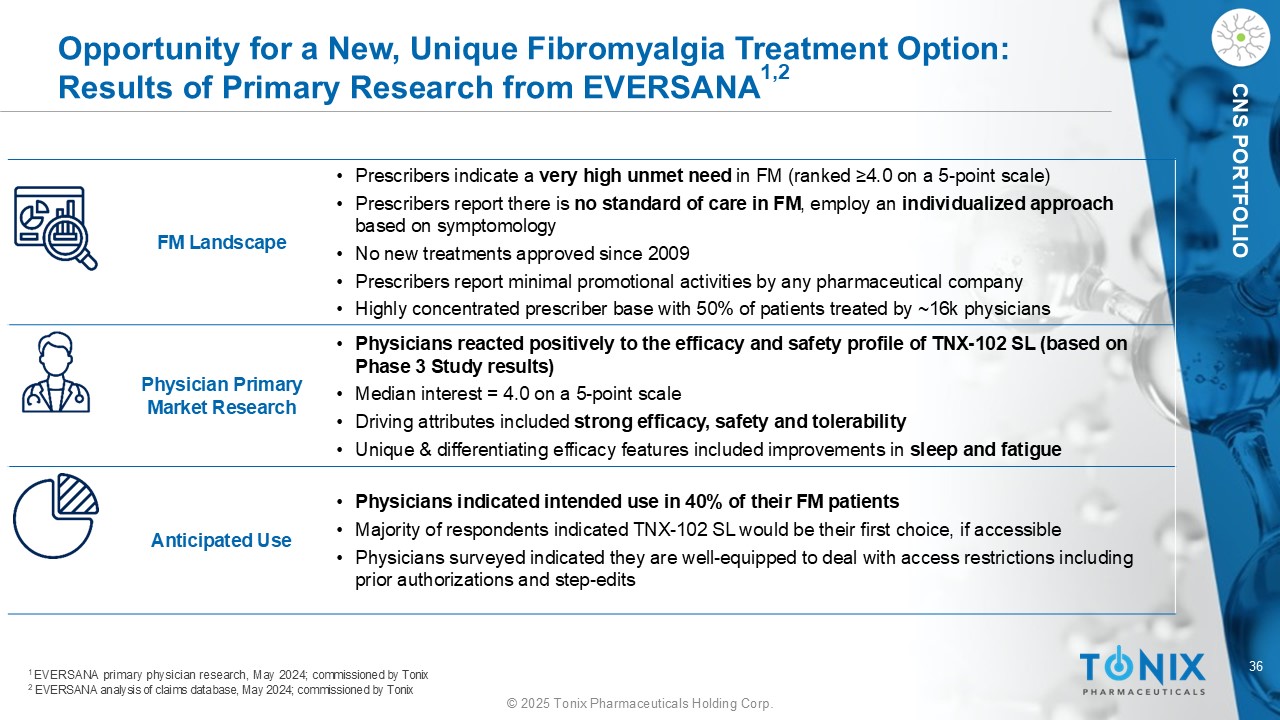
36 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Opportunity for a New, Unique Fibromyalgia Treatment Option: Results of Primary Research from EVERSANA 1,2 • Prescribers indicate a very high unmet need in FM (ranked ≥4.0 on a 5 - point scale) • Prescribers report there is no standard of care in FM , employ an individualized approach based on symptomology • No new treatments approved since 2009 • Prescribers report minimal promotional activities by any pharmaceutical company • Highly concentrated prescriber base with 50% of patients treated by ~16k physicians FM Landscape • Physicians reacted positively to the efficacy and safety profile of TNX - 102 SL (based on Phase 3 Study results) • Median interest = 4.0 on a 5 - point scale • Driving attributes included strong efficacy, safety and tolerability • Unique & differentiating efficacy features included improvements in sleep and fatigue Physician Primary Market Research • Physicians indicated intended use in 40% of their FM patients • Majority of respondents indicated TNX - 102 SL would be their first choice, if accessible • Physicians surveyed indicated they are well - equipped to deal with access restrictions including prior authorizations and step - edits Anticipated Use 1 EVERSANA primary physician research, May 2024; commissioned by Tonix 2 EVERSANA analysis of claims database, May 2024; commissioned by Tonix
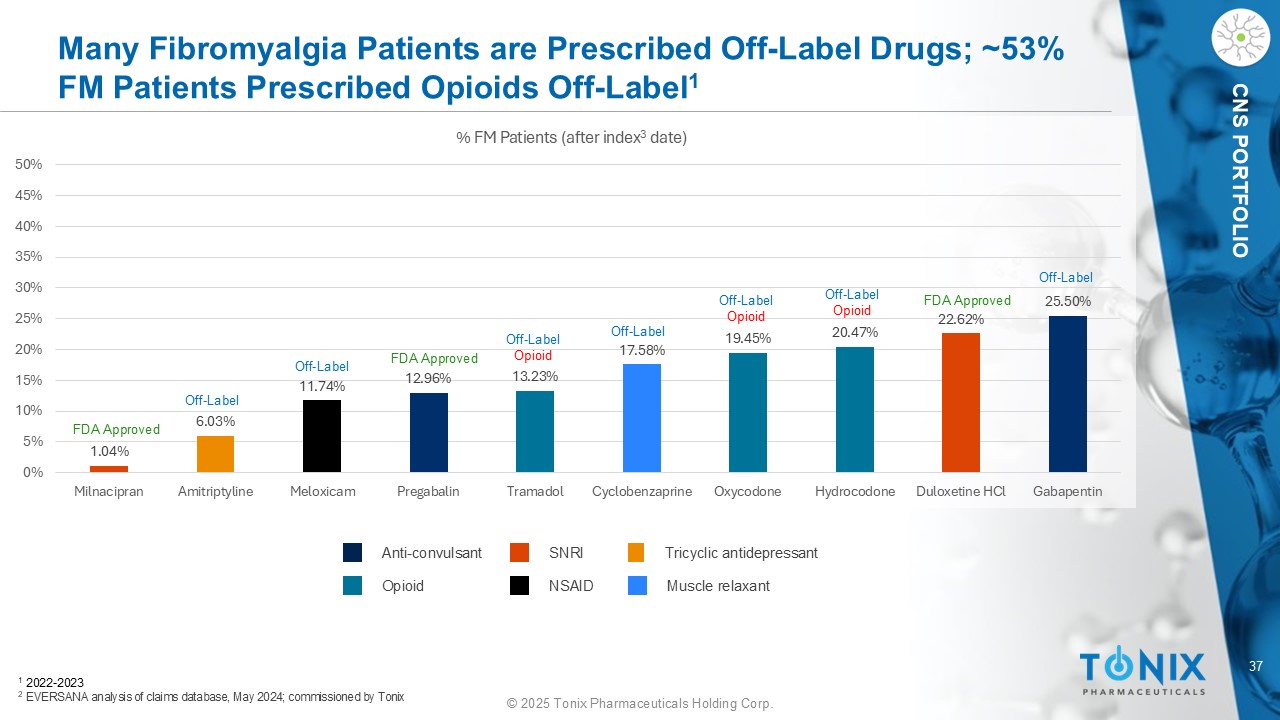
37 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Many Fibromyalgia Patients are Prescribed Off - Label Drugs; ~53% FM Patients Prescribed Opioids Off - Label 1 1.04% 6.03% 11.74% 12.96% 13.23% 17.58% 19.45% 20.47% 22.62% 25.50% 0% 5% 10% 15% 20% 25% 30% 35% 40% 45% 50% Milnacipran Amitriptyline Meloxicam Pregabalin Tramadol Cyclobenzaprine Oxycodone Hydrocodone Duloxetine HCl Gabapentin % FM Patients (after index 3 date) 1 2022 - 2023 2 EVERSANA analysis of claims database, May 2024; commissioned by Tonix Anti - convulsant SNRI Opioid Tricyclic antidepressant NSAID Muscle relaxant FDA Approved Off - Label Off - Label FDA Approved Off - Label Opioid Off - Label Off - Label Opioid Off - Label Opioid FDA Approved Off - Label

38 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO CNS PORTFOLIO Acute Stress Reaction (ASR) / Acute Stress Disorder (ASD) ASR/ASD are acute stress conditions resulting from trauma which c an affect both civilian and military populations. Large unmet need: • According to National Center for PTSD, about 60% of men and 50% of women in the US are exposed least one traumatic experience in their lives 1 • In the US alone, one - third of emergency department visits (40 - 50 million patients per year) are for evaluation after trauma exposures 2 Current standard of care: • No medications are currently available at or near the point of care to treat patients suffering from acute traumatic events and support long - term health 1 National Center for PTSD. How Common is PTSD in Adults? https://www.ptsd.va.gov/understand/common/common_adults.asp 2 Wisco et al. J Clin Psychiatry . 2014.75(12):1338 - 46
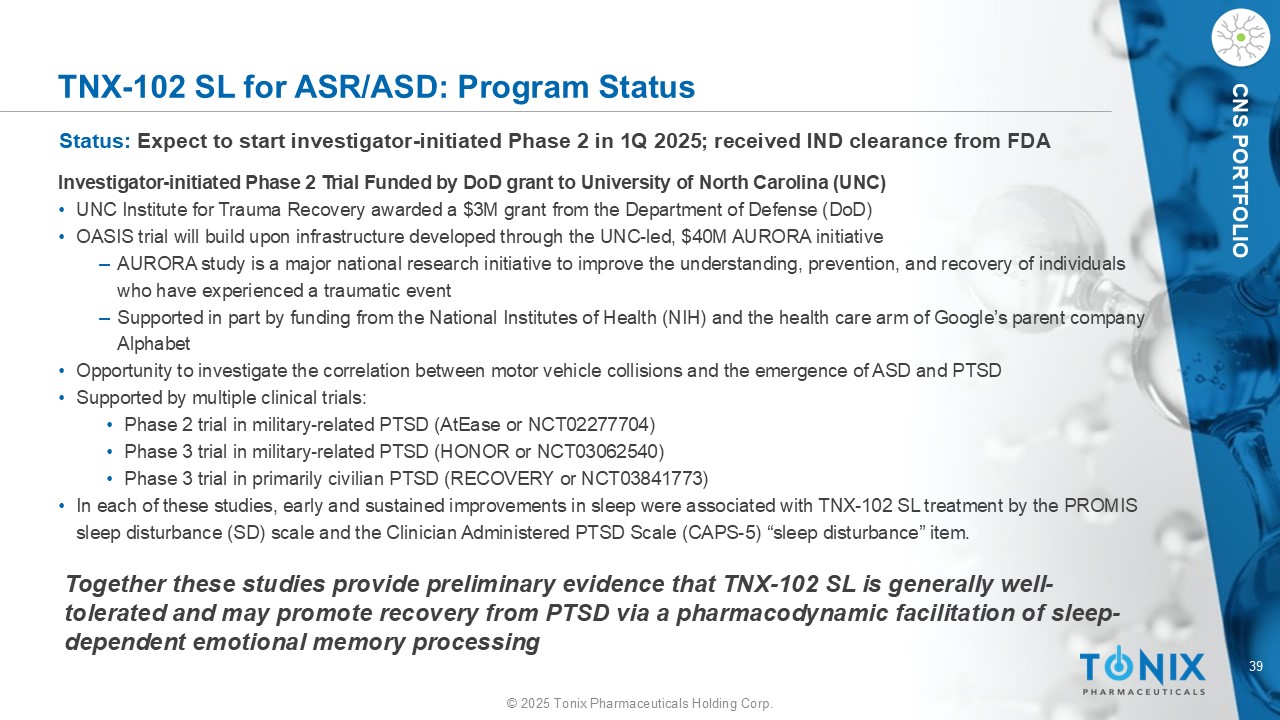
39 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 102 SL for ASR/ASD: Program Status Status: Expect to start investigator - initiated Phase 2 in 1Q 2025 ; received IND clearance from FDA Investigator - initiated Phase 2 Trial Funded by DoD grant to University of North Carolina (UNC) • UNC Institute for Trauma Recovery awarded a $3M grant from the Department of Defense (DoD) • OASIS trial will build upon infrastructure developed through the UNC - led, $40M AURORA initiative ‒ AURORA study is a major national research initiative to improve the understanding, prevention, and recovery of individuals who have experienced a traumatic event ‒ Supported in part by funding from the National Institutes of Health (NIH) and the health care arm of Google’s parent company Alphabet • Opportunity to investigate the correlation between motor vehicle collisions and the emergence of ASD and PTSD • Supported by multiple clinical trials: • Phase 2 trial in military - related PTSD ( AtEase or NCT02277704) • Phase 3 trial in military - related PTSD (HONOR or NCT03062540) • Phase 3 trial in primarily civilian PTSD (RECOVERY or NCT03841773) • In each of these studies, early and sustained improvements in sleep were associated with TNX - 102 SL treatment by the PROMIS sleep disturbance (SD) scale and the Clinician Administered PTSD Scale (CAPS - 5) “sleep disturbance” item. Together these studies provide preliminary evidence that TNX - 102 SL is generally well - tolerated and may promote recovery from PTSD via a pharmacodynamic facilitation of sleep - dependent emotional memory processing

40 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 102 SL for ASR/ASD: Phase 2 OASIS Study Design General s tudy c haracteristics: • Randomized, double - blind, placebo - controlle d study in Acute Stress Reaction (ASR) / Acute Stress Disorder (ASD) • The proposed O ptimizing A cute S tress reaction I nterventions with TNX - 102 S L (OASIS) trial will examine the safety and efficacy of TNX - 102 SL to reduce adverse posttraumatic neuropsychiatric sequelae among patients presenting to the emergency department after a motor ve hicle collision (MVC) • The trial will enroll approximately 180 individuals who acutely experienced trauma at study sites across the US • Participants will be randomized in the emergency department to receive a two - week course of either TNX - 102 SL or placebo • Investigator - initiated IND Objective: • Investigate the potential of Tonix’s TNX - 102 SL (cyclobenzaprine HCl sublingual tablets) to reduce the frequency and severity of the adverse effects of traumatic exposure, including acute stress reaction (ASR), acute stress disorder (ASD), and posttraumatic stress d iso rder (PTSD). • ASR refers to the body’s immediate response to trauma, whereas ASD is the short - term effects of trauma (within 1 month), and PTS D is the long - term effects of trauma (beyond 1 month) * First dose of TNX - 102 SL 5.6 mg versus placebo taken in the emergency department, and then daily at bedtime to finish 2 weeks of treatment A Phase 2 Study to Evaluate the Efficacy and Safety of TNX - 102 SL Taken Daily in Patients With ASR/ ASD (OASIS) • Primary outcome measure: Acute Stress Disorder Scale (ASDS) assessed at 7 and 21 days post MVC • Posttraumatic stress symptom severity assessed at 6 and 12 weeks post MVC using the PTSD Checklist for DSM - 5 (PCL - 5) • Standardized survey instruments of sleep disturbances, anxiety and depression symptoms, general physical and mental health, and clinical global improvement also employed • Detailed and brief neurocognitive assessments are performed from baseline to 12 weeks after MVC at specific timepoints throughout study participation period Placebo once - daily at bedtime 2 weeks TNX - 102 SL once - daily at bedtime 5.6 mg (2 x 2.8 mg tablets) *
41 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Significant Overlap between Fibromyalgia and Long - COVID National Institutes of Health (NIH) - sponsored RECOVER study found many Long COVID patients suffer from pain at multiple sites 1 • Suggests that many Long - COVID patients may meet the diagnostic criteria for fibromyalgia Tonix has previously presented its analysis of real - world evidence from the TriNetX claims database suggesting that over 40% of Long COVID patients present with a constellation of symptoms that overlap with fibromyalgia 2,3 1 Thaweethai T, et al. JAMA . 2023 329(22):1934 - 1946. 2 Feb 22, 2023 Tonix Pharmaceuticals Press Release. URL: https://ir.tonixpharma.com/news - events/press - releases/detail/1369/tonix - pharmaceuticals - describes - emerging - research - on - the 3 September 21, 2022, Tonix Pharmaceuticals Poster at the IASP, “Retrospective observational database study of patients with Lo ng COVID with multi - site pain, fatigue and insomnia”. URL: www.tonixpharma.com/wp - content/uploads/2022/09/Retrospective - Observational - Database - Study - of - Patients - with - Long - COVID - with - Multi - Site - Pain - Fatigue - and - Insomnia_A - Real - World - Analysis - of - Symptomatology - and - Opioid - Use.pdf
42 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO NASEM Definition of Long - COVID • In June 2024, the US National Academies of Sciences, Engineering and Medicine (NASEM) described fibromyalgia as a ‘ diagnosable condition’ in people suffering from Long COVID 1 • This definition provides guidance to government, healthcare professionals and industry that fibromyalgia can arise after infection with the SARS - CoV - 2 virus and can be diagnosed in Long COVID patients Fibromyalgia prevalence prior to COVID - 19 pandemic: >10M adults in the US 2 Long - COVID prevalence: 5.3% or ~14M adults in the US 3 Tonix believes that diagnosing fibromyalgia in Long COVID patients will increase the potential market for TNX - 102 SL as compared to market estimates from before the COVID - 19 pandemic 1 U.S. National Academies of Sciences, Engineering, and Medicine. 2024. A Long COVID Definition: A chronic, systemic disease state with profound consequences. Washington, DC: The National Academies Press. https://doi.org/10.17226/27768 . http://www.nationalacademies.org/long - covid - definition . 2 Vincent A, et al. Arthritis Care Res (Hoboken). 2013 65(5):786 - 92. doi : 10.1002 3 National Center for Health Statistics. U.S. Census Bureau, Household Pulse Survey, 2022 – 2024. Long COVID. Generated interactivel y: July 22, 2024 from https://www.cdc.gov/nchs/covid19/pulse/long - covid.htm

© 2025 Tonix Pharmaceuticals Holding Corp. TONIX MEDICINES: MARKETED PRODUCTS

44 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Tonix Medicines: Commercial - Stage Specialty Pharma Subsidiary • Tonix Medicines is a w holly - owned subsidiary of Tonix ( NASDAQ: TNXP) • Currently marketing two products indicated for the treatment of acute migraine: Zembrace ® SymTouch ® and Tosymra ® • Nascent commercial organization • Tonix Medicines is preparing to launch TNX - 102 SL for fibromyalgia • Fibromyalgia care is relatively concentrated to specialized providers • We believe prescribing physicians can be targeted effectively by a specialty sales force • Evolving landscape in commercial markets favors distribution channels such as specialty pharmacies

45 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Tonix Medicines Markets Two Proprietary Migraine Drugs Non - oral Formulations of Sumatriptan • Each indicated for the tr eatment of acute migraine with or without aura in adults • Sumatriptan remains the acute migraine ‘gold standard’ treatment for many patients and continues to represent the largest segment of the market in terms of unit sales 3 • Each may provide migraine pain relief in as few as 10 minutes for some patients 1,2,4,5 • Patents to 2036 ( Zembrace ) and 2031 ( Tosymra ) 1 Zembrace SymTouch [package insert] . For more information, talk to your provider and read the Patient Information and Instructions for Use . – Important Safety Information is provided in the appendix 2 Tosymra [package insert ]. For more information, talk to your provider and read the Patient Information and Instructions for use – Important Safety Information is provided in the appendix 3 Tonix Medicines, Inc.; Data On File, 2023 Zembrace® SymTouch ® (sumatriptan injection) 3 mg 1 Tosymra® (sumatriptan nasal spray) 10 mg 2 Tonix Medicines Commercial Subsidiary • Complete commercialization capability • Manage supply chain and contract manufacturer • Distribution • Trade, Managed Care & Government contracting • Team of professionals including Sales & Marketing personnel 4 Mathew NT, et al. Dose ranging efficacy and safety of subcutaneous sumatriptan in the acute treatment of migraine. US Sumatri pta n Research Group. Arch Neurol. 1992;49(12):1271 - 1276. 5 Wendt J, et al. A randomized, double - blind, placebo - controlled trial of the efficacy and tolerability of a 4 - mg dose of subcutan eous sumatriptan for the treatment of acute migraine attacks in adults. Clinical Therapeutics. 2006;28(4):517 - 526. Zembrace SymTouch and Tosymra are registered trademarks of Tonix Medicines, Inc. Intravail is a registered trademark of Aegis Therapeutics, LLC, a wholly owned subsidiary of Neurelis , Inc.
46 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Zembrace and Tosymra Bypass the GI Tract Bypassing gastrointestinal (GI) tract is potential advantage for treating acute migraine • GI absorption may be inconsistent in migraineurs due to gastric stasis (also called “gastroparesis”) 1 - 4 • Nausea and vomiting are symptoms of migraine 5 which can complicate oral treatment Existing Subcutaneous injectable products • Imitrex® SQ Injection (sumatriptan succinate) - 6mg and 4mg preparations • DHE 45 (dihydroergotamine mesylate) SQ Injection Existing intranasal products • Imitrex® nasal spray (sumatriptan) • Migranal ® (dihydroergotamine) nasal spray – developed by Novartis, sold by Bausch Health • Zavzpret ® ( zavegepant ) nasal spray, FDA approved in March, 2023 5 is the first intranasal gepant - marketed by Pfizer • Zomig ® nasal spray (zolmitriptan) • Onzetra ® Xsail ® (sumatriptan nasal powder) marketed by Currax • Trudhesa ® (dihydroergotamine) nasal spray 1 Zembrace SymTouch [package insert] . Maple Grove, MN : Upsher - Smith Laboratories, LLC : February 2021 . 2 Mathew NT, et al. Dose ranging efficacy and safety of subcutaneous sumatriptan in the acute treatment of migraine. US Sumatri pta n Research Group. Arch Neurol. 1992;49(12):1271 - 1276. 3 Landy, S. et al. Efficacy and safety of DFN - 11 (sumatriptan injection, 3 mg) in adults with episodic migraine: a multicenter, ra ndomized, double - blind, placebo - controlled study. J Headache Pain. 19, 69 (2018). 4 Brand - Schieber E, Munjal S, Kumar R, et al. Human factors validation study of 3 mg sumatriptan autoinjector, for migraine patien ts. Med Devices ( Auckl ). 2016;9:131 - 137. 5 Pfizer Press Release March 10, 2023. – https://www.pfizer.com/news/press - release/press - release - detail/pfizers - zavzprettm - zavegepant - migraine - nasal - spray

47 © 2025 Tonix Pharmaceuticals Holding Corp. Pipeline Programs and Strategy for Partnerships

48 © 2025 Tonix Pharmaceuticals Holding Corp. Pipeline Development Strategy Focusing on government and academic collaborations • Validates Tonix’s scientific expertise and technology • Reduces internal spend • Increases number of trials • Potentially speeds tim e to market • Grants, contracts, cost - sharing or “in - kind” arrangements

49 © 2025 Tonix Pharmaceuticals Holding Corp. External Partnerships Government partners providing direct funding, cost sharing or in - kind support include 1 : • National Institutes of Health (NIH) • National Institute of Allergy and Infectious Disease (NIAID) ▪ TNX - 1800 selected for Project NextGen • National Institute on Drug Abuse (NIDA) ▪ TNX - 1300 for cocaine intoxication; Phase 2 study funding • Department of Defense (DoD) • TNX - 4200 for the prevention or treatment of infections to improve the medical readiness of military personnel in biological threat environments ▪ TNX - 102 SL for ASD; investigator - initiated Phase 2 study funding Academic partners sponsoring clinical trials of Tonix’s investigational drug products include: • Massachusetts General Hospital (MGH) • University of Washington • University of North Carolina 1 Tonix’s product development candidates are investigational new drugs or biologics; their efficacy and safety have not been es tab lished and have not been approved for any indication.

50 © 2025 Tonix Pharmaceuticals Holding Corp. TNX - 1300: COCAINE INTOXICATION Key Partnerships TNX - 1500: ALLOGRAFT REJECTION TNX - 1800 : COVID - 19 VACCINE TNX - 2900: PRADER - WILLI SYNDROME TNX - 102 SL: ACUTE STRESS DISORDER TNX - 4200: BROAD - SPECTRUM ANTIVIRAL

51 © 2025 Tonix Pharmaceuticals Holding Corp. TNX - 801 Live Virus Vaccine Live virus vaccine platform with multitude of potential applications

52 © 2025 Tonix Pharmaceuticals Holding Corp. INFECTIOUS DISEASE PORTFOLIO Mpox Declared Public Health Emergency of International Concern (PHEIC) by WHO* on August 14, 2024: New Clade I = “Clade Ib ” • Clade Ib - first wave in Democratic Republic of Congo (DRC) • Affects children • New mutations • ~0.5% mortality • Affects both MSM (men who have sex with men) + heterosexual transmission primarily in adults • 2024 mpox epidemic has spread to 16 countries in Africa • Outside of Africa cases identified in Sweden, Thailand, Singapore, India, Germany and England • Two FDA ** - approved vaccines: • Jynneos ® (Bavarian - Nordic) – requires 2 dose regimen • Durability of neutralization antibody titers being studied 1 - 3 • Also approved for use in adults by the WHO 4 • ACAM 2000 (Emergent) – single - dose, reactogenic • Provides durable protection • Approved for people at high risk of mpox infection 5 *WHO = World Health Organization 1 Zaeck LM, Nat Med. 2023 29(1):270 - 278. doi : 10.1038/s41591 - 022 - 02090 2 Berens - Riha N, et al. Euro Surveill . 2022 27(48):2200894. doi : 10.2807/1560 - 7917.ES.2022.27.48.2200894. 3 Collier AY, et al. JAMA . 2024 Oct 3. doi : 10.1001/jama.2024.20951. Epub ahead of print. PMID: 39361499 . https://pubmed.ncbi.nlm.nih.gov/39361499/ 4 Keaton, J. Sept. 13, 2024. Associated Press. “WHO grants first mpox vaccine approval to ramp up response to disease in Africa.” URL: https://bit.ly/4e4yyeb 5 https://www.fda.gov/vaccines - blood - biologics/vaccines/key - facts - about - vaccines - prevent - mpox - disease#:~:text=ACAM2000%20Vaccine,for%20smallpox%20or%20mpox%20infection.
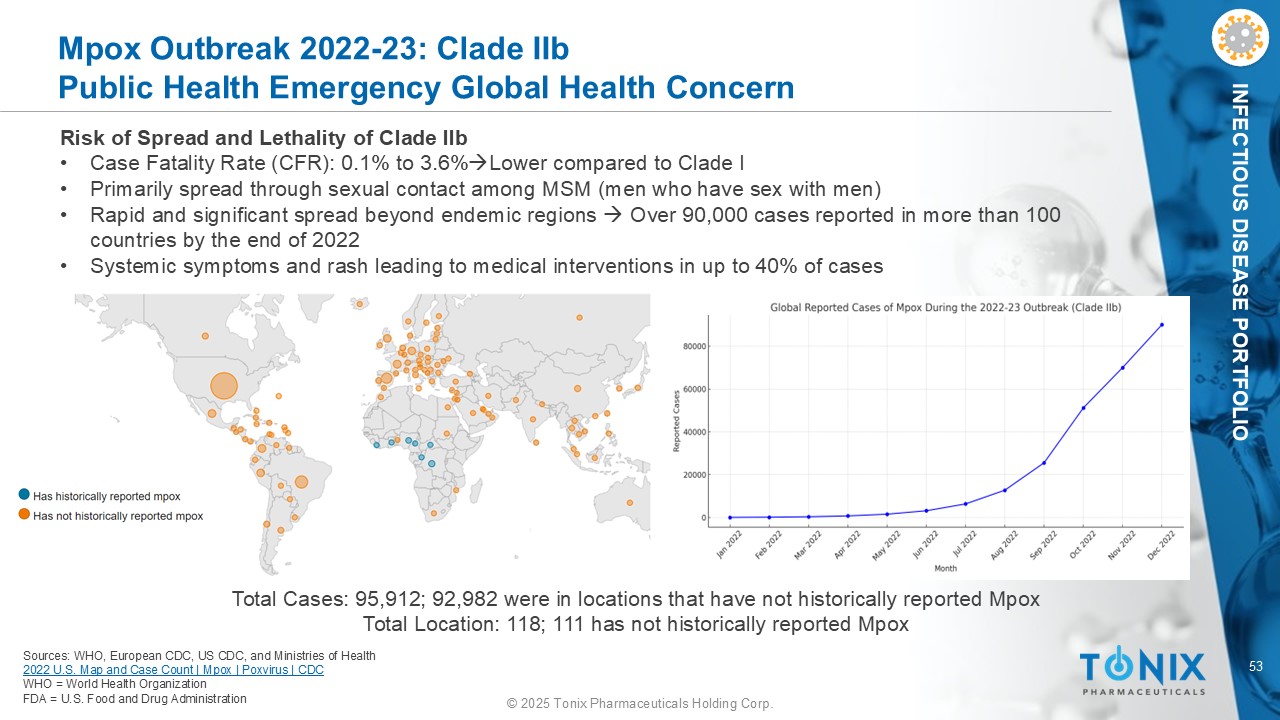
53 © 2025 Tonix Pharmaceuticals Holding Corp. INFECTIOUS DISEASE PORTFOLIO Mpox Outbreak 2022 - 23: Clade II b Public Health Emergency Global Health Concern Risk of Spread and Lethality of Clade IIb • Case Fatality Rate (CFR): 0.1% to 3.6% Lower compared to Clade I • Primarily spread through sexual contact among MSM (men who have sex with men) • Rapid and significant spread beyond endemic regions Over 90,000 cases reported in more than 100 countries by the end of 2022 • Systemic symptoms and rash leading to medical interventions in up to 40% of cases Sources: WHO, European CDC, US CDC, and Ministries of Health 2022 U.S. Map and Case Count | Mpox | Poxvirus | CDC WHO = World Health Organization FDA = U.S. Food and Drug Administration Total Cases: 95,912; 92,982 were in locations that have not historically reported Mpox Total Location: 118; 111 has not historically reported Mpox

54 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Monkeypox Headlines • Multiple recent statements by U.S. Agencies warning about smallpox and monkeypox 1 - 6 • U.S. National Academy of Sciences Consensus Report (March 2024) 6 ‒ “Additionally, safer, single - dose vaccines and a diverse set of therapeutic options against smallpox would improve the U.S. readiness and response posture for immediate containment and long - term protection in a smallpox emergency. ‒ “Smallpox vaccines that have improved safety across different population subgroups and are available as a single dose would support faster and more effective response to contain smallpox and other orthopoxvirus outbreaks. The development of novel smallpox vaccines using multi - vaccine platforms (i.e., use common vaccine vectors, manufacturing ingredients, and processes) would improve the capacity for rapid vaccine production in response to a smallpox event and reduce the need for stockpiling in the SNS at current levels. ‒ “Given the lack of commercially available orthopoxvirus diagnostics, vaccines, and therapeutics, planning for logistics and supply chain management considerations is critical. Efforts could give consideration to developing plans to increase the number of smallpox vaccine and therapeutics manufacturers as well as optimizing current manufacturing capacities should they be needed in the shorter term.” 1 Office of Science and Technology Policy (OSTP). American Pandemic Preparedness: Transforming Our Capabilities. September 2021 2 National Biodefense Science Board (NBSB). Prioritization of Product Attribute Categories to Maximize Access for Next Generati on COVID - 19 Vaccines and Therapeutics. August 2023 3 Office of Science and Technology Policy (OSTP). American Pandemic Preparedness: Transforming Our Capabilities. September 2021 4 National Biodefense Science Board (NBSB). Prioritization of Product Attribute Categories to Maximize Access for Next Generati on COVID - 19 Vaccines and Therapeutics. August 2023 5 BARDA Strategic Plan 2022 - 2026. 6 U.S. National Academy of Sciences. March 28, 2024. “Consensus Study Report: Future State of Smallpox Medical Countermeasures. ” https://nap.nationalacademies.org/catalog/27652/future - state - of - smallpox - medical - countermeasures
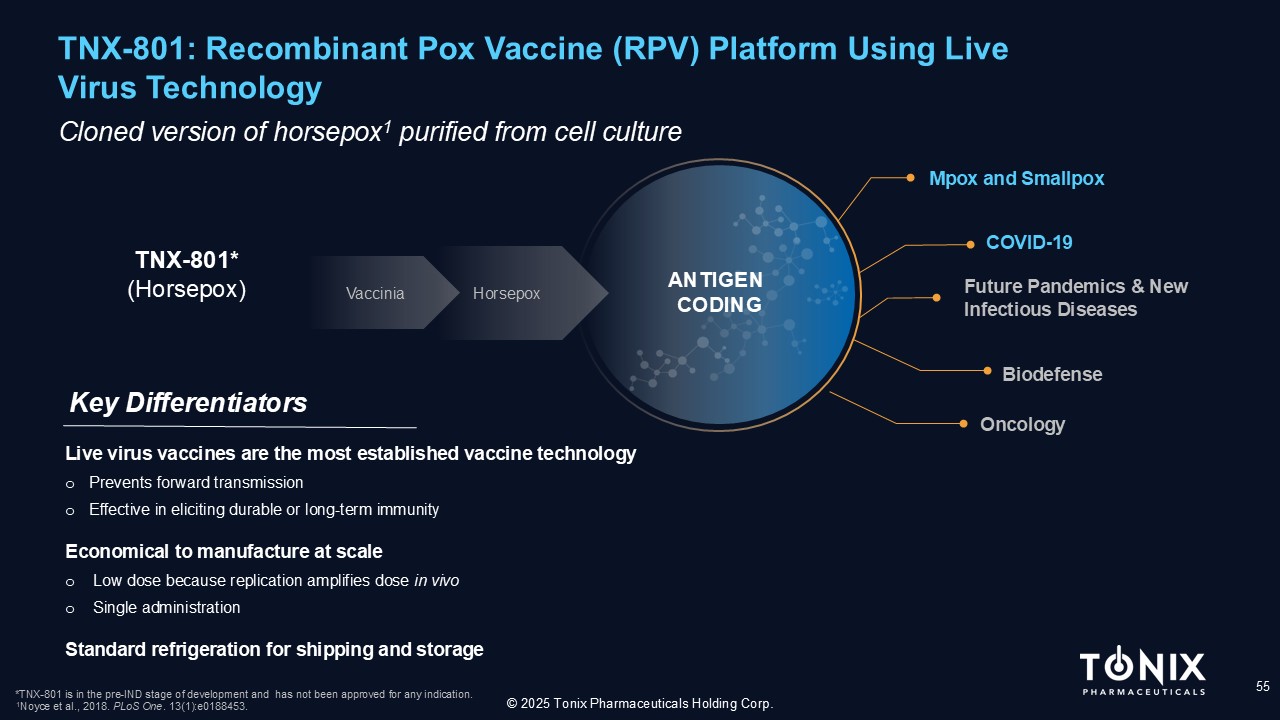
TNX - 801: Recombinant Pox Vaccine (RPV) Platform Using Live Virus Technology 55 C loned version of horsepox 1 purified from cell culture Key Differentiators © 2025 Tonix Pharmaceuticals Holding Corp. TNX - 801* (Horsepox) *TNX - 801 is in the pre - IND stage of development and has not been approved for any indication. 1 Noyce et al., 2018. PLoS One . 13(1):e0188453. Live virus vaccines are the most established vaccine technology o Prevents forward transmission o Effective in eliciting durable or long - term immunity Economical to manufacture at scale o Low dose because replication amplifies dose in vivo o Single administration Standard refrigeration for shipping and storage Mpox and Smallpox Future Pandemics & New Infectious Diseases COVID - 19 Biodefense Vaccinia Horsepox Oncology ANTIGEN CODING

56 © 2025 Tonix Pharmaceuticals Holding Corp. INFECTIOUS DISEASE PORTFOLIO TNX - 801: Pre - IND Ready Candidate Mpox Vaccine • Based on synthetic horsepox - vector, believed related to first smallpox vaccine used by Dr. Edward Jenner in 1796 1 • Single - dose percutaneous 2 • Attenuated live virus 3 • Expected durable T - cell immunity similar to 19 th Century vaccinia • Believed to be thermo - stable in ultimate lyophilized formulation • Eventual presentation using Microneedle Array Patch – working with developers R&D Center - Maryland Operational BSL - 3 capable Advanced Manufacturing Center - MA GMP - manufacturing capability* *GMP Suites currently decommissioned 1 Noyce RS, et al. PLoS One. 2018 Jan 19;13(1):e0188453. doi : 10.1371/journal.pone.0188453. PMID: 29351298; PMCID: PMC5774680. 2 Noyce RS, et al. Viruses . 2023 Jan 26;15(2):356. Doi: 10.3390/v15020356. PMID: 36851570; PMCID: PMC9965234 3 Trefry SV, et al. mSphere . 2024 Nov 13:e0026524. doi : 10.1128/msphere.00265 - 24. Epub ahead of print. PMID: 39535212.
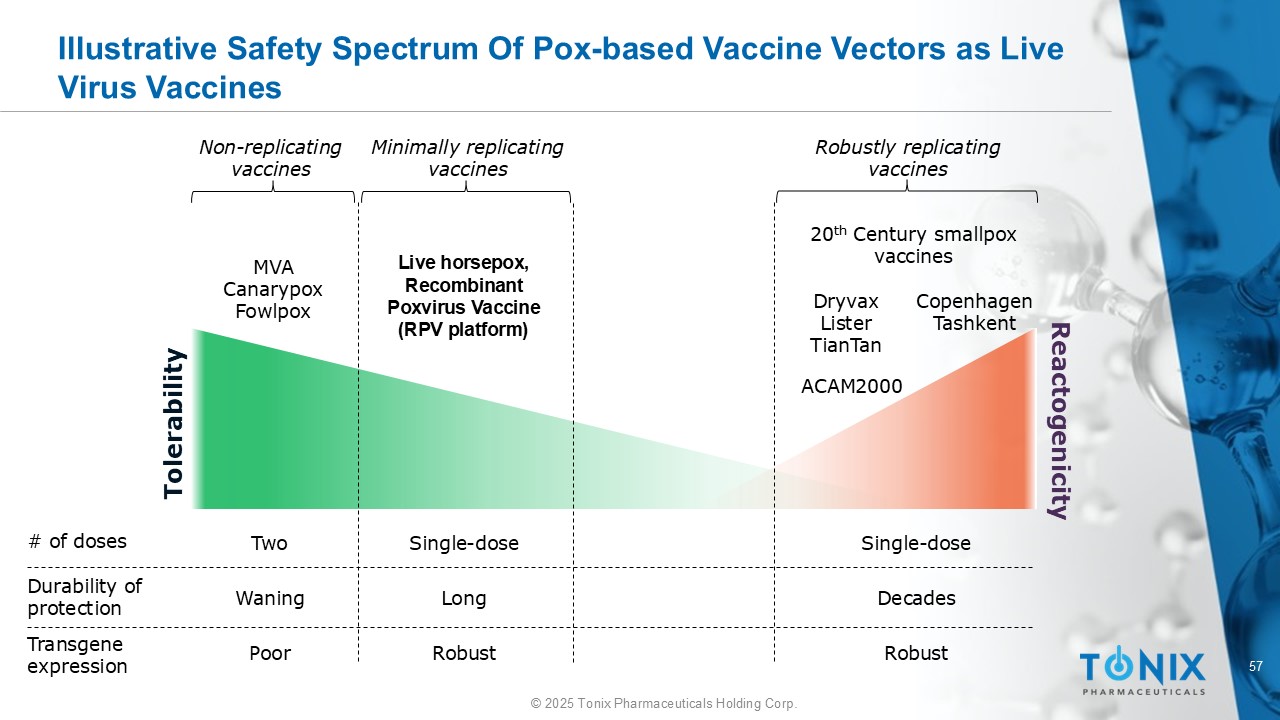
57 © 2025 Tonix Pharmaceuticals Holding Corp. Illustrative Safety Spectrum Of Pox - based Vaccine Vectors as Live Virus Vaccines 20 th Century smallpox vaccines Dryvax Lister TianTan Copenhagen Tashkent MVA Canarypox Fowlpox Reactogenicity Tolerability Non - replicating vaccines Live horsepox, Recombinant Poxvirus Vaccine (RPV platform) Minimally replicating vaccines Robustly replicating vaccines # of doses Durability of protection Transgene expression Two Waning Poor Single - dose Long Robust Single - dose Decades Robust ACAM2000
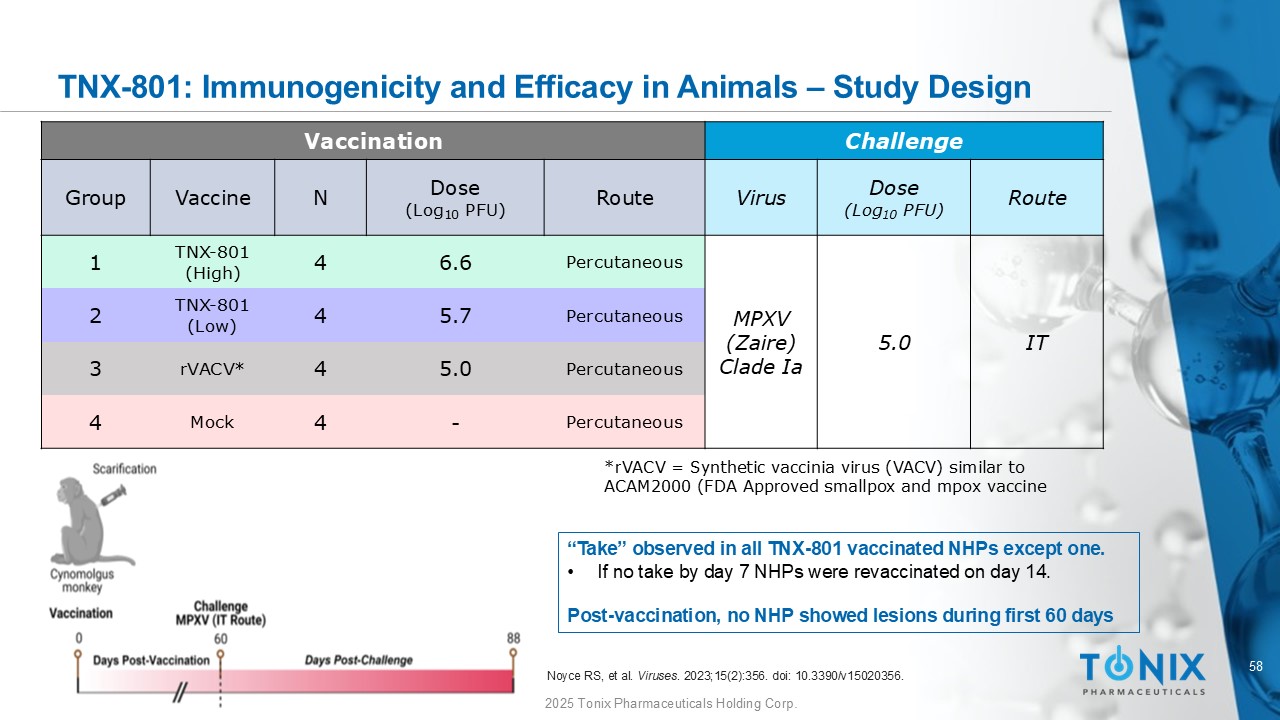
58 © 2025 Tonix Pharmaceuticals Holding Corp. Challenge Vaccination Route Dose (Log 10 PFU) Virus Route Dose (Log 10 PFU) N Vaccine Group IT 5.0 MPXV (Zaire) Clade Ia Percutaneous 6.6 4 TNX - 801 (High) 1 Percutaneous 5.7 4 TNX - 801 (Low) 2 Percutaneous 5.0 4 rVACV * 3 Percutaneous - 4 Mock 4 “Take” observed in all TNX - 801 vaccinated NHPs except one. • If no take by day 7 NHPs were revaccinated on day 14. Post - vaccination, no NHP showed lesions during first 60 days * rVACV = Synthetic vaccinia virus (VACV) similar to ACAM2000 (FDA Approved smallpox and mpox vaccine Noyce RS, et al. Viruses . 2023;15(2):356. doi : 10.3390/v15020356. TNX - 801: Immunogenicity and Efficacy in Animals – Study Design

59 © 2025 Tonix Pharmaceuticals Holding Corp. Days Post Vaccination Baseline 28 56 TNX - 801 (HD) TNX - 801 (LD) rVACV Mock 100% Seroconversion in TNX - 801 vaccinated groups 100% Seroconversion in TNX - 801 vaccinated groups HD = “High Dose” LD = “Low Dose” Noyce RS, et al. Viruses . 2023;15(2):356. doi : 10.3390/v15020356. TNX - 801 Vaccination – Followed by Lethal Monkeypox Clade I Challenge, Immunogenicity: Immunogenicity: Total IgG (ELISA)
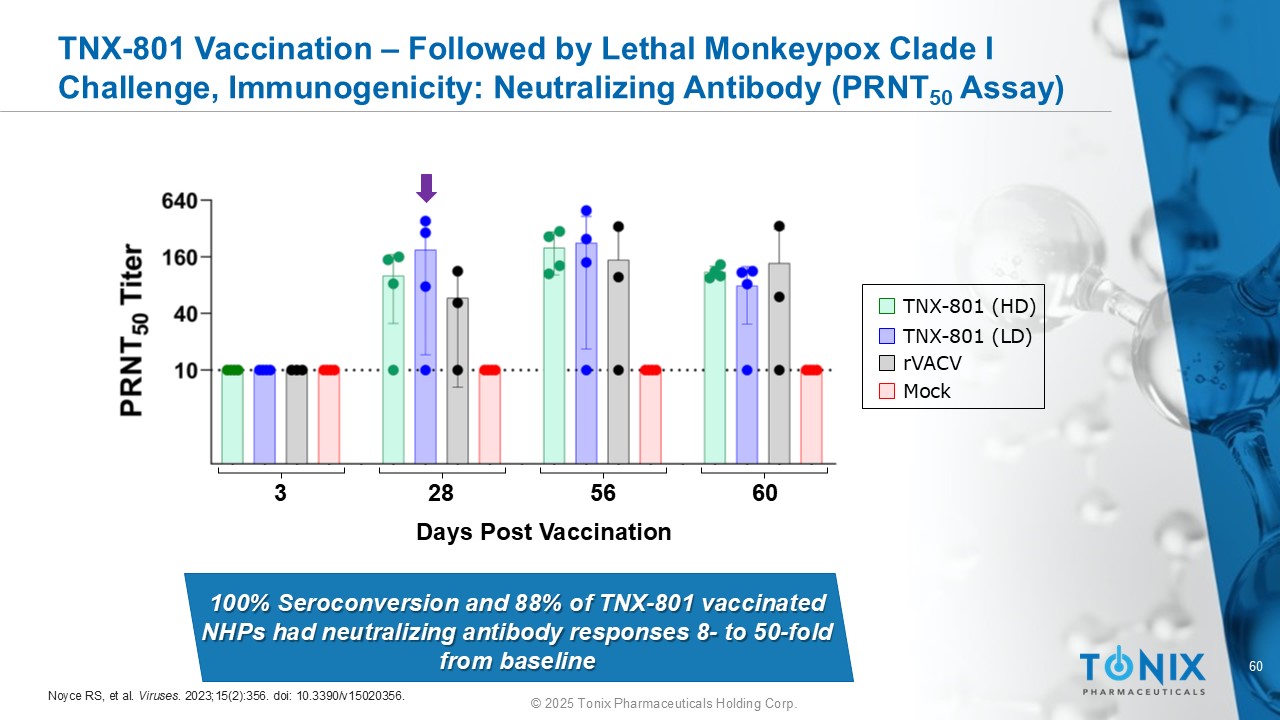
60 © 2025 Tonix Pharmaceuticals Holding Corp. TNX - 801 Vaccination – Followed by Lethal Monkeypox Clade I Challenge, Immunogenicity: Neutralizing Antibody ( PRNT 50 A ssay) Days Post Vaccination 3 28 56 60 100% Seroconversion and 88% of TNX - 801 vaccinated NHPs had neutralizing antibody responses 8 - to 50 - fold from baseline TNX - 801 (HD) TNX - 801 (LD) rVACV Mock Noyce RS, et al. Viruses . 2023;15(2):356. doi : 10.3390/v15020356.
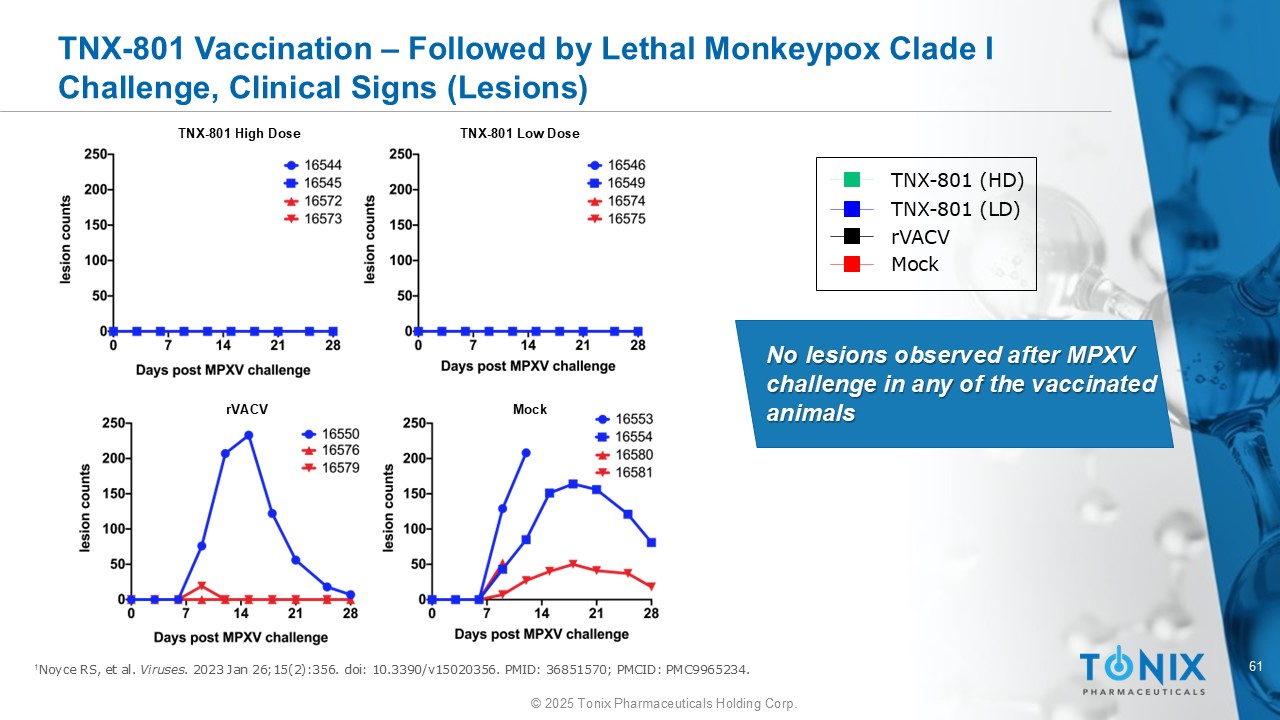
61 © 2025 Tonix Pharmaceuticals Holding Corp. TNX - 801 Vaccination – Followed by Lethal Monkeypox Clade I Challenge, Clinical Signs (Lesions) 1 Noyce RS, et al. Viruses . 2023 Jan 26;15(2):356. doi : 10.3390/v15020356. PMID: 36851570; PMCID: PMC9965234. TNX - 801 High Dose TNX - 801 Low Dose rVACV Mock No lesions observed after MPXV challenge in any of the vaccinated animals TNX - 801 (HD) TNX - 801 (LD) rVACV Mock
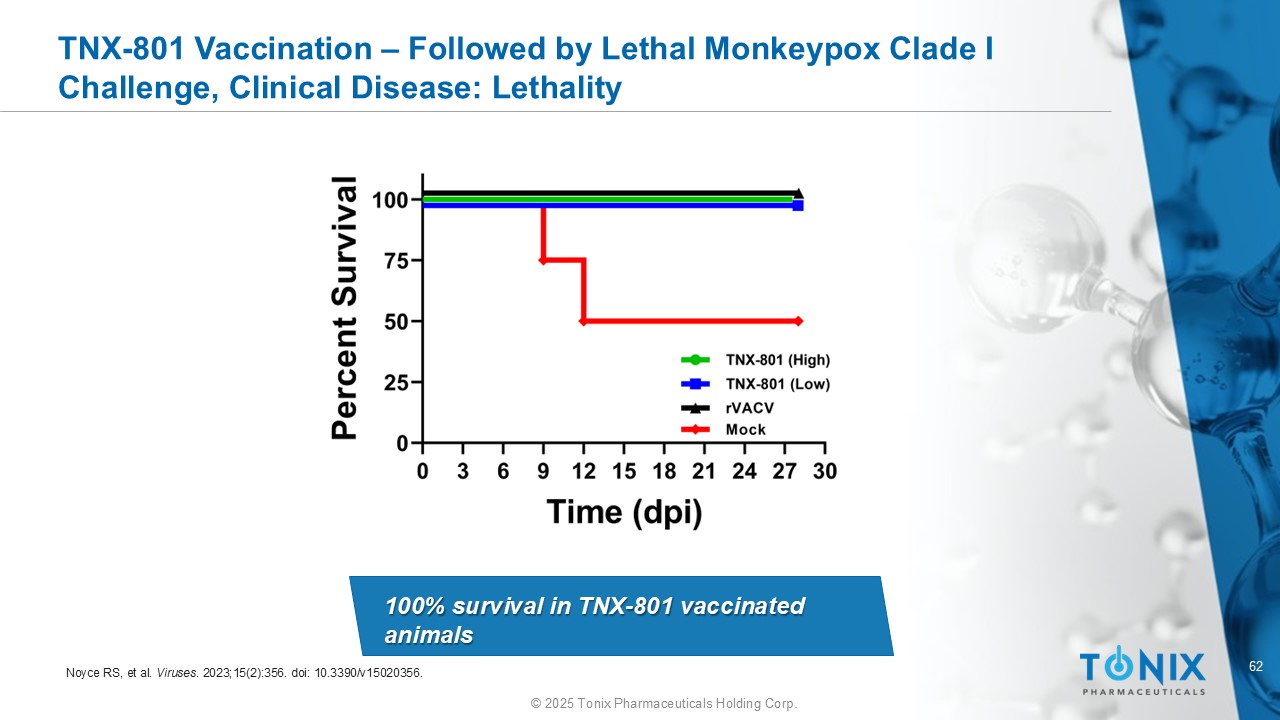
62 © 2025 Tonix Pharmaceuticals Holding Corp. TNX - 801 Vaccination – Followed by Lethal Monkeypox Clade I Challenge, Clinical Disease: Lethality No deaths in TNX - 801 vaccinated groupsx 100% survival in TNX - 801 vaccinated animals Noyce RS, et al. Viruses . 2023;15(2):356. doi : 10.3390/v15020356.
63 © 2025 Tonix Pharmaceuticals Holding Corp. INFECTIOUS DISEASE PORTFOLIO TNX - 1800*: Designed to Express the SARs - CoV - 2 Spike Protein TNX - 1800 (recombinant horsepox virus) is a live virus vaccine based on Tonix’s TNX - 801 that is designed to express the spike protein of the SARS - CoV - 2 virus and to elicit a predominant T cell response • I mmunogenic and well tolerated 1 • S howed promise in protecting animals from challenge with SARS - CoV - 2 delivered directly into the lungs 1 Status: National Institute of Allergy and Infectious Diseases (NIAID) will conduct a Phase 1 clinical trial with TNX - 1800 • First vaccine candidate using Tonix’s live virus recombinant pox virus (RPV) platform technology to enter clinical trials • “Project NextGen” is an initiative by the U.S. Department of Health and Human Services (HHS) to advance a pipeline of new, innovative vaccines and therapeutics for COVID - 19. NIAID will be conducting clinical trials to evaluate several early - stage vaccine candidates, including TNX - 1800 • Phase 1 study is designed to assess safety and immunogenicity in approximately 60 healthy adult volunteers • Upon completion of the trial, NIAID and Tonix will assess the results and determine the next steps for the development of TNX - 1800 1 Awasthi, M. et al. Viruses . 2023. 15(10):2131. 2 Awasthi, M. et al. Vaccines (Basel) . 2023. 11(11):1682. *TNX - 1800 is in the pre - IND stage of development and has not been approved for any indication

© 2025 Tonix Pharmaceuticals Holding Corp. INFECTIOUS DISEASE: KEY CANDIDATES

65 © 2025 Tonix Pharmaceuticals Holding Corp. INFECTIOUS DISEASE PORTFOLIO Host - directed antiviral discovery programs • TNX - 4200*: CD45 targeted therapeutics o Small molecule therapeutics that reduce endogenous levels of CD45, a protein tyrosine phosphatase o Reduction in CD45 protects against many viruses including the Ebola virus • Cathepsin inhibitors o Small molecule therapeutics that inhibit essential cathepsins which are required by viruses such as coronaviruses and filoviruses to infect cells o Activity as monotherapy and in combination with other antivirals Virus - directed antivirals discovery program • Viral glycan - targeted engineered biologics o B ind to viral densely branched high - mannose (DBH) glycans o Neutralize circulating virus and stop the entry of the progeny virus into cells o Antiviral activity against a broad range of RNA viruses o Activity as monotherapy and in combination with other antivirals R&D Center (RDC): Frederick, MD • Accelerated development of vaccines and antiviral drugs against infectious diseases • ~48,000 square feet, BSL - 2 with some areas designated BSL - 3 Broad - Spectrum Antiviral Discovery Programs *TNX - 4200 is in the pre - IND stage of development and has not been approved for any indication

66 © 2025 Tonix Pharmaceuticals Holding Corp. INFECTIOUS DISEASE PORTFOLIO Tonix Awarded Contract from DoD Defense Threat Reduction Agency (DTRA) contract is expected to advance development of Tonix’s broad - spectrum oral antiviral program, TNX - 4200, for medical countermeasures • Other Transaction Agreement (OTA) with a potential for up to $34 million over five years • Objective is to develop small molecule, broad - spectrum antiviral agents for the prevention or treatment of infections to improve the medical readiness of military personnel in biological threat environments • Tonix’s focus is to d evelop an orally available CD45 antagonist with broad - spectrum efficacy against a range of viral families through preclinical evaluation o P rogram is expected to establish physicochemical properties, pharmacokinetics, and safety attributes to support an IND submission and to fund a first - in - human Phase 1 clinical study

67 © 2025 Tonix Pharmaceuticals Holding Corp. TNX - 1300 Cocaine Esterase Fast acting antidote for life threatening cocaine intoxication
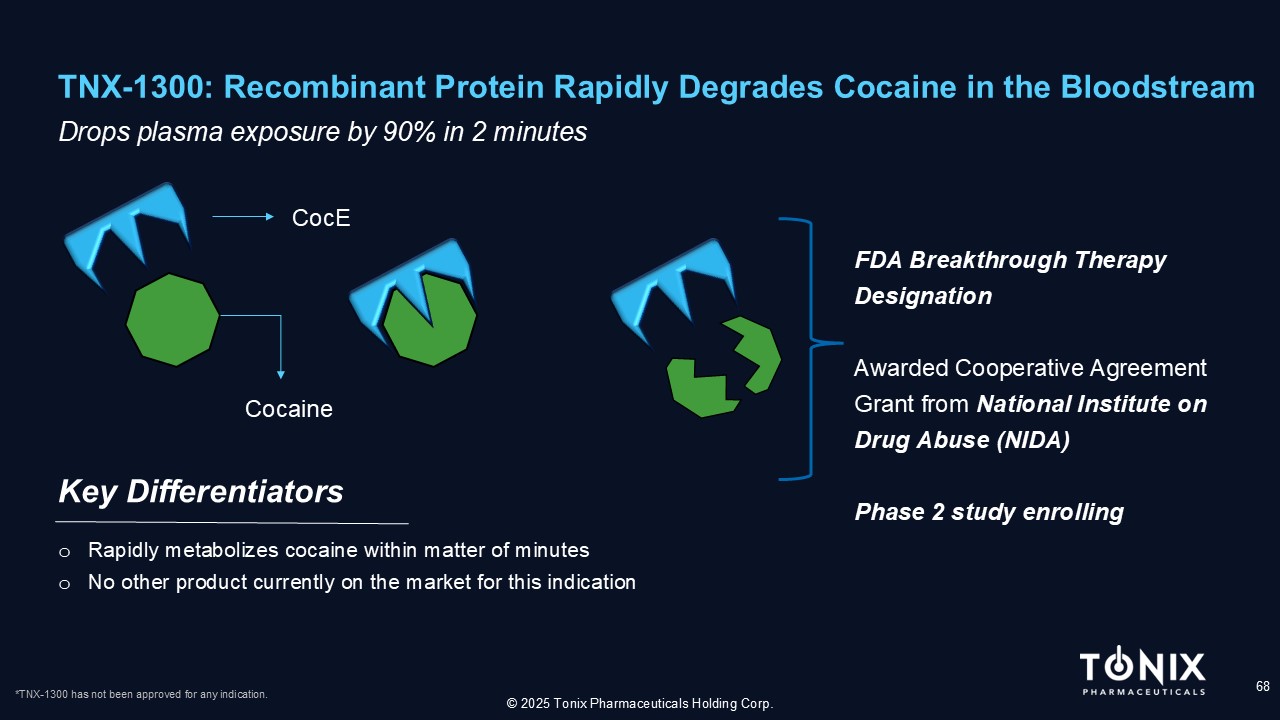
TNX - 1300: Recombinant Protein Rapidly Degrades Cocaine in the Bloodstream 68 Drops plasma exposure by 90% in 2 minutes Key Differentiators © 2025 Tonix Pharmaceuticals Holding Corp. o Rapidly metabolizes cocaine within matter of minutes o N o other product currently on the market for this indication CocE Cocaine FDA Breakthrough Therapy Designation Awarded Cooperative Agreement Grant from National Institute on Drug Abuse (NIDA) Phase 2 study enrolling *TNX - 1300 has not been approved for any indication.
69 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO CNS PORTFOLIO Over 500,000 emergency department visits for cocaine, annually 3,4 About Cocaine Intoxication Over 5 million Americans reported current cocaine use in 2020, which is almost 2% of the population 1 . In 2021, more than 24,900 individuals in the US died from drug overdose deaths involving cocaine 2 500k 1 Substance Abuse and Mental Health Services Administration. (2021). Results from the 2020 National Survey on Drug Use and Health: Detailed Tables: Prevalence Estimates, Standard Errors, and Sample Sizes. 2 Centers for Disease Control and Prevention (CDC) - https://www.cdc.gov/nchs/nvss/vsrr/drug - overdose - data.htm 3 Substance Mental Health Services Administration, Drug Abuse Warning Network, 2011: National Estimates of Drug - Related Emergency Department Visits. HHS Publication No. (SMA) 13 - 4760, DAWN Series D - 39. Rockville, MD: Substance Abuse and Mental Health Services Administration, 2013. 4 Drug Abuse Warning Network, 2011: Selected Tables of National Estimates of Drug - Related Emergency Department Visits. Rockville, MD: Center for Behavioral Health Statistics and Quality, SAMHSA, 2013. Current standard of care: • Patients are currently managed only by supportive care for the adverse effects of cocaine intoxication on the cardiovascular and central nervous systems Large unmet need: • N o other product currently on the market for this indication • TNX - 1300 could significantly reduce the time and resources required for other detox services • Potentially r educes the risk of morbidity and mortality

© 2025 Tonix Pharmaceuticals Holding Corp. IMMUNOLOGY: KEY CANDIDATES

71 © 2025 Tonix Pharmaceuticals Holding Corp. TNX - 1500 Anti - CD40L Monoclonal Antibody Next Generation mAb preserves efficacy without risk of thrombosis
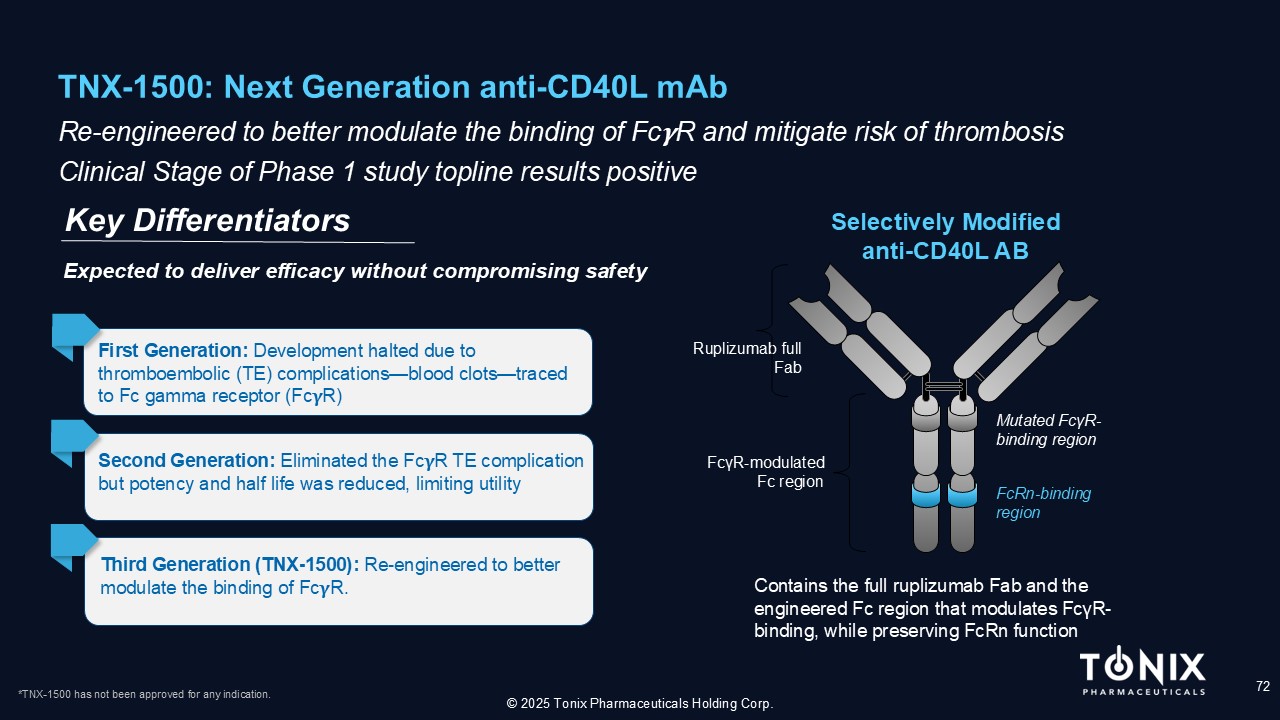
TNX - 1500: Next Generation anti - CD40L mAb 72 Re - engineered to better modulate the binding of Fc R and mitigate risk of thrombosis Clinical Stage of Phase 1 study topline results positive Key Differentiators © 2025 Tonix Pharmaceuticals Holding Corp. Selectively Modified anti - CD40L AB Ruplizumab full Fab Contains the full ruplizumab Fab and the engineered Fc region that modulates Fc γ R - binding, while preserving FcRn function Mutated Fc γ R - binding region FcRn - binding region Fc γ R - modulated Fc region First Generation: Development halted due to thromboembolic (TE) complications — blood clots — traced to Fc gamma receptor (Fc R) Second Generation: Eliminated the Fc R TE complication but potency and half life was reduced, limiting utility Third Generation (TNX - 1500): Re - engineered to better modulate the binding of Fc R. Expected to deliver efficacy without compromising safety *TNX - 1500 has not been approved for any indication.

73 © 2025 Tonix Pharmaceuticals Holding Corp. IMMUNOLOGY PORTFOLIO TNX - 1500 Phase 1 Topline Results and Conclusions Phase 1 design – single ascending dose study in healthy participants • Goals: Evaluate safety, pharmacodynamics and pharmacokinetics • At total of 26 participants were enrolled in three cohorts – Cohort 1: n=4 at 3 mg/kg, n=2 placebo; Cohort 3: n=8 at 10 mg/kg, n=2 placebo; Cohort 3: n=8 at 30 mg/kg, n=2 placebo Topline results • TNX - 1500 blocked the primary and secondary antibody responses to a test antigen (KLH) at the 10 and 30 mg/kg IV doses • Preliminary pharmacokinetic results showed mean half - life (t 1/2 ) for the 10 mg/kg and 30 mg/kg doses of 34 - 38 days • TNX - 1500 was generally well - tolerated with a favorable safety profile Conclusions • Results support proceeding to develop Phase 2 trial for the prevention of kidney transplant rejection • Fc modifications we engineered to TNX - 1500 for safety did not attenuate the potency of TNX - 1500 relative to humanized 5c8 (hu5c8, ruplizumab, BG9588) 1 - 3 • We believe the results of this study and our prior animal studies 4,5 indicate that TNX - 1500 is potentially best - in - class among anti - CD40L mAbs in development 1. Lederman S, et al, J Exp Med . 1992 Apr 1;175(4):1091 - 101. doi : 10.1084/jem.175.4.1091. PMID: 1348081; PMCID: PMC2119166. 2. Boumpas DT, et. al. Arthritis Rheum . 2003;48(3):719 - 27. doi : 10.1002/art.10856. PMID: 12632425. 3. Pierson RN 3rd, et al. Transplantation . 1999;68(11):1800 - 5. doi : 10.1097/00007890 - 199912150 - 00026. PMID: 10609959. 4. Lassiter G, et al. Am J Transplant . 2023;23(8):1171 - 1181. doi : 10.1016/j.ajt.2023.03.022. 5. Miura S, et al. Am J Transplant . 2023;23(8):1182 - 1193. doi : 10.1016/j.ajt.2023.03.025.

74 © 2025 Tonix Pharmaceuticals Holding Corp. IMMUNOLOGY PORTFOLIO TNX - 1500 Phase 1 Results and Methods Topline Results ▪ Tolerability : TNX - 1500 was generally well - tolerated with a favorable safety and tolerability profile . The only TEAE occurring in ≥ 3 participants among all TNX - 1500 groups was Aphthous ulcer, occurring in one participant each in the 3 mg/kg, 10 mg/kg, and 30 mg/kg groups ; all were rated as mild, possibly related, and resolved in 2 - 10 days . There were no TEAEs assessed as related to KLH administration . No TEAEs led to study discontinuation . There were no serious adverse events . There were no thromboembolic events, which were prespecified as TEAEs of special interest . ▪ Pharmacodynamics : TNX - 1500 at 10 mg/kg and 30 mg/kg, on average, completely suppressed both the primary and secondary anti - KLH Ab responses, evidenced by the mean Ab level at all sampled timepoints (through Day 120 ) being below the lower limit of quantitation ( 400 µ g/L) . TNX - 1500 at 3 mg/kg completely suppressed the primary response to KLH Day 2 challenge and reduced the peak secondary response to KLH Day 29 challenge by approximately two thirds ( 69 % ) relative to the peak response to placebo . ▪ Pharmacokinetics : The mean (SD) half - life (t 1 / 2 ) of TNX - 1500 was : 3 mg/kg, 19 . 6 ( 9 . 29 ) days ; 10 mg/kg, 37 . 8 ( 5 . 46 ) days ; and 30 mg/kg, 33 . 7 ( 4 . 83 ) days . Methods ▪ Dosing : TNX - 1500 solution for IV infusion was infused over a period of one hour to achieve doses of 3 , 10 , and 30 mg/kg . Participants were observed in the clinic for one day and followed with periodic clinic visits to Day 120 . ▪ Keyhole Limpet Hemocyanin (KLH) Challenge : To evaluate the immune modulation potency of TNX - 1500 , participants received an antigen challenge with KLH ( Immucothel ® ) administered subcutaneously (SC) on Day 2 and Day 29 of the study . Samples for anti - KLH antibody (Ab) were obtained on Days 1 (pre - challenge), 8 , 15 , 29 , 36 , 50 , 64 , 78 , and 120 . ▪ Disposition : At total of 26 participants were enrolled in three Cohorts (Cohort 1 : n= 4 at 3 mg/kg, n= 2 placebo ; Cohort 3 : n= 8 at 10 mg/kg, n= 2 placebo ; Cohort 3 : n= 8 at 30 mg/kg, n= 2 placebo) . A total of 24 participants completed the study and two discontinued early (one placebo participant was lost to follow - up and one on TNX - 1500 withdrew consent) . 1 Pierson RN 3rd, et al. Transplantation . 1999;68(11):1800 - 5. doi : 10.1097/00007890 - 199912150 - 00026. PMID: 10609959.

75 © 2025 Tonix Pharmaceuticals Holding Corp. IMMUNOLOGY PORTFOLIO TNX - 1500 Preclinical Data and Publications Non - human Primate Kidney Allo - Transplantation • TNX - 1500 monotherapy consistently prevents kidney transplant rejection with n o thrombosis observed • April 2023 Publication: Lassiter, G., et al. (2023). TNX - 1500, an Fc - modified Anti - CD154 Antibody, Prolongs Nonhuman Primate Renal Allograft Survival. American Journal of Transplantation . www.sciencedirect.com/science/article/pii/S1600613523003714 Non - human Primate Heart Heterotopic Allo - Transplantation • TNX - 1500 monotherapy consistently prevents heart transplant rejection. Similar activity to chimeric hu5c8 1 during treatment phase in prior studies • April 2023 Publication: Miura, S., et al. (2023) TNX - 1500, an Fc - modified Anti - CD154 Antibody, Prolongs Nonhuman Primate Cardiac Allograft Survival. American Journal of Transplantation. www.sciencedirect.com/science/article/pii/S1600613523003969 Non - Human Primate Kidney Xenograft Transplantation • TNX - 1500 therapy is part of a regiment to prevent rejection in kidney xenograft transplants ‒ Anand, R.P., Layer, J.V., Heja , D. et al. (2023). Design and testing of a humanized porcine donor for xenotransplantation. Nature. https://www.nature.com/articles/s41586 - 023 - 06594 - 4 ‒ Kozlov, M. (2023). Monkey survives two years after gene - edited pig - kidney transplant. Nature. https://www.nature.com/articles/d41586 - 023 - 03176 - 2 ‒ Mohiuddin, M. (2023). Pig - to - primate organ transplants require genetic modifications of donor. Nature. https://www.nature.com/articles/d41586 - 023 - 02817 - w 1 Pierson RN 3rd, et al. Transplantation . 1999;68(11):1800 - 5. doi : 10.1097/00007890 - 199912150 - 00026. PMID: 10609959.
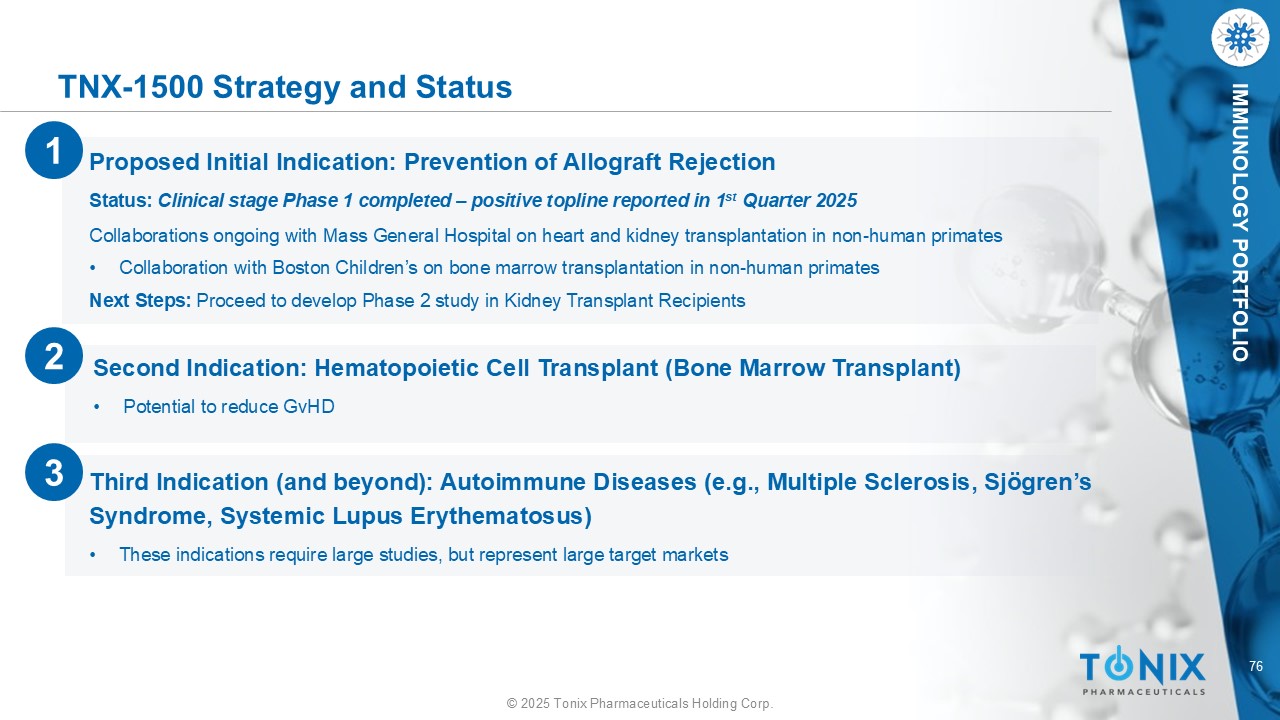
76 © 2025 Tonix Pharmaceuticals Holding Corp. IMMUNOLOGY PORTFOLIO TNX - 1500 Strategy and Status Third Indication (and beyond): Autoimmune Diseases ( e.g., Multiple Sclerosis, Sj ö gren’s Syndrome, Systemic Lupus Erythematosus) • These indications require large studies, but represent large target markets Proposed Initial Indication: Prevention of Allograft Rejection Status: Clinical stage Phase 1 completed – positive topline reported in 1 st Quarter 2025 Collaborations ongoing with Mass General Hospital on heart and kidney transplantation in non - human primates • Collaboration with Boston Children’s on bone marrow transplantation in non - human primates Next Steps: Proceed to develop Phase 2 study in Kidney Transplant Recipients 1 2 Second Indication: Hematopoietic Cell Transplant (Bone Marrow Transplant) • Potential to reduce GvHD 3

77 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO ࢻ - CD40L Headlines • Mass General Hospital just transplanted a genetically engineered pig kidney into a living human 1 • Boston Globe, March 21, 2024 • Patient’s death announced May 11, 2024 2 • The patient was being treated with anti - CD40L mAb tegoprubart from Eledon 1 • The preclinical work was performed with TNX - 1500 3 1 Massachusetts General Hospital press release. March 21, 2024. “World’s First Genetically Edited Pig Kidney Transplant into Living Recipient Performed at Massachusetts General Hospital.” www.massgeneral.org/news/press - release/worlds - first - genetically - edited - pig - kidney - transplant - into - living - recipient (accessed March 29, 2024) 2 Stoico , N. Boston Globe . May 11, 2023. “Mass Man who received first kidney transplant from genetically engineered pig has died, family says”. 3 Anand, R.P., et al Nature. 622, 393 – 401 (2023). https://doi.org/10.1038/s41586 - 023 - 06594 - 4

78 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Anti - CD40L Headlines • Sanofi published their Phase 2 data on their frexalimab in multiple sclerosis in the the New England Journal of Medicine 1 • Sanofi projects its Fc - modified humanized anti - CD40L mAb frexalimab will exceed €5 B per year in peak sales 2 • Like frexalimab , TNX - 1500 is Fc - modified to reduce/eliminate the risk of thrombosis seen with “first generation” anti - CD40L mAbs . 1 Vermersch P, et al. N Engl J Med. 2024 Feb 15;390(7):589 - 600. doi : 10.1056/NEJMoa2309439. PMID: 38354138. 2 Dunn, A. Endpoints. December 7, 2023. “Sanofi CEO Paul Hudson pitches 12 blockbusters in a bid to convince investors on boost ing R&D spend”.https ://endpts.com/sanofi - rd - day - ceo - paul - hudson - touts - 12 - blockbusters - ups - rdspend/

79 © 2025 Tonix Pharmaceuticals Holding Corp. TNX - 1700 Recombinant Trefoil Factor Family Member 2 (rTFF2 - HSA) Fusion Protein Targeting the toxic tumor micro - environment
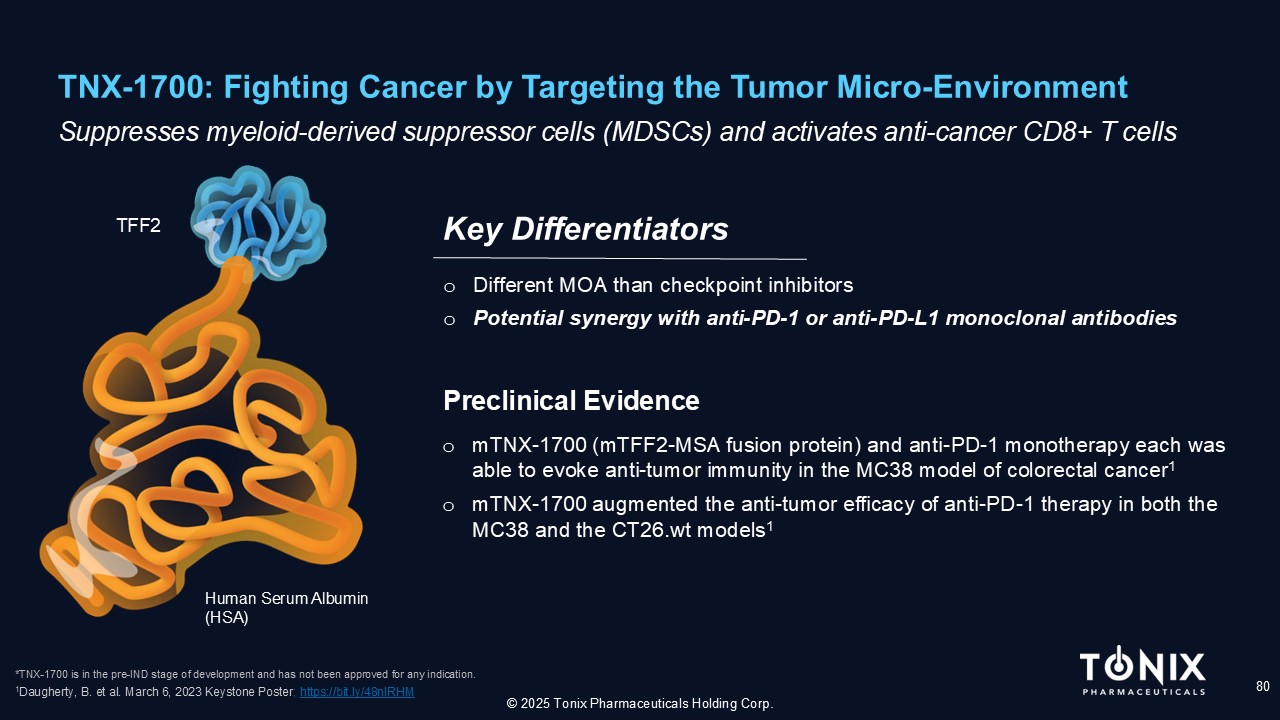
TNX - 1700: Fighting Cancer by Targeting the Tumor Micro - Environment 80 S uppresses myeloid - derived suppressor cells (MDSCs) and activates anti - cancer CD8+ T cells o Different MOA than checkpoint inhibitors o Potential synergy with anti - PD - 1 or anti - PD - L1 monoclonal antibodies Key Differentiators © 2025 Tonix Pharmaceuticals Holding Corp. *TNX - 1700 is in the pre - IND stage of development and has not been approved for any indication. Human Serum Albumin (HSA) TFF2 o mTNX - 1700 (mTFF2 - MSA fusion protein) and anti - PD - 1 monotherapy each was able to evoke anti - tumor immunity in the MC38 model of colorectal cancer 1 o mTNX - 1700 augmented the anti - tumor efficacy of anti - PD - 1 therapy in both the MC38 and the CT26.wt models 1 Prec linical Evidence 1 Daugherty, B. et al. March 6, 2023 Keystone Poster ; https://bit.ly/48nIRHM
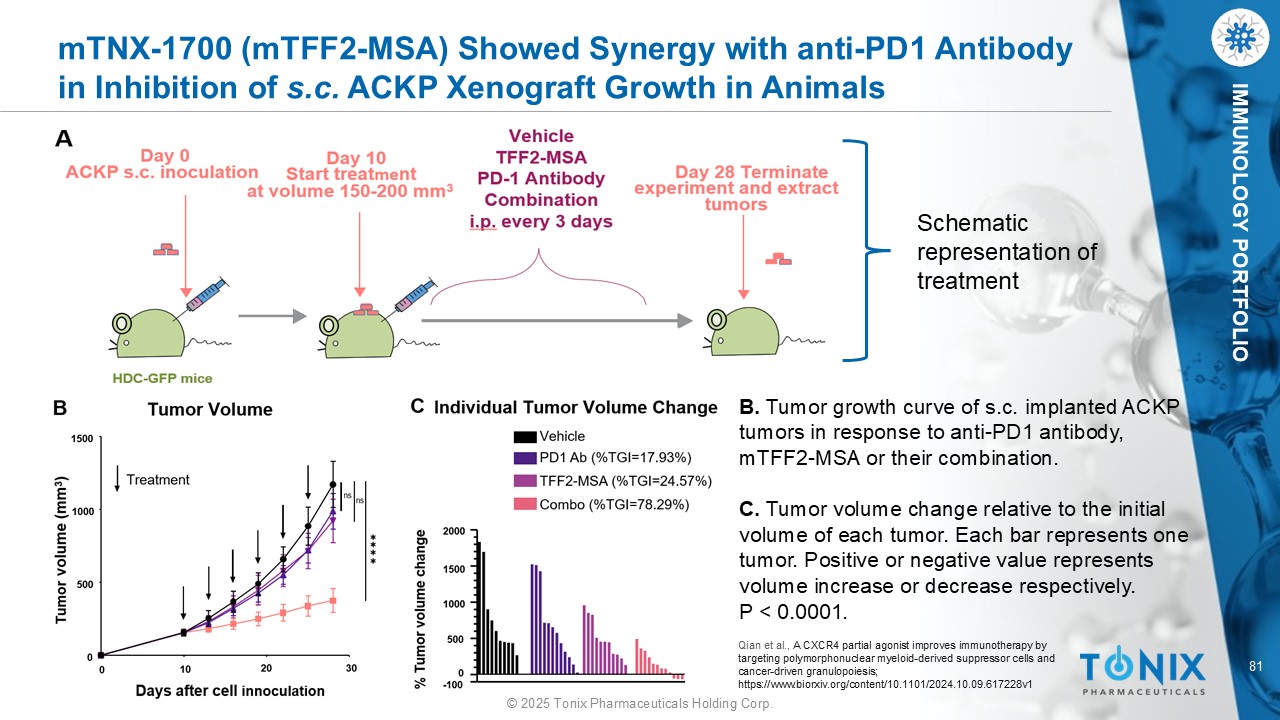
81 © 2025 Tonix Pharmaceuticals Holding Corp. IMMUNOLOGY PORTFOLIO m TNX - 1700 ( m TFF2 - MSA) Showed Synergy with anti - PD1 Antibody in Inhibition of s.c. ACKP Xenograft Growth in Animals B. Tumor growth curve of s.c. implanted ACKP tumors in response to anti - PD1 antibody, mTFF2 - MSA or their combination. C. Tumor volume change relative to the initial volume of each tumor. Each bar represents one tumor. Positive or negative value represents volume increase or decrease respectively. P < 0.0001. Schematic representation of treatment Qian et al., A CXCR4 partial agonist improves immunotherapy by targeting polymorphonuclear myeloid - derived suppressor cells and cancer - driven granulopoiesis; https://www.biorxiv.org/content/10.1101/2024.10.09.617228v1
82 © 2025 Tonix Pharmaceuticals Holding Corp. IMMUNOLOGY PORTFOLIO People living with colorectal cancer in the US 2 About Gastric and Colorectal Cancer Gastric and colorectal cancer are both leading cancers in the US. C olorectal cancer is the 3 rd leading cause of cancer - related deaths in both men and women. 1 >1.3M Current standard of care: • PD - 1 blockade − However, gastric and colorectal cancer are relatively unresponsive Large unmet need: • Gastric and colorectal cancer have a relative 5 - year survival rate of 35.7% and 65%, respectively − Despite advances in the field, patients are still in need of life saving treatment 1 American Cancer Society, accessed September 2023 - https://www.cancer.org/cancer/types/colon - rectal - cancer/about/key - statistics.html 2 NIH, accessed September 2023 - https://seer.cancer.gov/statfacts/html/colorect.html 3 NIH, accessed September 2023 - https://seer.cancer.gov/statfacts/html/stomach.html >125k People living with gastric cancer in the US 3

© 2025 Tonix Pharmaceuticals Holding Corp. TEAM, NETWORK, & UPCOMING MILESTONES
84 © 2025 Tonix Pharmaceuticals Holding Corp. Management Team Seth Lederman, MD Co - Founder, CEO & Chairman Jessica Morris Chief Operating Officer Gregory Sullivan, MD Chief Medical Officer Bradley Saenger, CPA Chief Financial Officer

85 © 2025 Tonix Pharmaceuticals Holding Corp. Milestones: Recently Completed and Upcoming TNX - 102 SL for the Management of Fibromyalgia Milestones FDA Fast Track Designation granted by FDA □ 3 rd Quarter 2024 Submitted NDA to FDA for TNX - 102 SL for fibromyalgia in October 2024 □ October 2024 FDA assigned a PDUFA* goal date of August 15, 2025 □ December 2024 FDA decision expected on marketing authorization □ August 15, 2025 Other Key Program Milestones U.S. DoD / DTRA Awarded up to $34 M contract (over 5 years) for broad spectrum antiviral development (TNX - 4200) □ 3 rd Quarter 2024 Initiate Phase 2 study of TNX - 1300 for the treatment of cocaine intoxication □ 3 rd Quarter 2024 Topline results from First in Human Phase 1 Pharmacokinetic and Pharmacodynamic study of TNX - 1500 (in development for prevention of organ transplant and treatment of autoimmunity □ 1 st Quarter 2025 Initiate Phase 2 Investigator - Initiated study at UNC of TNX - 102 SL for the treatment of Acute Stress Disorder (ASD) / Acute Stress Reaction (ASR) □ 1 st Quarter 2025 Topline results from Phase 2 study of TNX - 1300 for the treatment of cocaine intoxication □ 3 rd Quarter 2025 x x x x *PDUFA = Prescription Drug User Fee Act x x

© 2025 Tonix Pharmaceuticals Holding Corp. THANK YOU

87 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Zembrace ® Important Safety Information (1 of 2) Zembrace SymTouch ( Zembrace ) can cause serious side effects, including heart attack and other heart problems, which may lead to death. Stop use and get emergency help if you have any signs of a heart attack: D iscomfort in the center of your chest that lasts for more than a few minutes or goes away and comes back; severe tightness, pain, pressure, or heaviness in your chest, throat, neck, or jaw; pain or discomfort in your arms, back, neck, jaw or stomach ; shortness of breath with or without chest discomfort ; breaking out in a cold sweat ; nausea or vomiting ; feeling lightheaded Zembrace is not for people with risk factors for heart disease (high blood pressure or cholesterol, smoking, overweight, diabetes, family history of heart disease) unless a heart exam shows no problem. Do not use Zembrace if you have: H istory of heart problems ; narrowing of blood vessels to your legs, arms, stomach, or kidney (peripheral vascular disease) ; uncontrolled high blood pressure ; hemiplegic or basilar migraines. If you are not sure if you have these, ask your provider. H ad a stroke, transient ischemic attacks (TIAs), or problems with blood circulation ; severe liver problems ; taken any of the following medicines in the last 24 hours: almotriptan , eletriptan , frovatriptan , naratriptan, rizatriptan, ergotamines , dihydroergotamine ; are taking certain antidepressants, known as monoamine oxidase (MAO) - A inhibitors or it has been 2 weeks or less since you stopped taking a MAO - A inhibitor. Ask your provider for a list of these medicines if you are not sure. A n allergy to sumatriptan or any of the components of Zembrace Tell your provider about all of your medical conditions and medicines you take, including vitamins and supplements. Zembrace can cause dizziness, weakness, or drowsiness. If so, do not drive a car, use machinery, or do anything where you need to be alert.

88 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Zembrace ® Important Safety Information (2 of 2) Zembrace may cause serious side effects including: • Changes in color or sensation in your fingers and toes; sudden or severe stomach pain, stomach pain after meals, weight loss, na usea or vomiting, constipation or diarrhea, bloody diarrhea, fever; cramping and pain in your legs or hips; feeling of heaviness or t igh tness in your leg muscles; burning or aching pain in your feet or toes while resting; numbness, tingling, or weakness in your legs; cold fe eli ng or color changes in one or both legs or feet; increased blood pressure including a sudden severe increase even if you have no history of high blood pressure; medication overuse headaches from using migraine medicine for 10 or more days each month. If your headaches g et worse, call your provider. • Serotonin syndrome, a rare but serious problem that can happen in people using Zembrace , especially when used with anti - depressant medicines called SSRIs or SNRIs. Call your provider right away if you have: mental changes such as seeing things that are not th ere (hallucinations), agitation, or coma; fast heartbeat; changes in blood pressure; high body temperature; tight muscles; or tro ubl e walking. • Hives (itchy bumps); swelling of your tongue, mouth, or throat • Seizures even in people who have never had seizures before The most common side effects of Zembrace include: pain and redness at injection site; tingling or numbness in your fingers or toes; dizziness; warm, hot, burning feeling to your face (flushing); discomfort or stiffness in your neck; feeling weak, drowsy, or ti red. Tell your provider if you have any side effect that bothers you or does not go away. These are not all the possible side effe cts of Zembrace . For more information, ask your provider. This is the most important information to know about Zembrace but is not comprehensive. For more information, talk to your provider and read the Patient Information and Instructions for Use . For full Prescribing Information, visit: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=6e5b104f - 2b9e - 416e - 92fb - ef1bdaea867d You are encouraged to report adverse effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1 - 800 - FDA - 1088. Zembrace is a prescription medicine used to treat acute migraine headaches with or without aura in adults who have been diagnosed with migraine. Zembrace is not used to prevent migraines. It is not known if it is safe and effective in children under 18 years of age.

89 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Tosymra® Important Safety Information (1 of 2) Tosymra® can cause serious side effects, including heart attack and other heart problems, which may lead to death. Stop Tosymra and get emergency medical help if you have any signs of heart attack: • D iscomfort in the center of your chest that lasts for more than a few minutes or goes away and comes back ; severe tightness, pain, pressure, or heaviness in your chest, throat, neck, or jaw ; pain or discomfort in your arms, back, neck, jaw, or stomach ; shortness of breath with or without chest discomfort; breaking out in a cold sweat ; nausea or vomiting ; feeling lightheaded Tosymra is not for people with risk factors for heart disease (high blood pressure or cholesterol, smoking, overweight, diabetes, family history of heart disease) unless a heart exam is done and shows no problem. Do not use Tosymra if you have: • H istory of heart problems ; narrowing of blood vessels to your legs, arms, stomach, or kidney (peripheral vascular disease) ; uncontrolled high blood pressure ; severe liver problems ; hemiplegic or basilar migraines. If you are not sure if you have these, ask your healthcare provider. • H ad a stroke, transient ischemic attacks (TIAs), or problems with blood circulation ; taken any of the following medicines in the last 24 hours: almotriptan , eletriptan , frovatriptan , naratriptan, rizatriptan, ergotamines , or dihydroergotamine. Ask your provider if you are not sure if your medicine is listed above • are taking certain antidepressants, known as monoamine oxidase (MAO) - A inhibitors or it has been 2 weeks or less since you stopped taking a MAO - A inhibitor . A sk your provider for a list of these medicines if you are not sure • A n allergy to sumatriptan or any ingredient in Tosymra Tell your provider about all of your medical conditions and medicines you take, including vitamins and supplements. Tosymra can cause dizziness, weakness, or drowsiness. If so, do not drive a car, use machinery, or do anything where you need to be alert.

90 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Tosymra may cause serious side effects including: • Changes in color or sensation in your fingers and toes; sudden or severe stomach pain, stomach pain after meals, weight loss, nausea or vomiting, constipation or diarrhea, bloody diarrhea, fever; cramping and pain in your legs or hips, feeling of heaviness or tightness in your leg muscles, burning or aching pain in your feet or toes while resting, numbness, tingling, or weakness in your legs, cold feeling or color changes in one or both legs or feet; increased blood pressure including a sudden severe increase even if you have no history of high blood pressure; medication overuse headaches from using migraine medicine for 10 or more days each month. If your headaches get worse, call your provider . • Serotonin syndrome, a rare but serious problem that can happen in people using Tosymra , especially when used with anti - depressant medicines called SSRIs or SNRIs. Call your provider right away if you have : mental changes such as seeing things that are not there (hallucinations), agitation, or coma; fast heartbeat; changes in blood pressure; high body temperature; tight muscles; or trouble walking. • Seizures even in people who have never had seizures before The most common side effects of Tosymra include : tingling, dizziness, feeling warm or hot, burning feeling, feeling of heaviness, feeling of pressure, flushing, feeling of ti ght ness, numbness, application site (nasal) reactions, abnormal taste, and throat irritation. Tell your provider if you have any side effect that bothers you or does not go away. These are not all the possible side effects of Tosymra . For more information, ask your provider. This is the most important information to know about Tosymra but is not comprehensive. For more information, talk to your provider and read the Patient Information and Instructions for use . For full Prescribing Information, visit: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=015a5cf9 - f246 - 48bc - b91e - cd730a53d8aa . You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch , or call 1 - 800 - FDA - 1088. Tosymra is a prescription medicine used to treat acute migraine headaches with or without aura in adults. Tosymra is not used to treat other types of headaches such as hemiplegic or basilar migraines or cluster headaches. Tosymra is not used to prevent migraines. It is not known if Tosymra is safe and effective in children under 18 years of age. Tosymra ® Important Safety Information (2 of 2)