
Tonix Pharmaceuticals Form 8-K
Exhibit 99.02

© 2025 Tonix Pharmaceuticals Holding Corp. Controversies in Fibromyalgia 2025 NASDAQ: TNXP Version P06048 March 3, 2025 (Doc 1564 ) Transmucosal Sublingual Cyclobenzaprine (TNX - 102 SL) Treatment of Fibromyalgia at Bedtime to Target Non - Restorative Sleep Showed Durable Pain Reduction in Two Double - Blind Randomized Phase 3 Studies March 3, 2025

2 © 2025 Tonix Pharmaceuticals Holding Corp. Cautionary Note on Forward - Looking Statements Certain statements in this presentation regarding strategic plans, expectations and objectives for future operations or results are “forward - looking statements” as defined by the Private Securities Litigation Reform Act of 1995. These statements may be identified by the use of forward - looking words such as “anticipate,” “believe,” “forecast,” “estimate” and “intend,” among others. These forward - looking statements are based on Tonix’s current expectations and actual results could differ materially. There are a number of factors that could cause actual events to differ materially from those indicated by such forward - looking statements. These factors include, but are not limited to, the risks related to failure to obtain FDA clearances or approvals and noncompliance with FDA regulations; risks related to the failure to successfully market any of our products; risks related to the timing and progress of clinical development of our product candidates; our need for additional financing; uncertainties of patent protection and litigation; uncertainties of government or third party payor reimbursement; limited research and development efforts and dependence upon third parties; and substantial competition. As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products. The forward - looking statements in this presentation are made as of the date of this presentation, even if subsequently made available by Tonix on its website or otherwise. Tonix does not undertake an obligation to update or revise any forward - looking statement, except as required by law. Investors should read the risk factors set forth in the Annual Report on Form 10 - K for the year ended December 31, 2023, as filed with the Securities and Exchange Commission (the “SEC”) on April 1, 2024, and periodic reports and current reports filed with the SEC on or after the date thereof. All of Tonix's forward - looking statements are expressly qualified by all such risk factors and other cautionary statements.
3 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 102 SL (Cyclobenzaprine HCl Sublingual Tablets) 1 • Non - opioid analgesic designed for long - term daily bedtime use in fibromyalgia patients ‒ Targets non - restorative sleep ‒ Potent binding and antagonist activities at the serotonin - 5 - HT2A, α1 - adrenergic, histaminergic - H1, and muscarinic - M1 receptors ‒ No recognized risk for abuse • Improves sleep quality , does not increase sleep quantity : ‒ Not a traditional hypnotic or sedative • Proprietary, sublingual transmucosal formulation of cyclobenzaprine designed to optimize delivery and absorption ‒ P rotectic ® formulation based on eutectic composition of matter • Rapid a bsorption • Decrease in major metabolite by bypassing first - pass hepatic metabolism 1 *TNX - 102 SL has not been approved for any indication.
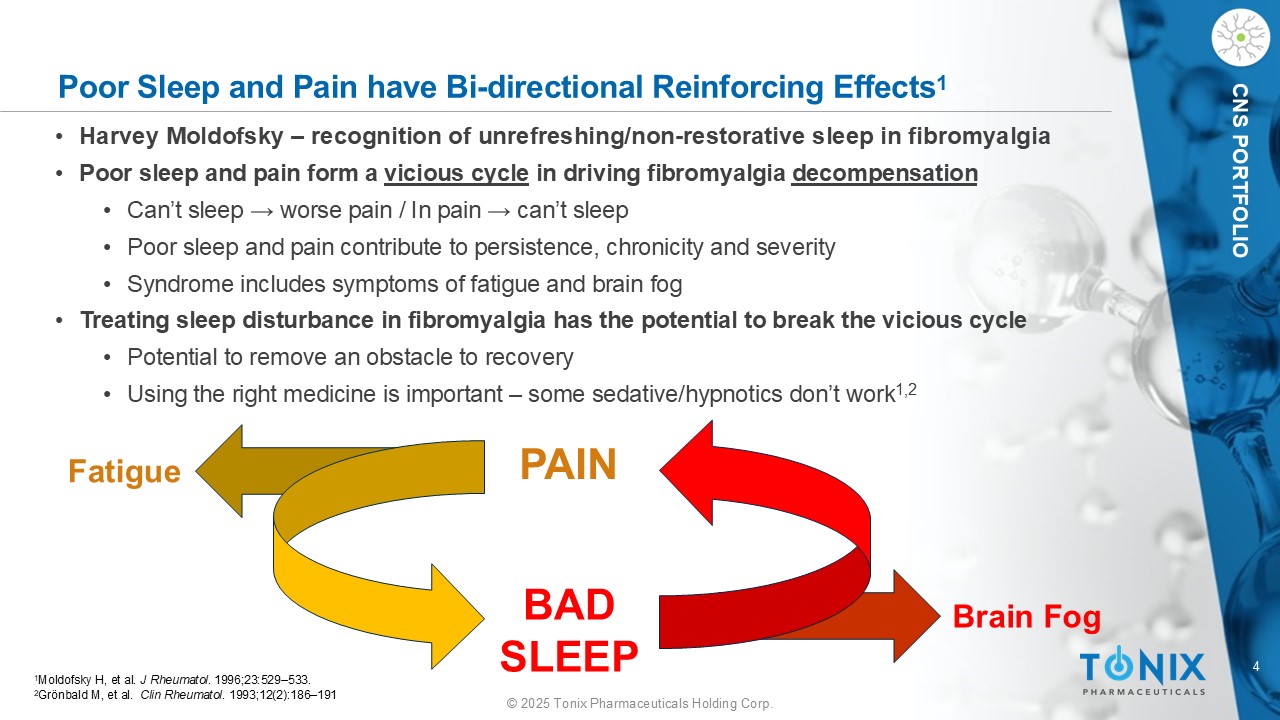
4 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Poor Sleep and Pain have Bi - directional Reinforcing Effects 1 1 Moldofsky H, et al. J Rheumatol . 1996;23:529 – 533. 2 Grönbald M, et al. Clin Rheumatol . 1993;12(2):186 – 191 • Harvey Moldofsky – recognition of unrefreshing/non - restorative sleep in fibromyalgia • Poor sleep and pain form a vicious cycle in driving fibromyalgia decompensation • Can’t sleep → worse pain / In pain → can’t sleep • Poor sleep and pain contribute to persistence, chronicity and severity • Syndrome includes symptoms of fatigue and brain fog • Treating sleep disturbance in fibromyalgia has the potential to break the vicious cycle • Potential to remove an obstacle to recovery • Using the right medicine is important – some sedative/hypnotics don’t work 1,2 PAIN BAD SLEEP Fatigue Brain Fog

5 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Fibromyalgia: Unrefreshing Sleep and Cyclobenzaprine Treatment 1 Moldofsky H et al. Psychosom Med. 1975. 37:341 - 51. 2 Moldofsky H and Scarisbrick P. Psychosom Med. 1976. 38:35 - 44. 3 Bennett RM, et al. Arthritis Rheum 1988 . 31 : 1535 – 42 . 4 Quimby LG, et al . J Rheumatol Suppl, 1989 Nov ; 19 : 140 – 3 . 5 Reynolds WJ, et al. J Rheumatol . 1991 . 18 : 452 – 4 . 6 Santandrea S, et al . J Int Med Res. 1993 . 21 : 74 – 80 . • Non - restorative sleep 1,2 ‒ Harvey Moldofsky – recognition of unrefreshing/non - restorative sleep: ▪ Symptom ▪ Potential causative or potentiating factor • Cyclobenzaprine 3,9 ‒ With amitriptyline one of the earliest drugs studied in fibromyalgia as an oral swallowed agent ‒ Studies showed equivocal effects and tolerability issues at “muscle spasm” doses • Bedtime, low - dose cyclobenzaprine targeting non - restorative sleep 10 - 11 ‒ Recognition of unrefreshing sleep as a target of therapy ‒ Primitive oral, swallowed formulation – “flat” pharmacokinetics • Bedtime, sublingual transmucosal cyclobenzaprine targeting non - restorative sleep 12 ‒ Dynamic pharmacokinetic profile, rapid absorption, decrease in major metabolite ‒ Two studies (Phase 2 and Phase 3) at 2.8 mg; three Phase 3 studies at 5.6 mg. 7 Cantini F, et al . Minerva Med. 1994 . 85 : 97 – 100 . 8 Carette S, et al. Arthritis Rheum. 1994 . 37 : 32 – 40 . 9 Tofferi JK, et al . Arthritis Rheum. 2004 . 51 : 9 – 13 . 1 10 Iglehart IW. 2003; US Patent 6,541,523. 11 Moldofsky et al. J Rheumatol . 2011. 38:2653 - 2663 12 Lederman S et al. Arthritis Care Res . 2023. 75:2359 - 2368.
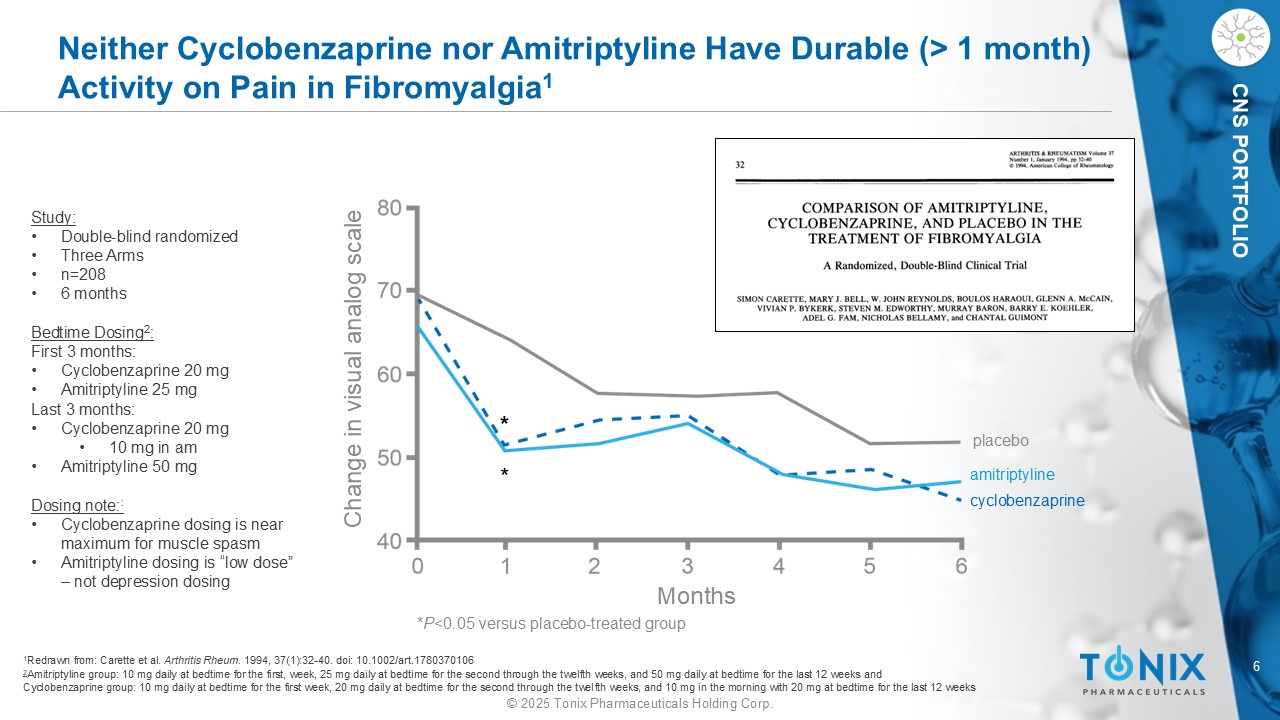
6 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Neither Cyclobenzaprine nor Amitriptyline Have Durable (> 1 month) Activity on Pain in Fibromyalgia 1 Months Change in visual analog scale * * * P <0.05 versus placebo - treated group placebo cyclobenzaprine amitriptyline 1 Redrawn from: Carette et al. Arthritis Rheum. 1994, 37(1):32 - 40. doi : 10.1002/art.1780370106 2 Amitriptyline group: 10 mg daily at bedtime for the first, week, 25 mg daily at bedtime for the second through the twelfth we eks , and 50 mg daily at bedtime for the last 12 weeks and Cyclobenzaprine group: 10 mg daily at bedtime for the first week, 20 mg daily at bedtime for the second through the twelfth w eek s, and 10 mg in the morning with 20 mg at bedtime for the last 12 weeks Study: • Double - blind randomized • Three Arms • n=208 • 6 months Bedtime Dosing 2 : First 3 months: • Cyclobenzaprine 20 mg • Amitriptyline 25 mg Last 3 months: • Cyclobenzaprine 20 mg • 10 mg in am • Amitriptyline 50 mg Dosing note: : • Cyclobenzaprine dosing is near maximum for muscle spasm • Amitriptyline dosing is “low dose” – not depression dosing
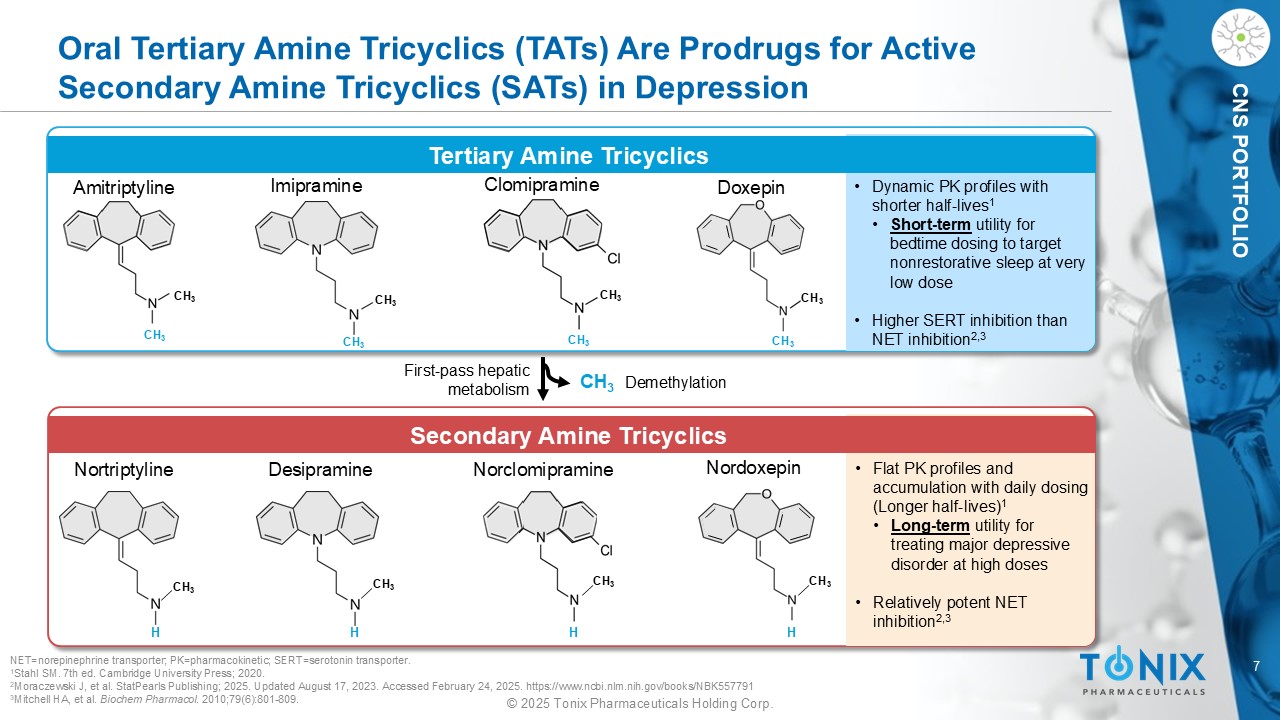
7 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Oral Tertiary Amine Tricyclics (TATs) Are Prodrugs for Active Secondary Amine Tricyclics (SATs) in Depression Tertiary Amine Tricyclics Amitriptyline Nortriptyline Imipramine Desipramine Norclomipramine Doxepin Nordoxepin CH 3 CH 3 CH 3 First - pass hepatic metabolism Demethylation Secondary Amine Tricyclics • Dynamic PK profiles with shorter half - lives 1 • Short - term utility for bedtime dosing to target nonrestorative sleep at very low dose • Higher SERT inhibition than NET inhibition 2,3 • Flat PK profiles and accumulation with daily dosing (Longer half - lives) 1 • Long - term utility for treating major depressive disorder at high doses • Relatively potent NET inhibition 2,3 NET=norepinephrine transporter; PK = pharmacokinetic; SERT=serotonin transporter . 1 Stahl SM. 7th ed. Cambridge University Press; 2020. 2 Moraczewski J, et al. StatPearls Publishing; 2025. Updated August 17, 2023. Accessed February 24, 2025. https://www.ncbi.nlm.nih.gov/books/NBK557791 3 Mitchell HA, et al. Biochem Pharmacol . 2010;79(6):801 - 809. CH 3 CH 3 Clomipramine CH 3 CH 3 CH 3 CH 3 H CH 3 CH 3 H CH 3 H CH 3 H
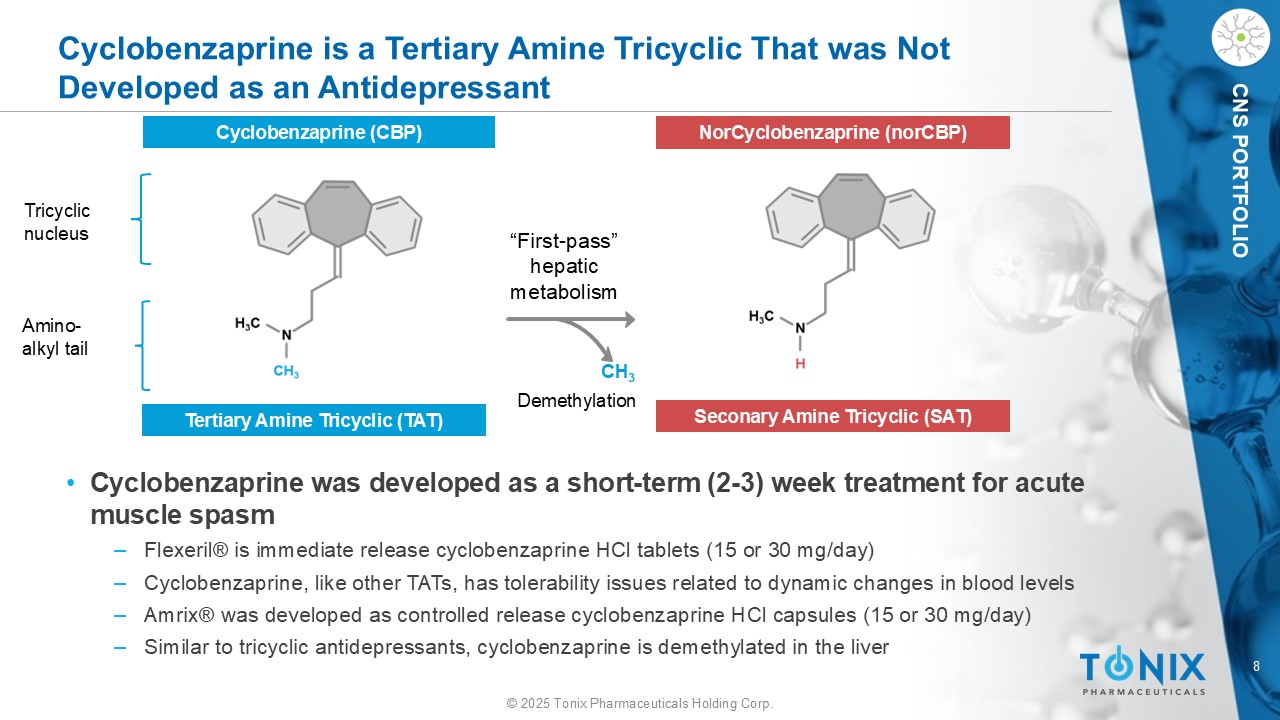
8 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Cyclobenzaprine is a Tertiary Amine Tricyclic That was Not Developed as an Antidepressant CH 3 “First - pass” hepatic metabolism D emethylation • Cyclobenzaprine was developed as a short - term (2 - 3) week treatment for acute muscle spasm ‒ Flexeril® is immediate release cyclobenzaprine HCl tablets (15 or 30 mg/day) ‒ Cyclobenzaprine, like other TATs, has tolerability issues related to dynamic changes in blood levels ‒ Amrix ® was developed as controlled release cyclobenzaprine HCl capsules (15 or 30 mg/day) ‒ Similar to tricyclic antidepressants, cyclobenzaprine is demethylated in the liver Cyclobenzaprine (CBP) N orCyclobenzaprine ( norCBP ) Tertiary Amine Tricyclic (TAT) Seconary Amine Tricyclic (SAT) Tricyclic nucleus Amino - alkyl tail
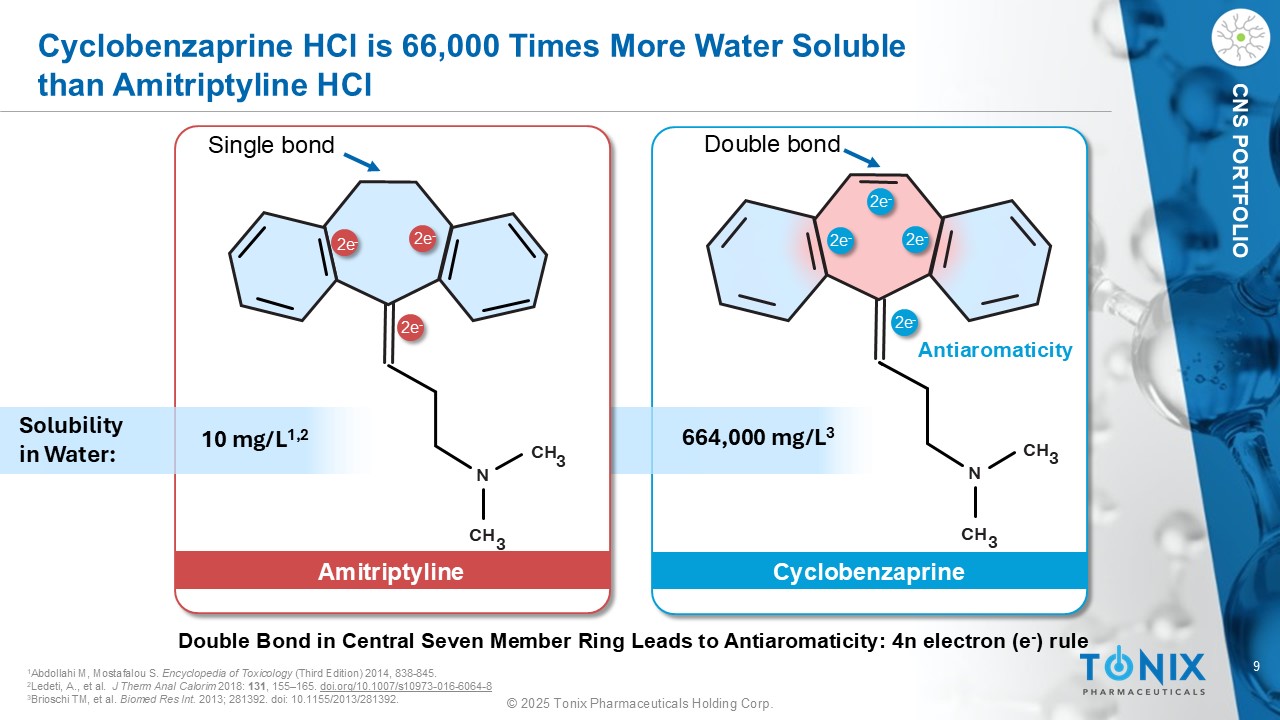
9 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Cyclobenzaprine Amitriptyline N CH 3 CH 3 N CH 3 CH 3 Cyclobenzaprine HCl is 66,000 Times More Water Soluble than Amitriptyline HCl 2e - 2e - 2e - 2e - Antiaromaticity 10 mg/L 1,2 664,000 mg/L 3 1 Abdollahi M, Mostafalou S. Encyclopedia of Toxicology (Third Edition) 2014, 838 - 845. 2 Ledeti, A., et al. J Therm Anal Calorim 2018: 131 , 155 – 165. doi.org/10.1007/s10973 - 016 - 6064 - 8 3 Brioschi TM, et al. Biomed Res Int. 2013; 281392. doi : 10.1155/2013/281392. Solubility in Water: 2e - 2e - 2e - Double bond Single bond Double Bond in Central Seven Member Ring Leads to Antiaromaticity: 4n electron (e - ) rule
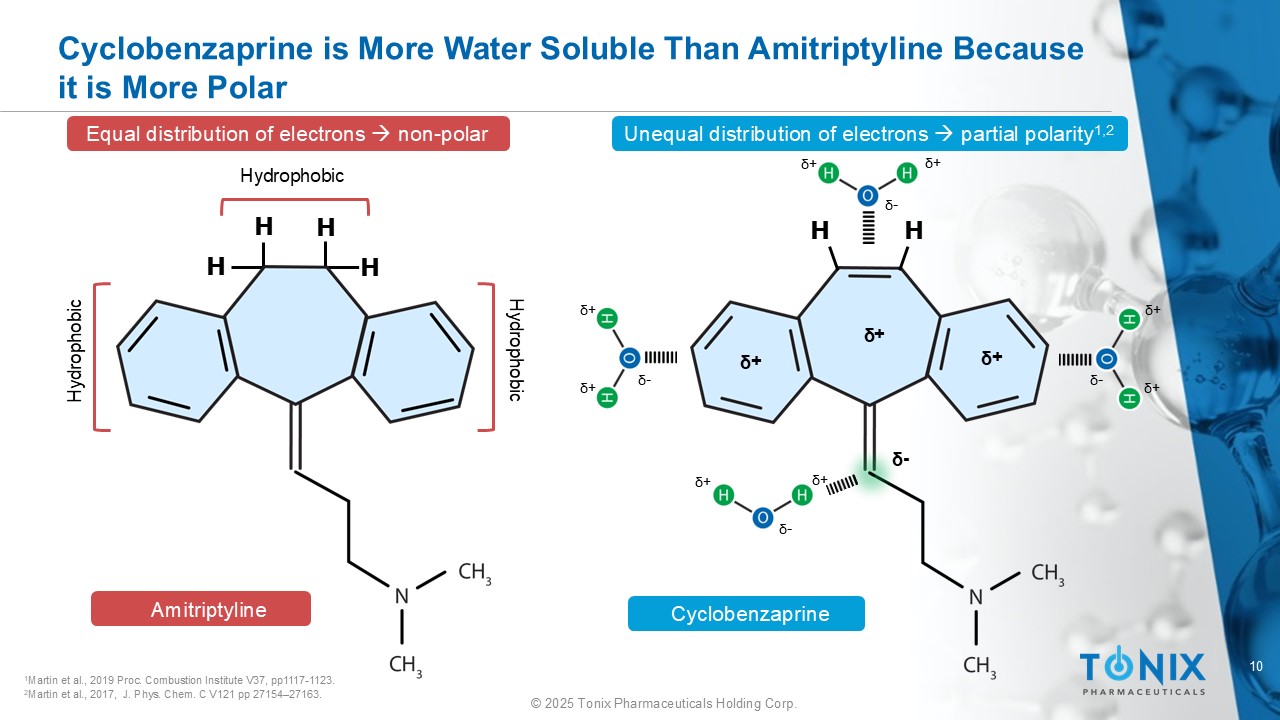
10 © 2025 Tonix Pharmaceuticals Holding Corp. Cyclobenzaprine is More Water Soluble Than Amitriptyline Because it is More Polar H H H H ᵟ - H H Unequal distribution of electrons partial polarity 1,2 Equal distribution of electrons non - polar Hydrophobic Hydrophobic 1 Martin et al., 2019 Proc. Combustion Institute V37, pp1117 - 1123. 2 Martin et al., 2017, J. Phys. Chem. C V121 pp 27154 – 27163. Hydrophobic ᵟ + ᵟ + ᵟ - ᵟ + ᵟ + ᵟ - ᵟ + ᵟ + ᵟ + ᵟ + ᵟ - ᵟ + ᵟ + ᵟ - Amitriptyline Cyclobenzaprine ᵟ +
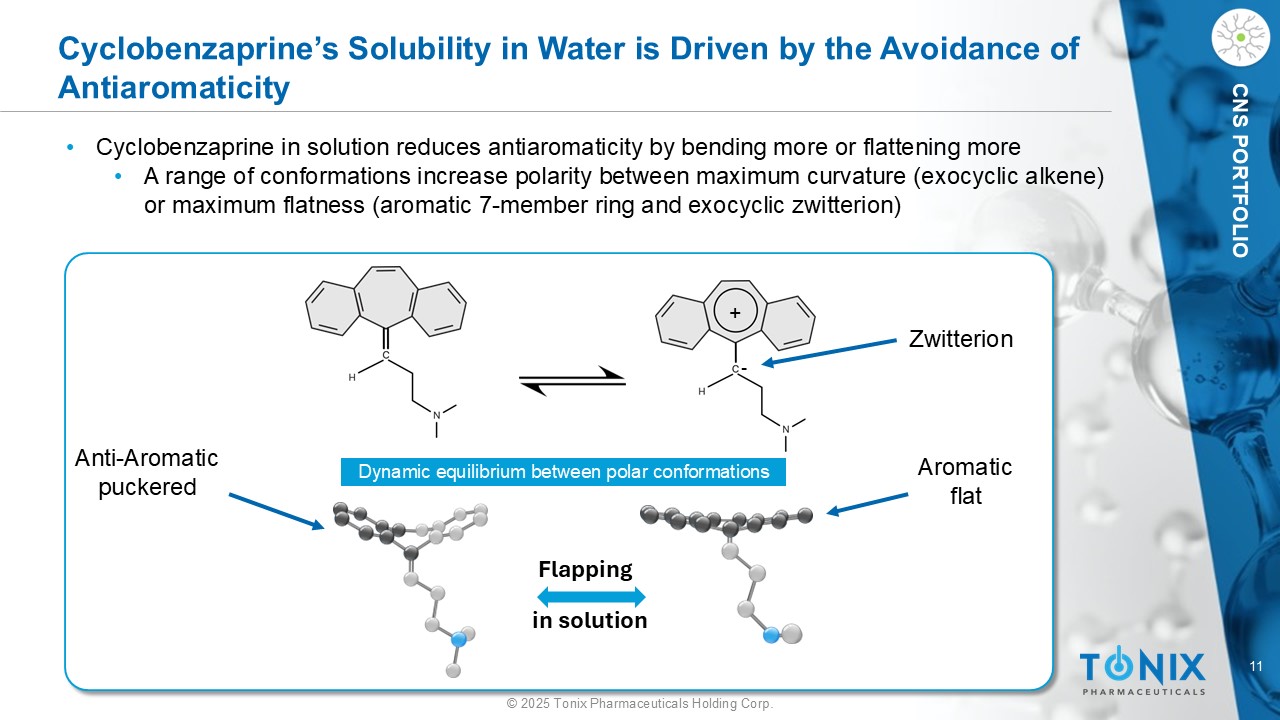
11 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Cyclobenzaprine’s Solubility in Water is Driven by the Avoidance of Antiaromaticity Dynamic equilibrium between polar conformations Flapping i n solution • Cyclobenzaprine in solution reduces antiaromaticity by bending more or flattening more • A range of conformations increase polarity between maximum curvature (exocyclic alkene) or maximum flatness (aromatic 7 - member ring and exocyclic zwitterion) + - Zwitterion Aromatic flat Anti - Aromatic puckered
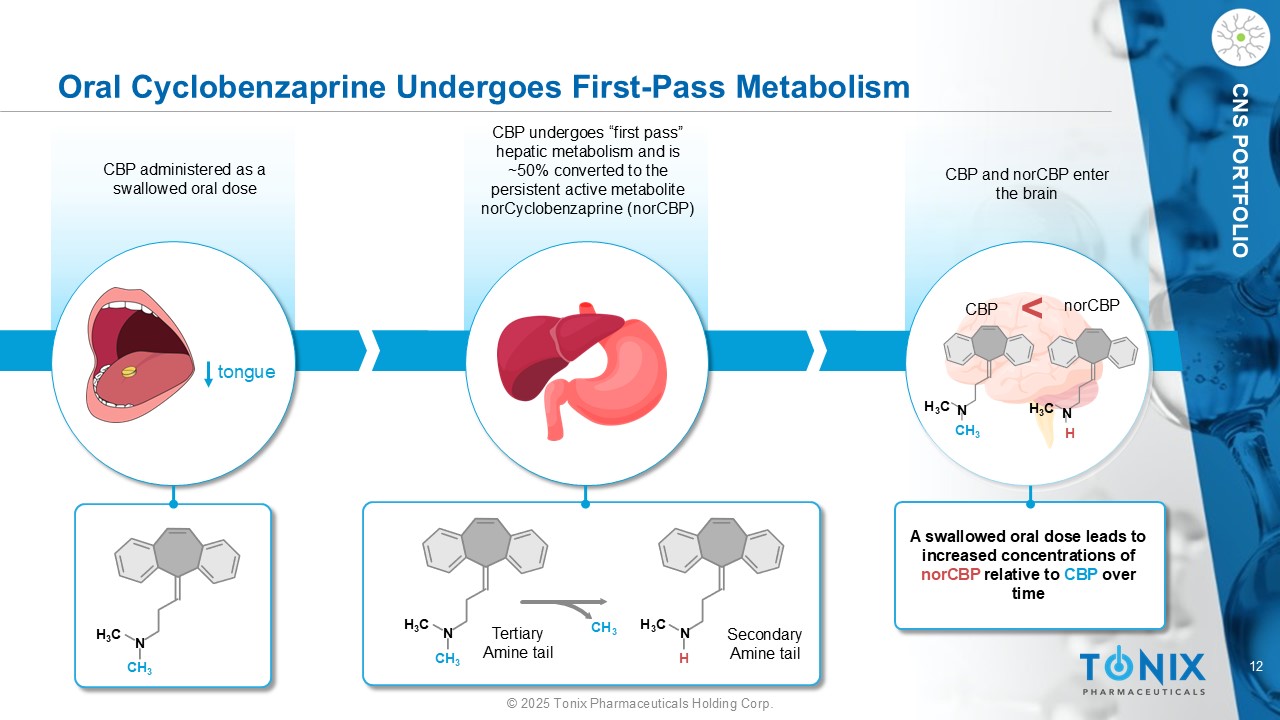
12 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO CBP and norCBP enter the brain Oral Cyclobenzaprine Undergoes First - Pass Metabolism tongue CBP administered as a swallowed oral dose CBP undergoes “first pass” hepatic metabolism and is ~50% converted to the persistent active metabolite norCyclobenzaprine ( norCBP ) H 3 C N CH 3 H 3 C N CH 3 CH 3 H 3 C N H H 3 C N CH 3 H 3 C N H A swallowed oral dose leads to increased concentrations of norCBP relative to CBP over time < CBP norCBP Secondary Amine tail Tertiary Amine tail
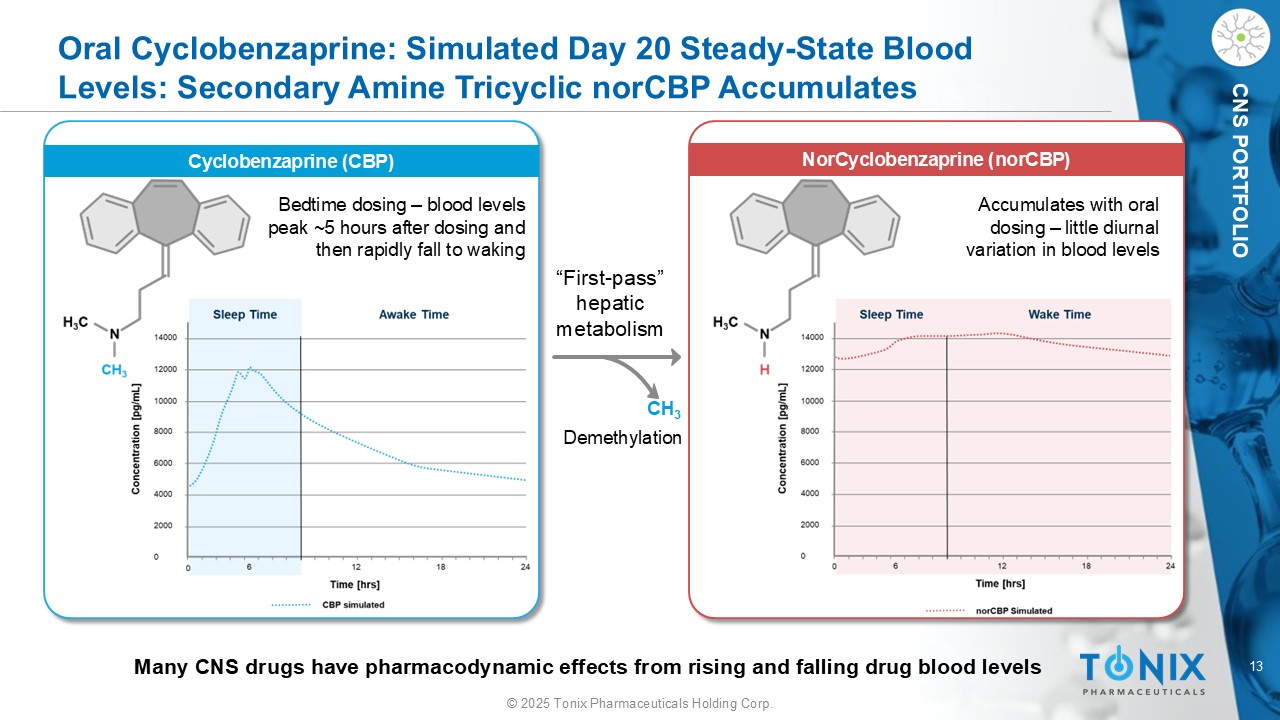
13 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Oral Cyclobenzaprine: Simulated Day 20 Steady - State Blood Levels: Secondary Amine Tricyclic norCBP Accumulates CH 3 “First - pass” hepatic metabolism Many CNS drugs have pharmacodynamic effects from rising and falling drug blood levels D emethylation Cyclobenzaprine (CBP) Bedtime dosing – blood levels peak ~5 hours after dosing and then rapidly fall to waking Accumulates with oral dosing – little diurnal variation in blood levels N orCyclobenzaprine ( norCBP )
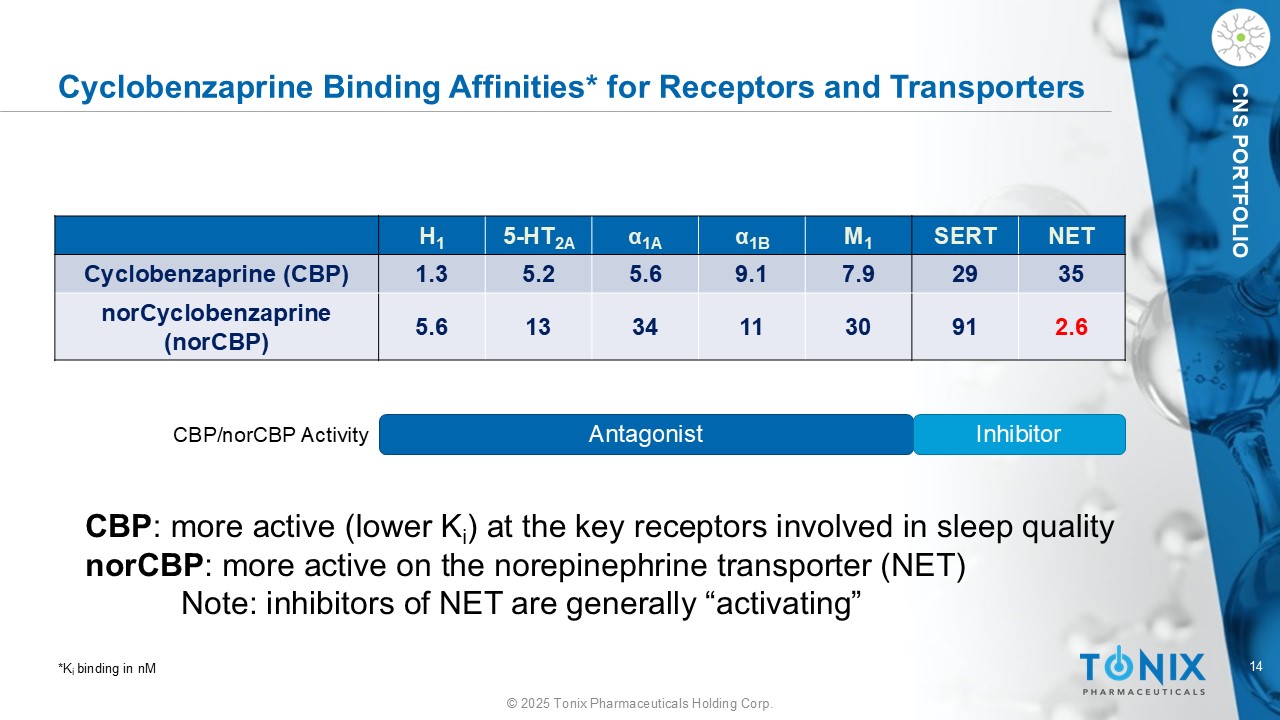
14 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Cyclobenzaprine Binding Affinities* for Receptors and Transporters NET SERT M 1 α 1B α 1A 5 - HT 2A H 1 35 29 7.9 9.1 5.6 5.2 1.3 Cyclobenzaprine (CBP) 2.6 91 30 11 34 13 5.6 norCyclobenzaprine ( norCBP ) Antagonist Inhibitor CBP/ norCBP Activity CBP : more active (lower K i ) at the key receptors involved in sleep quality norCBP : more active on the norepinephrine transporter (NET) Note: inhibitors of NET are generally “activating” *K i binding in nM
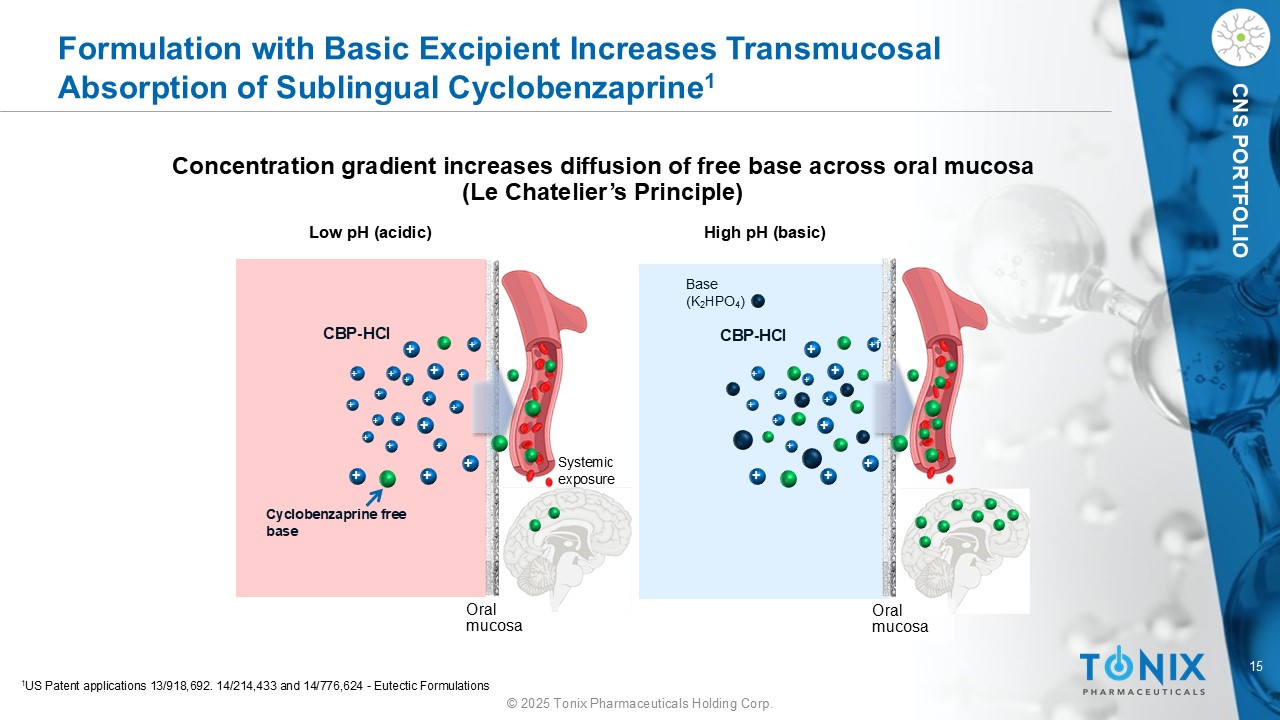
15 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Formulation with Basic Excipient Increases Transmucosal Absorption of Sublingual Cyclobenzaprine 1 Concentration gradient increases diffusion of free base across oral mucosa (Le Chatelier’s Principle) CBP - HCl Cyclobenzaprine free base Oral mucosa CBP - HCl Low pH (acidic) High pH (basic) Systemic exposure Base (K 2 HPO 4 ) + + + + + + + + + + + + + + + + + + + + + + + + + + +f + + + + + + + 1 US Patent applications 13/918,692. 14/214,433 and 14/776,624 - Eutectic Formulations Oral mucosa
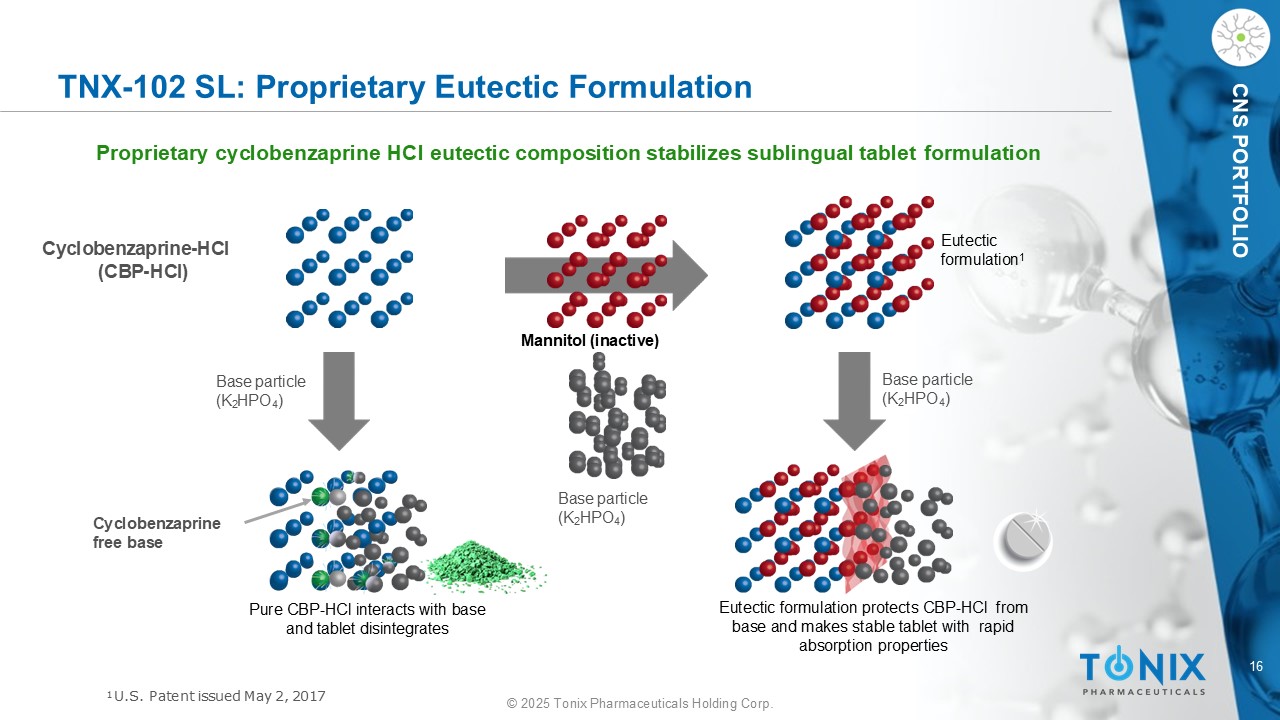
16 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 102 SL: Proprietary Eutectic Formulation Proprietary cyclobenzaprine HCl eutectic composition stabilizes sublingual tablet formulation Base particle (K 2 HPO 4 ) Base particle (K 2 HPO 4 ) Base particle (K 2 HPO 4 ) C y cl o be n z a p r i n e - HCl (CBP - HCl) Eutectic formulation protects CBP - HCl from base and makes stable tablet with rapid absorption properties Pure CBP - HCl interacts with base and tablet disintegrates Cy c l ob en zapr ine free base Eutectic formulation 1 Mannitol (inactive) 1 U.S. Patent issued May 2, 2017
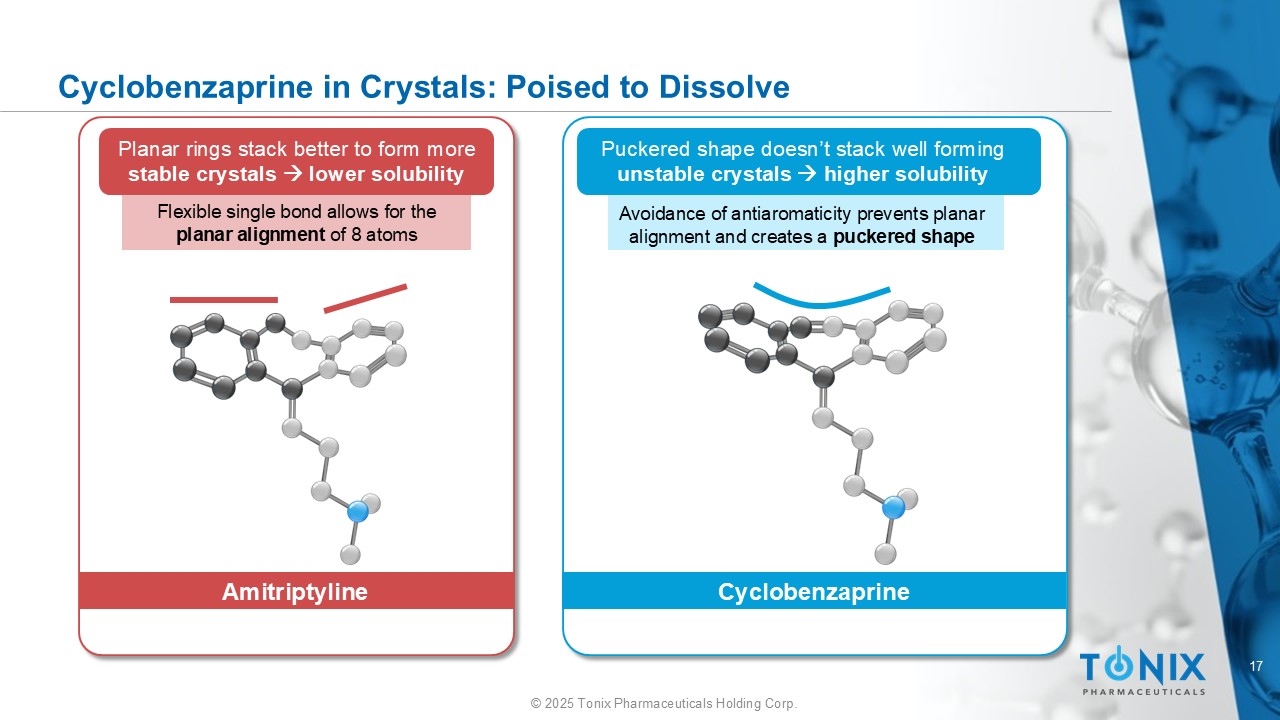
17 © 2025 Tonix Pharmaceuticals Holding Corp. Cyclobenzaprine in Crystals: Poised to Dissolve Puckered shape doesn’t stack well forming unstable crystals higher solubility Planar rings stack better to form more stable crystals lower solubility Flexible single bond allows for the planar alignment of 8 atoms Avoidance of antiaromaticity prevents planar alignment and creates a puckered shape Amitriptyline Cyclobenzaprine
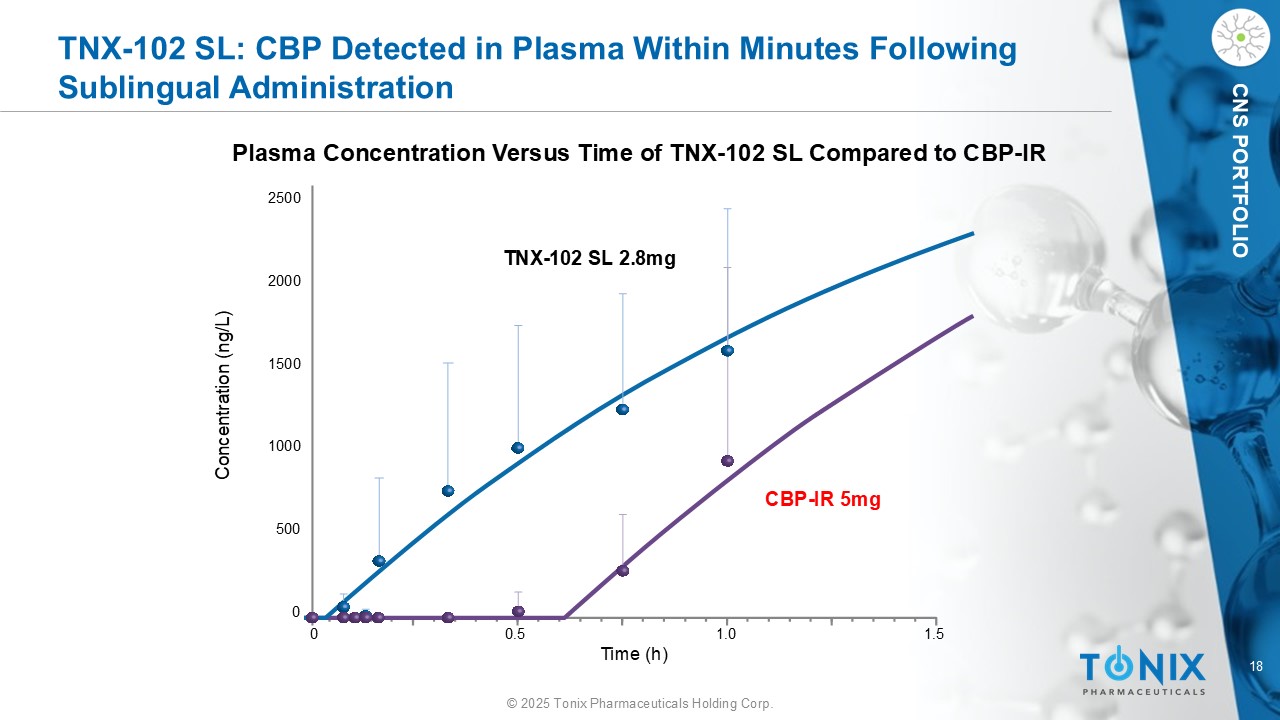
18 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 102 SL: CBP Detected in Plasma Within Minutes Following Sublingual Administration Plasma Concentration Versus Time of TNX - 102 SL Compared to CBP - IR 0 0.5 1.0 1.5 Concentration (ng/L) 2500 1500 1000 500 2000 0 TNX - 102 SL 2.8mg CBP - IR 5mg Time (h)
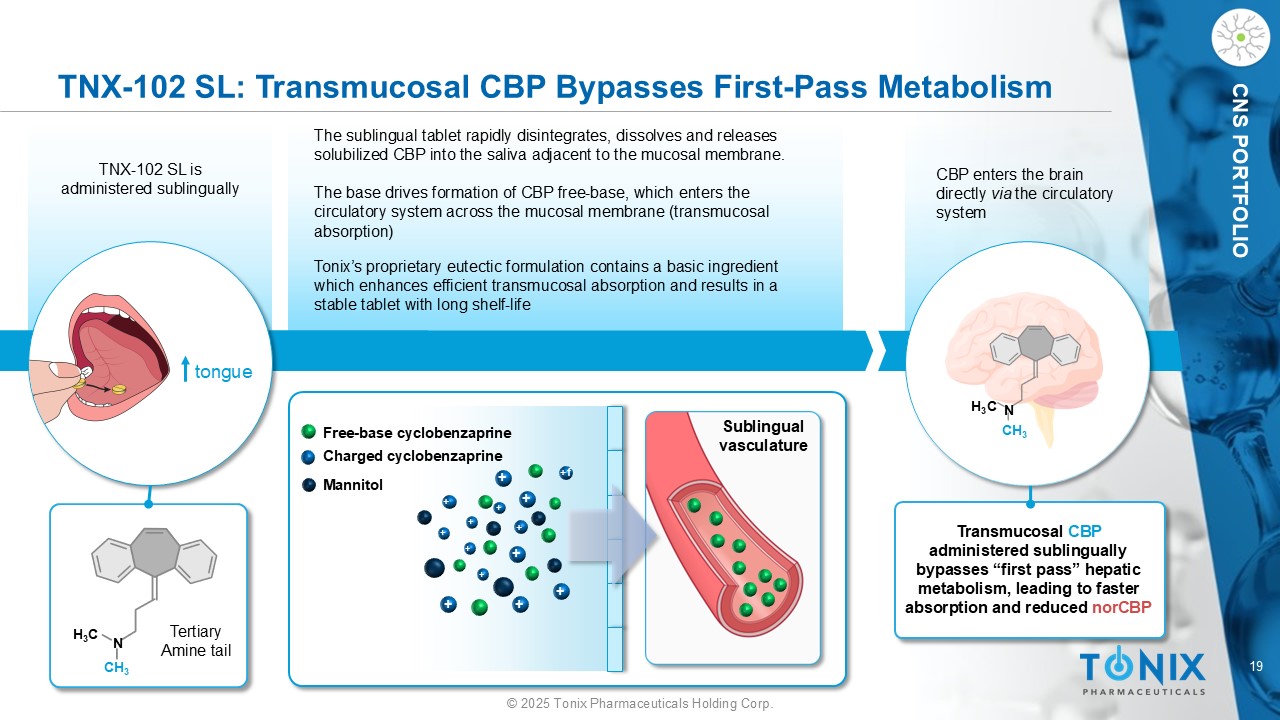
19 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO CBP enters the brain directly via the circulatory system TNX - 102 SL: Transmucosal CBP Bypasses First - Pass Metabolism tongue TNX - 102 SL is administered sublingually The sublingual tablet rapidly disintegrates, dissolves and releases solubilized CBP into the saliva adjacent to the mucosal membrane. The base drives formation of CBP free - base, which enters the circulatory system across the mucosal membrane (transmucosal absorption) H 3 C N CH 3 H 3 C N CH 3 Transmucosal CBP administered sublingually bypasses “first pass” hepatic metabolism, leading to faster absorption and reduced norCBP + + + + + + + + +f + + + + + Mannitol Free - base cyclobenzaprine Charged cyclobenzaprine Tonix’s proprietary eutectic formulation contains a basic ingredient which enhances efficient transmucosal absorption and results in a stable tablet with long shelf - life Sublingual vasculature Tertiary Amine tail
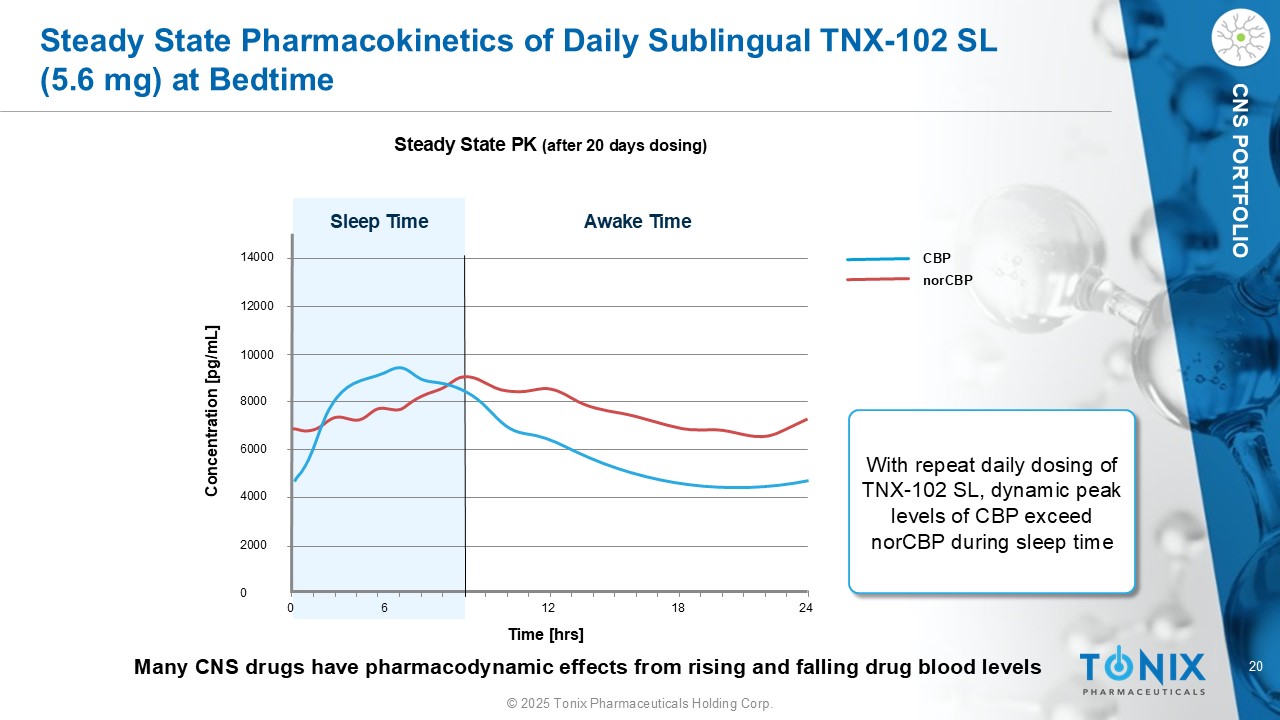
20 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Steady State Pharmacokinetics of Daily Sublingual TNX - 102 SL (5.6 mg) at Bedtime Steady State PK (after 20 days dosing) 14000 12000 10000 8000 6000 4000 2000 0 0 6 12 18 24 Sleep Time Awake Time Concentration [ pg /mL] Time [ hrs ] CBP norCBP With repeat daily dosing of TNX - 102 SL, dynamic peak levels of CBP exceed norCBP during sleep time Many CNS drugs have pharmacodynamic effects from rising and falling drug blood levels
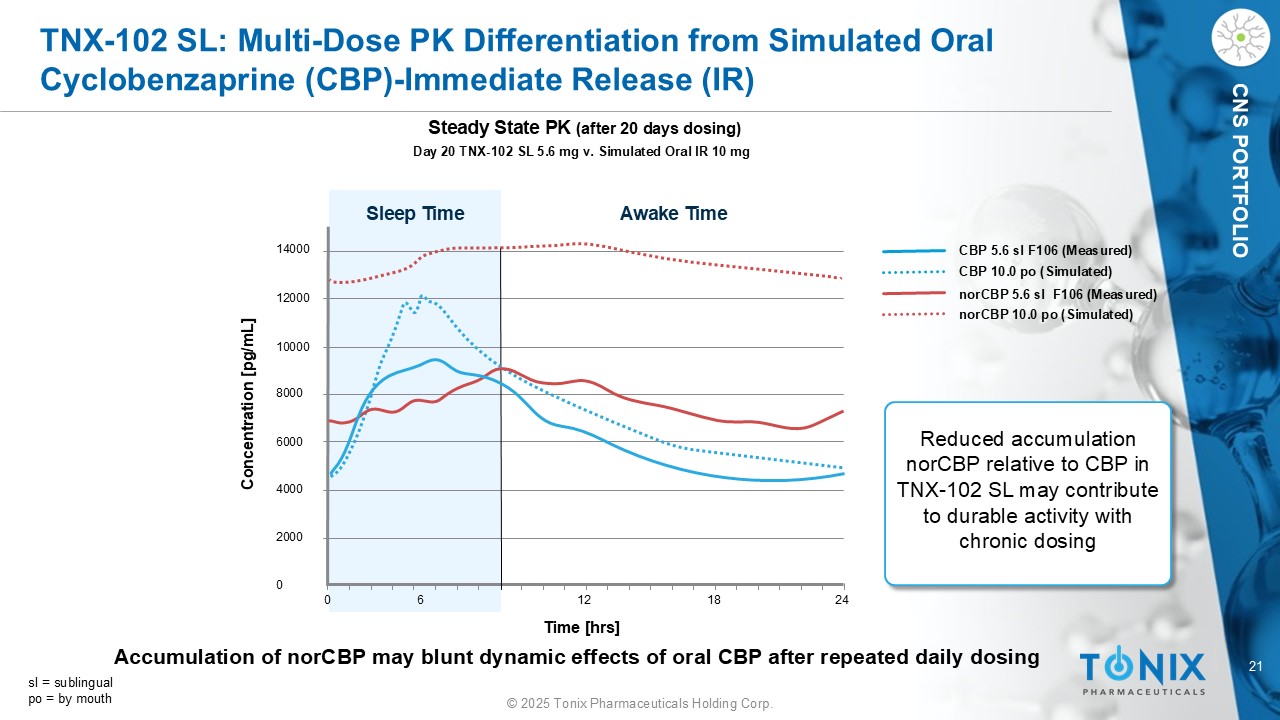
21 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 102 SL: Multi - Dose PK Differentiation from Simulated Oral Cyclobenzaprine (CBP) - Immediate Release (IR) Day 20 TNX - 102 SL 5.6 mg v. Simulated Oral IR 10 mg Steady State PK (after 20 days dosing) 14000 12000 10000 8000 6000 4000 2000 0 0 6 12 18 24 Sleep Time Awake Time Concentration [ pg /mL] Time [ hrs ] CBP 5.6 sl F106 (Measured) CBP 10.0 po (Simulated) norCBP 5.6 sl F106 (Measured) norCBP 10.0 po (Simulated) Reduced accumulation norCBP relative to CBP in TNX - 102 SL may contribute to durable activity with chronic dosing sl = sublingual po = by mouth Accumulation of norCBP may blunt dynamic effects of oral CBP after repeated daily dosing

22 © 2025 Tonix Pharmaceuticals Holding Corp. TNX - 102 SL* ( Cyclobenzaprine HCl Sublingual Tablets) 5.6 mg A unique, sublingual formulation of cyclobenzaprine (CBP) designed to optimize absorption and delivery *5.6 mg once - daily at bedtime, TNX - 102 SL is an investigational new drug, its efficacy and safety have not been established and it has not been approved for any indication norCBP = norCyclobenzaprine • Non - opioid analgesic – Tertiary Amine Tricyclic (TAT) • Rapid drug exposure following once - nightly sublingual administration • Reduction in persistent active metabolite norCBP with chronic dosing • Durable (14 week) reduction in fibromyalgia pain in two pivotal studies • Generally well tolerated • PDUFA goal date August 15, 2025
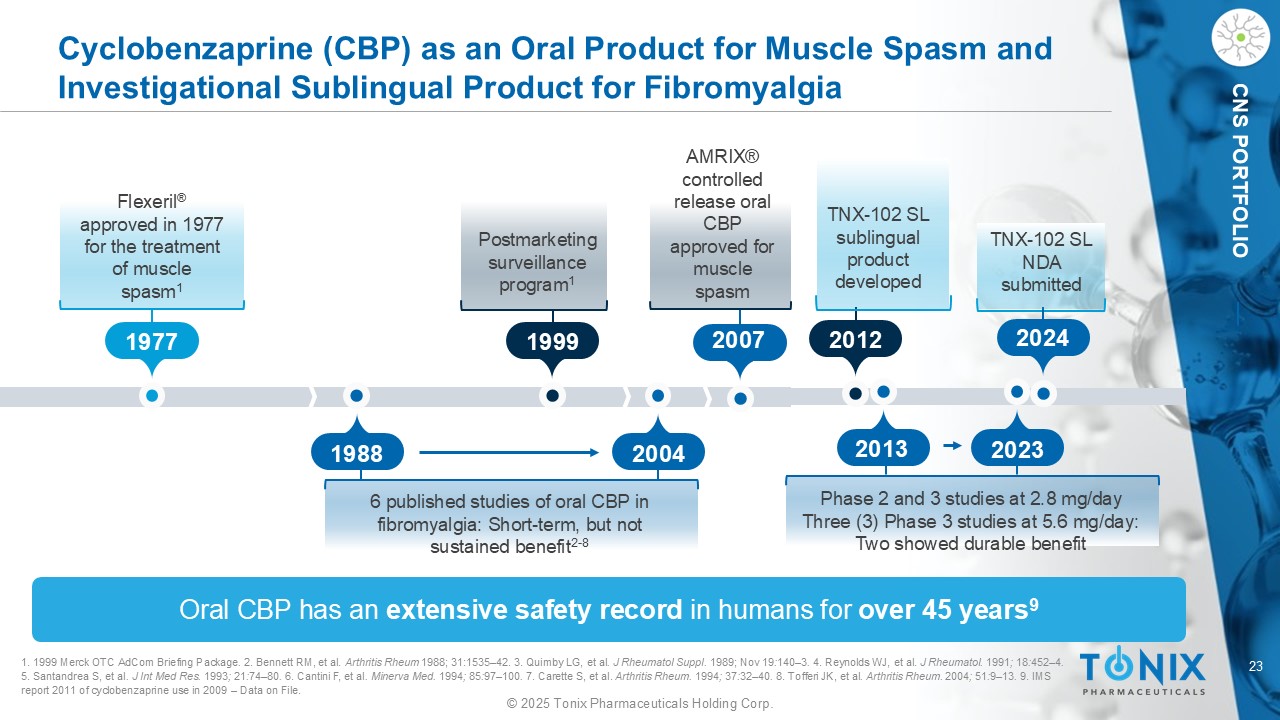
23 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Cyclobenzaprine (CBP) as an Oral Product for Muscle Spasm and Investigational Sublingual Product for Fibromyalgia Oral CBP has an extensive safety record in humans for over 45 years 9 1. 1999 Merck OTC AdCom Briefing Package . 2. Bennett RM, et al. Arthritis Rheum 1988 ; 31 : 1535 – 42 . 3. Quimby LG, et al . J Rheumatol Suppl. 1989; Nov 19 : 140 – 3 . 4. Reynolds WJ, et al. J Rheumatol . 1991 ; 18 : 452 – 4 . 5. Santandrea S, et al . J Int Med Res. 1993 ; 21 : 74 – 80 . 6. Cantini F, et al . Minerva Med. 1994 ; 85 : 97 – 100 . 7. Carette S, et al. Arthritis Rheum. 1994 ; 37 : 32 – 40 . 8. Tofferi JK, et al . Arthritis Rheum. 2004 ; 51 : 9 – 13 . 9. IMS report 2011 of cyclobenzaprine use in 2009 – Data on File. 1977 1988 2004 1999 Flexeril ® approved in 1977 for the treatment of muscle spasm 1 6 published studies of oral CBP in fibromyalgia: Short - term, but not sustained benefit 2 - 8 Postmarketing surveillance program 1 2007 AMRIX® controlled release oral CBP approved for muscle spasm 2012 2024 TNX - 102 SL s ublingual product developed TNX - 102 SL NDA submitted Phase 2 and 3 studies at 2.8 mg/day Three (3) Phase 3 studies at 5.6 mg/day : Two showed durable benefit 2013 2023

24 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 102 SL (5.6 mg) Fibromyalgia Pivotal Clinical Trial Results • Activity • First pivotal Phase 3 study ( RELIEF ) reported – December 2020 1 ‒ Statistically significant reduction in daily pain compared to placebo ( p = 0.010) • Second Phase 3 study ( RALLY ) missed primary endpoint – July 2021 • Confirmatory pivotal Phase 3 study ( RESILIENT ) reported – December 2023 ‒ Statistically significant reduction in daily pain compared to placebo ( p = 0.00005) • Tolerability in two pivotal trials • G enerally well tolerated with an adverse event profile comparable to prior studies and with no new safety signals observed • The most common treatment - emergent adverse event was tongue or mouth numbness at the administration site, which was temporally related to dosing, self - limited, never rated as severe, and rarely led to study discontinuation (one participant in each study) • Excluding COVID - 19, rates of systemic adverse events in each of the two studies were all below 4.0% 1 Lederman S, et al. Arthritis Care Res (Hoboken) . 2023 Nov;75(11):2359 - 2368. doi : 10.1002.
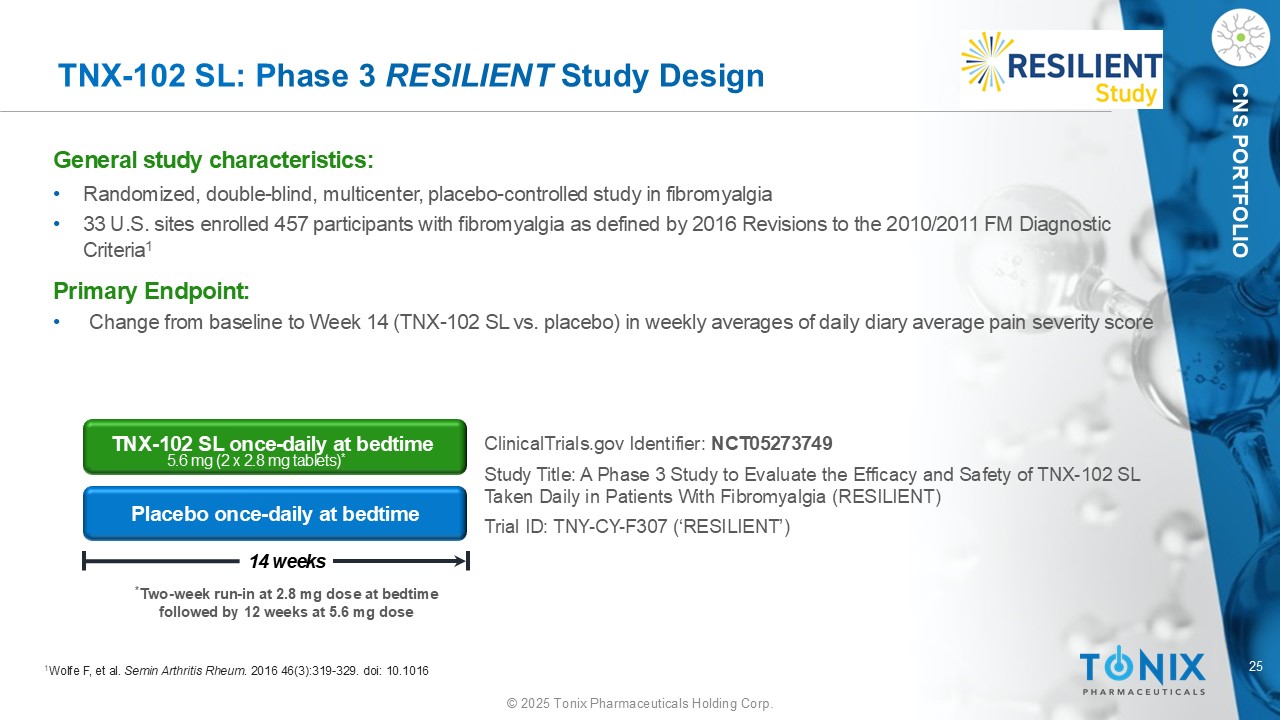
25 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 102 SL: P hase 3 RESILIENT Study Design General s tudy c haracteristics: • Randomized, double - blind, multicenter, placebo - controlle d study in fibromyalgia • 33 U.S. sites enrolled 457 participants with fibromyalgia as defined by 2016 Revisions to the 2010/2011 FM Diagnostic C riteria 1 Primary Endpoint: • Change from baseline to Week 14 (TNX - 102 SL vs. placebo) in weekly averages of daily diary average pain severity score Placebo once - daily at bedtime TNX - 102 SL once - daily at bedtime 5.6 mg (2 x 2.8 mg tablets) * * Two - week run - in at 2.8 mg dose at bedtime followed by 12 weeks at 5.6 mg dose ClinicalTrials.gov Identifier: NCT05273749 Study Title: A Phase 3 Study to Evaluate the Efficacy and Safety of TNX - 102 SL Taken Daily in Patients With Fibromyalgia (RESILIENT) Trial ID: TNY - CY - F307 (‘RESILIENT’) 14 weeks 1 Wolfe F, et al. Semin Arthritis Rheum . 2016 46(3):319 - 329. doi : 10.1016
26 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO RESILIENT Pre - Specified Primary Endpoint Summary 1 • TNX - 102 SL demonstrated statistically significant improvement in mean weekly pain scores over placebo at Week 14 • P - value of 0.00005 is highly statistically significant Additional Findings • Cohen’s d effect size 0.38 • All pre - specified sensitivity analyses of the primary endpoint show statistical significance ( p ≤ 0.001) • Rapid onset of action: p - values <0.01 at each weekly time point, including Week 1 1 The Company plans to publish the results in a journal later this year
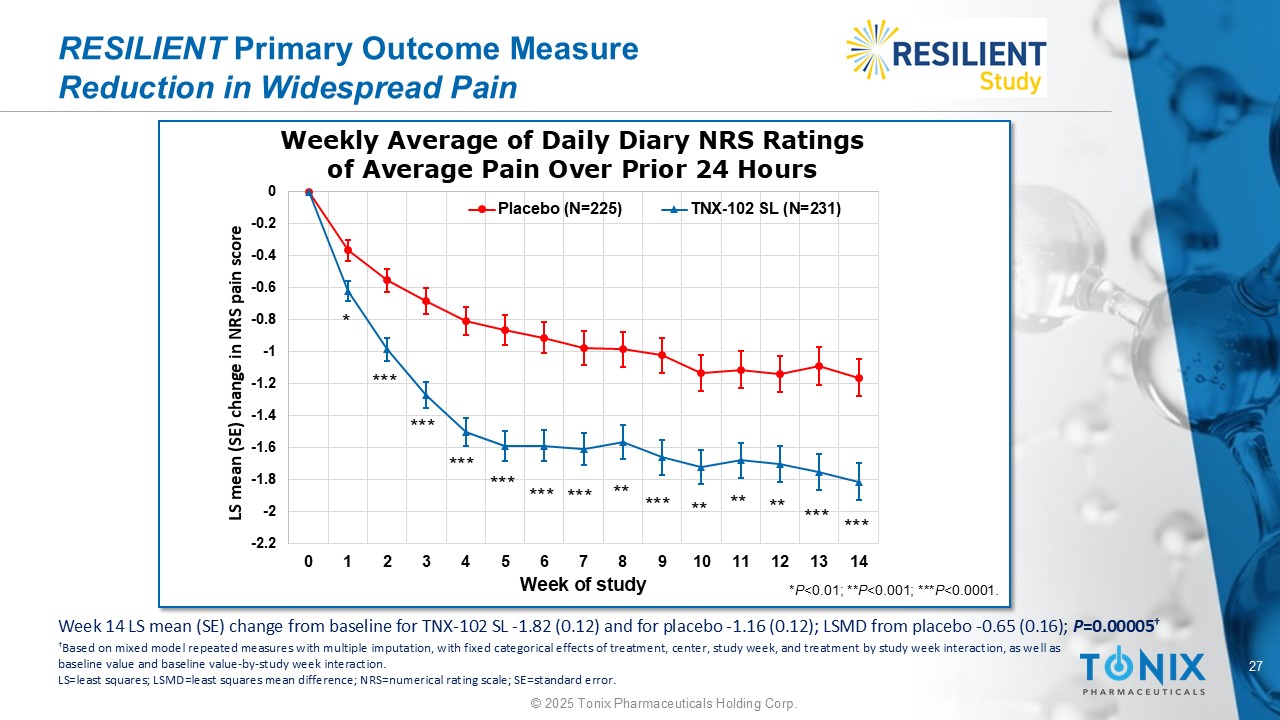
27 © 2025 Tonix Pharmaceuticals Holding Corp. RESILIENT Primary Outcome Measure Reduction in Widespread Pain Weekly Average of Daily Diary NRS Ratings of Average Pain Over Prior 24 Hours -2.2 -2 -1.8 -1.6 -1.4 -1.2 -1 -0.8 -0.6 -0.4 -0.2 0 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 LS mean (SE) change in NRS pain score Week of study Placebo (N=225) TNX-102 SL (N=231) * *** *** ** ** ** *** *** *** *** *** *** *** ** * P <0.01; ** P <0.001; *** P <0.0001. Week 14 LS mean (SE) change from baseline for TNX - 102 SL - 1.82 (0.12) and for placebo - 1.16 (0.12); LSMD from placebo - 0.65 (0.1 6); P =0.00005 † † Based on mixed m odel r epeated m easures with multiple i mputation , with fixed categorical effects of treatment, center, study week, and treatment by study week interaction, as well as baseline value and baseline value - by - study week interaction. LS = least squares; LSMD = least squares mean difference; NRS = numerical rating scale; SE = standard error.
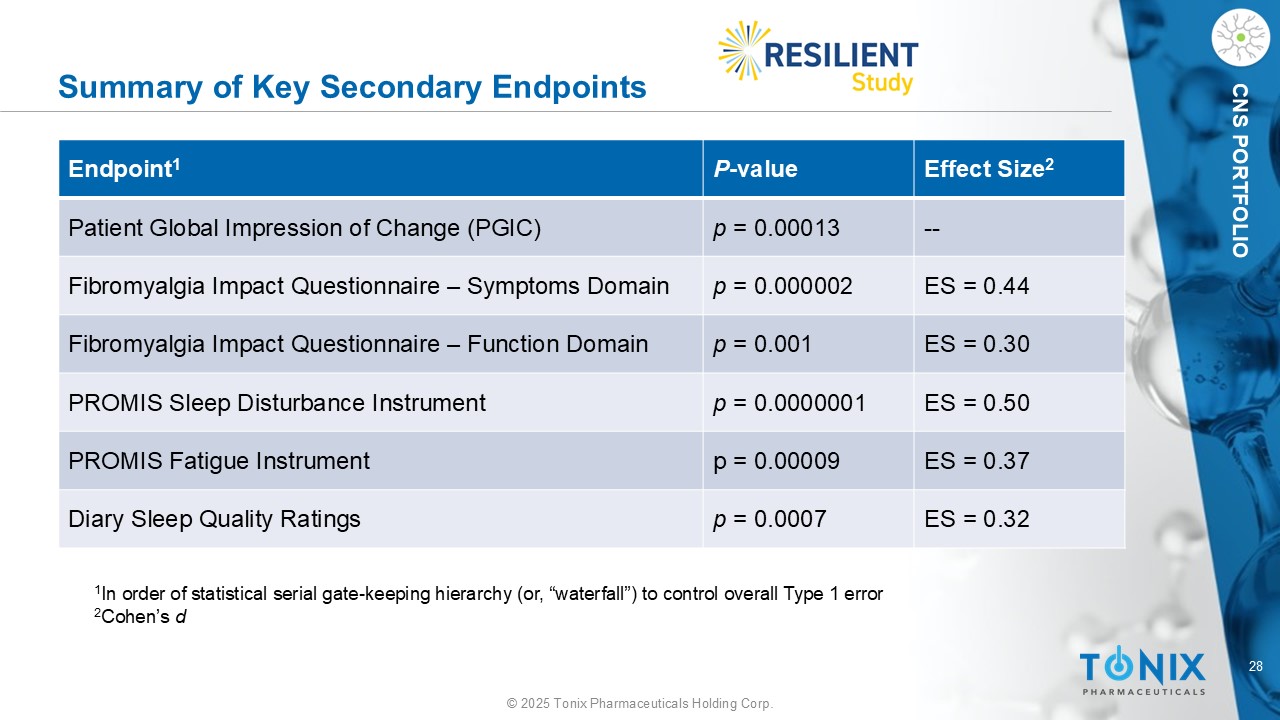
28 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Summary of Key Secondary Endpoints Effect Size 2 P - value Endpoint 1 -- p = 0.00013 Patient Global Impression of Change (PGIC) ES = 0.44 p = 0.000002 Fibromyalgia Impact Questionnaire – Symptoms Domain ES = 0.30 p = 0.001 Fibromyalgia Impact Questionnaire – Function Domain ES = 0.50 p = 0.0000001 PROMIS Sleep Disturbance Instrument ES = 0.37 p = 0.00009 PROMIS Fatigue Instrument ES = 0.32 p = 0.0007 Diary Sleep Quality Ratings 1 In order of statistical serial gate - keeping hierarchy (or, “waterfall”) to control overall Type 1 error 2 Cohen’s d
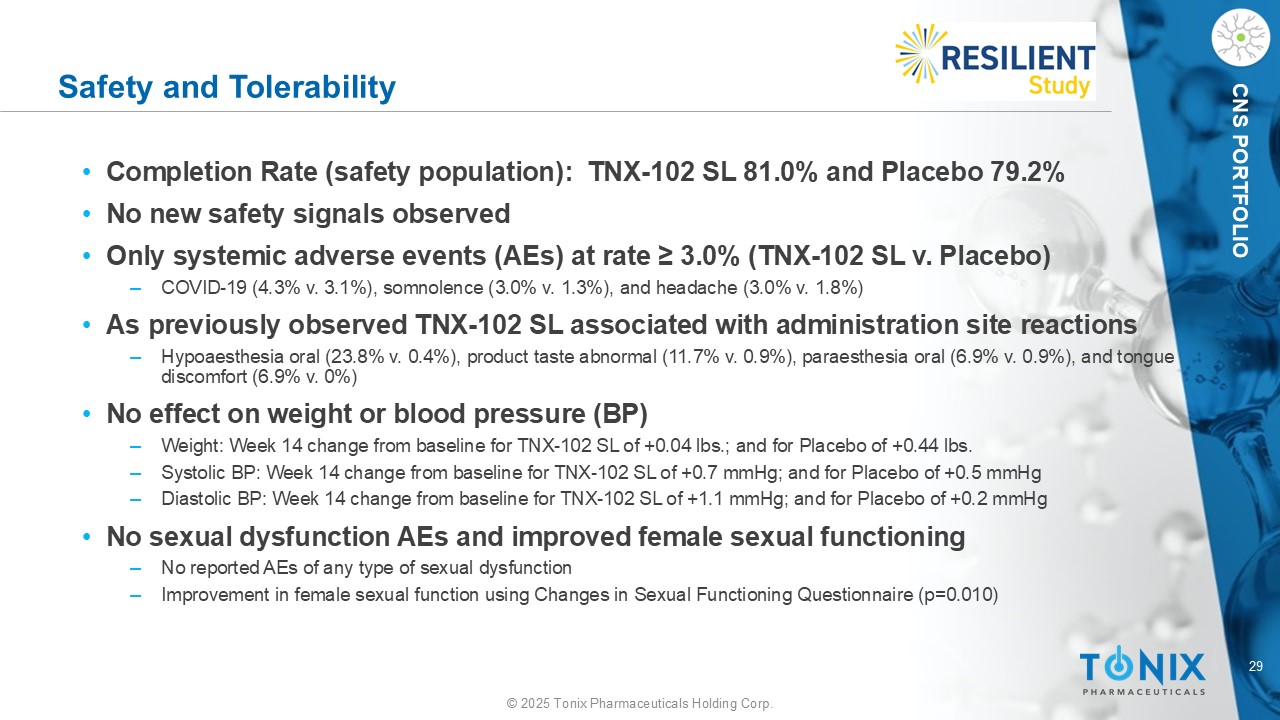
29 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Safety and Tolerability • Completion Rate (safety population): TNX - 102 SL 81.0% and Placebo 79.2% • No new safety signals observed • Only systemic adverse events (AEs) at rate ≥ 3.0% (TNX - 102 SL v. Placebo) ‒ COVID - 19 (4.3% v. 3.1%), somnolence (3.0% v. 1.3%), and headache (3.0% v. 1.8%) • As previously observed TNX - 102 SL associated with administration site reactions ‒ Hypoaesthesia oral (23.8% v. 0.4%), product taste abnormal (11.7% v. 0.9%), paraesthesia oral (6.9% v. 0.9%), and tongue discomfort (6.9% v. 0%) • No effect on weight or blood pressure (BP) ‒ Weight: Week 14 change from baseline for TNX - 102 SL of +0.04 lbs.; and for Placebo of +0.44 lbs. ‒ Systolic BP: Week 14 change from baseline for TNX - 102 SL of +0.7 mmHg; and for Placebo of +0.5 mmHg ‒ Diastolic BP: Week 14 change from baseline for TNX - 102 SL of +1.1 mmHg; and for Placebo of +0.2 mmHg • No sexual dysfunction AEs and improved female sexual functioning ‒ No reported AEs of any type of sexual dysfunction ‒ Improvement in female sexual function using Changes in Sexual Functioning Questionnaire (p=0.010)
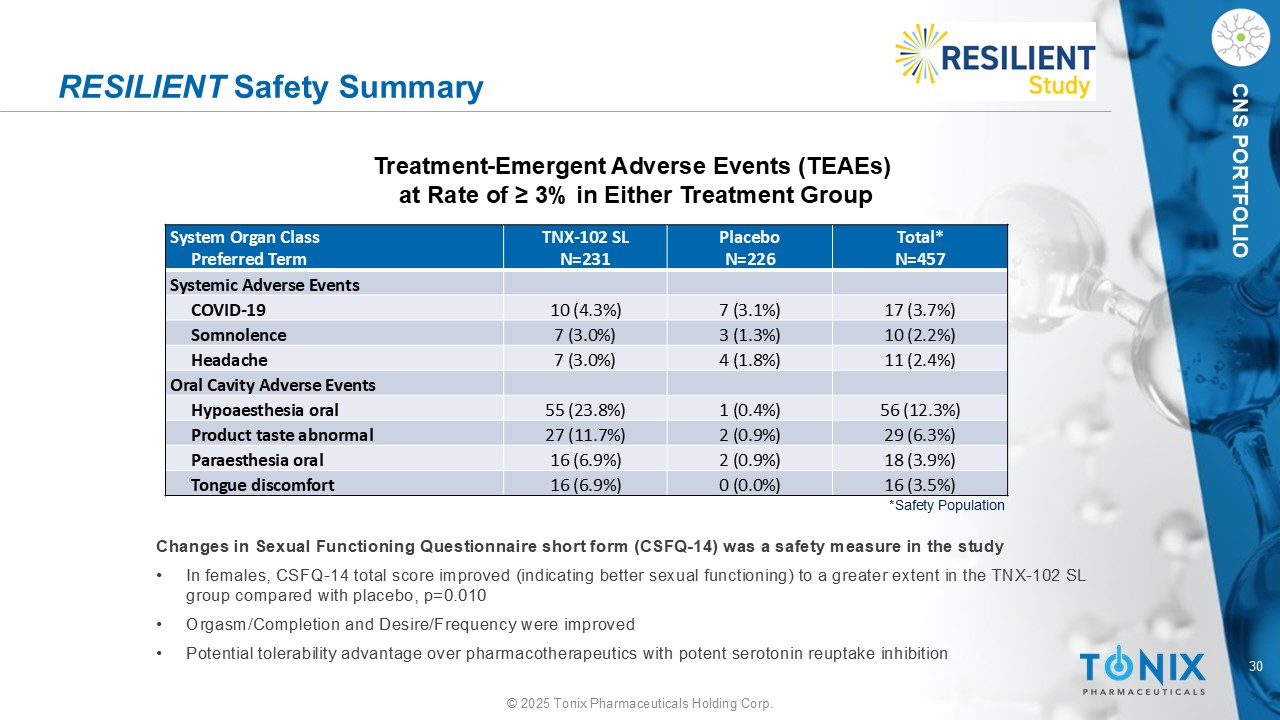
30 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO RESILIENT Safety Summary *Safety Population Treatment - Emergent Adverse Events (TEAEs) at Rate of ≥ 3% in Either Treatment Group Changes in Sexual Functioning Questionnaire short form (CSFQ - 14) was a safety measure in the study • In females, CSFQ - 14 total score improved (indicating better sexual functioning) to a greater extent in the TNX - 102 SL group compared with placebo, p=0.010 • Orgasm/Completion and Desire/Frequency were improved • Potential tolerability advantage over pharmacotherapeutics with potent serotonin reuptake inhibition Total* N=457 Placebo N=226 TNX - 102 SL N=231 System Organ Class Preferred Term Systemic Adverse Events 17 (3.7%) 7 (3.1%) 10 (4.3%) COVID - 19 10 (2.2%) 3 (1.3%) 7 (3.0%) Somnolence 11 (2.4%) 4 (1.8%) 7 (3.0%) Headache Oral Cavity Adverse Events 56 (12.3%) 1 (0.4%) 55 (23.8%) Hypoaesthesia oral 29 (6.3%) 2 (0.9%) 27 (11.7%) Product taste abnormal 18 (3.9%) 2 (0.9%) 16 (6.9%) Paraesthesia oral 16 (3.5%) 0 (0.0%) 16 (6.9%) Tongue discomfort
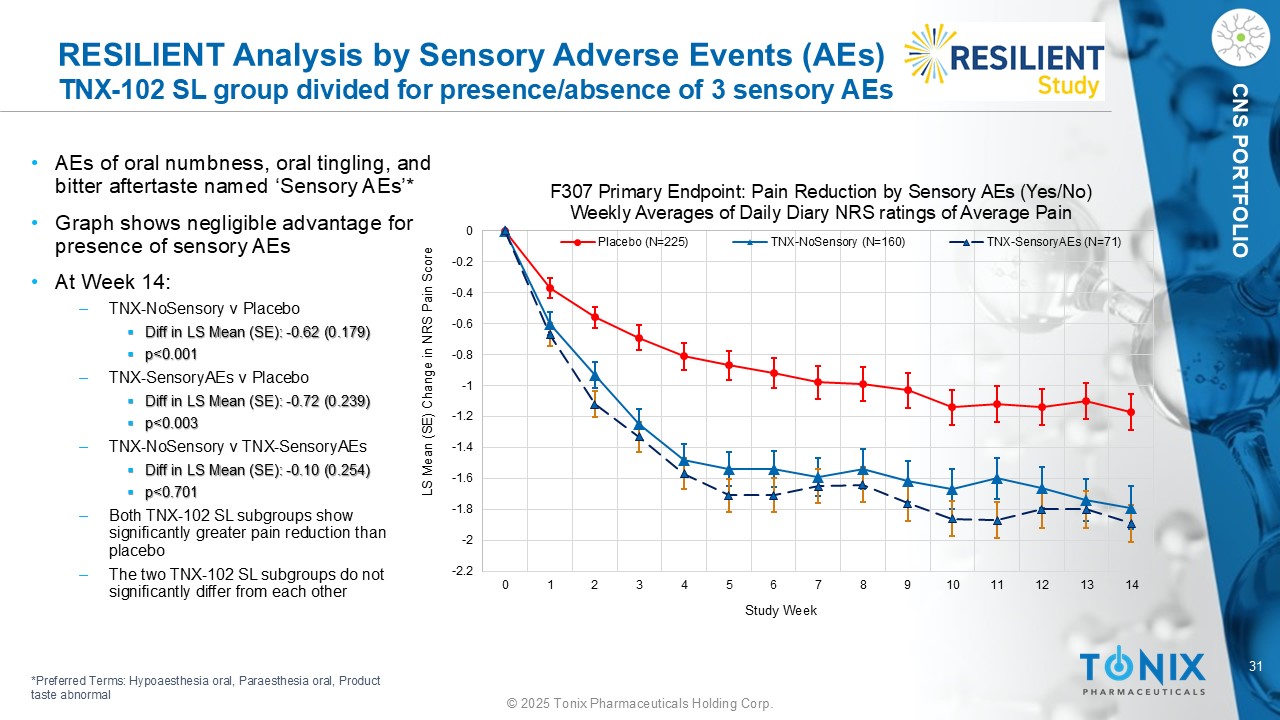
31 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO RESILIENT Analysis by Sensory Adverse Events (AEs) TNX - 102 SL group divided for presence/absence of 3 sensory AEs • AEs of oral numbness, oral tingling, and bitter aftertaste named ‘Sensory AEs’* • Graph shows negligible advantage for presence of sensory AEs • At Week 14: ‒ TNX - NoSensory v Placebo ▪ Diff in LS Mean (SE): - 0.62 (0.179) ▪ p<0.001 ‒ TNX - SensoryAEs v Placebo ▪ Diff in LS Mean (SE): - 0.72 (0.239) ▪ p<0.003 ‒ TNX - NoSensory v TNX - SensoryAEs ▪ Diff in LS Mean (SE): - 0.10 (0.254) ▪ p<0.701 ‒ Both TNX - 102 SL subgroups show significantly greater pain reduction than placebo ‒ The two TNX - 102 SL subgroups do not significantly differ from each other -2.2 -2 -1.8 -1.6 -1.4 -1.2 -1 -0.8 -0.6 -0.4 -0.2 0 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 LS Mean (SE) Change in NRS Pain Score Study Week F307 Primary Endpoint: Pain Reduction by Sensory AEs (Yes/No) Weekly Averages of Daily Diary NRS ratings of Average Pain Placebo (N=225) TNX-NoSensory (N=160) TNX-SensoryAEs (N=71) *Preferred Terms: Hypoaesthesia oral, Paraesthesia oral, Product taste abnormal
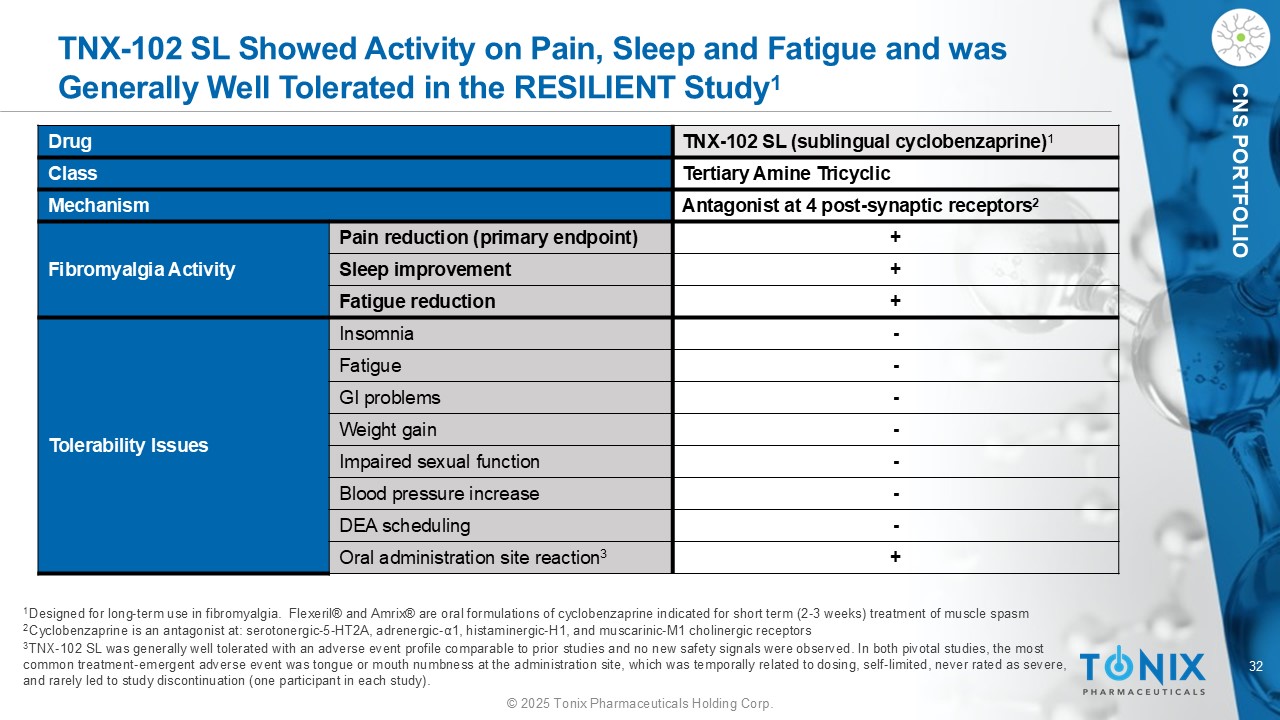
32 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 102 SL Showed Activity on Pain, Sleep and Fatigue and was Generally Well Tolerated in the RESILIENT Study 1 1 Designed for long - term use in fibromyalgia. Flexeril® and Amrix ® are oral formulations of cyclobenzaprine indicated for short term (2 - 3 weeks) treatment of muscle spasm 2 Cyclobenzaprine is an antagonist at: serotonergic - 5 - HT2A, adrenergic - α1, histaminergic - H1, and muscarinic - M1 cholinergic recepto rs 3 TNX - 102 SL was generally well tolerated with an adverse event profile comparable to prior studies and no new safety signals were observed. In both pivotal studies, the most common treatment - emergent adverse event was tongue or mouth numbness at the administration site, which was temporally related to dosing, self - limited, never rated as severe, and rarely led to study discontinuation (one participant in each study). TNX - 102 SL (sublingual cyclobenzaprine) 1 Drug Tertiary Amine Tricyclic Class Antagonist at 4 post - synaptic receptors 2 Mechanism + Pain reduction (primary endpoint) Fibromyalgia Activity + Sleep improvement + Fatigue reduction - Insomnia Tolerability Issues - Fatigue - GI problems - Weight gain - Impaired sexual function - Blood pressure increase - DEA scheduling + Oral administration site reaction 3
33 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 102 SL: Sublingual Formulation is Designed for Long - Term Daily Administration at Bedtime and Transmucosal Absorption • TNX - 102 SL: Proprietary sublingual formulation of cyclobenzaprine (CBP) with transmucosal absorption ‒ Rapid systemic exposure of CBP – Tertiary Amine Tricyclic (TAT) ‒ Innovation by design with patent - protected eutectic formulation ‒ Increases cyclobenzaprine bioavailability during sleep ‒ Avoids first - pass metabolism ‒ Lowers exposure to persistent active major metabolite, norCyclobenzaprine ( norCBP ) • norCBP is generated in the liver by de - methylation ‒ Long half - life (~72 hours) – Secondary Amine Tricyclic (SAT) ‒ Reduced levels of norCBP after TNX - 102 SL administration relative oral CBP ‒ Less selective for sleep - promoting post - synaptic target receptors (5 - HT2A, α1 - adrenergic, histamine H1) ‒ Active inhibitor of norepinephrine transporter (NET)
34 © 2025 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Summary and Conclusions • Neither oral cyclobenzaprine nor oral amitriptyline provide durable reduction in fibromyalgia pain ‒ Short - term (1 month), but not long - term (3 months) benefit ‒ Chasing loss - of - effect with higher and higher doses was of no benefit • TNX - 102 SL (cyclobenzaprine HCl tablets) with transmucosal absorption has the potential to provide durable reduction in fibromyalgia pain ‒ Cyclobenzaprine is expected to be a first - in - class Tertiary Amine Tricyclic (TAT) ‒ Proposed indication: for the management of fibromyalgia ‒ Non - opioid analgesic ‒ Decreased exposure to long - lived active major metabolite, norCyclobenzaprine ( norCBP ) is believed to contribute to durability of pain reduction ▪ Wash - out of oral c yclobenzaprine or amitriptyline required for clinical trial enrollment ‒ Cyclobenzaprine is a TAT that is amenable to sublingual transmucosal dosing, in part because it is 66,000 times more soluble than amitriptyline ‒ Transmucosal delivery of c yclobenzaprine requires a basic excipient to produce free - base

© 2024 Tonix Pharmaceuticals Holding Corp. THANK YOU