
Tonix Pharmaceuticals Holding Corp. 8-K
Exhibit 99.02

A NOVEL SINGLE - DOSE, LIVE ATTENUATED, MINIMALLY REPLICATIVE MPOX VACCINE Farooq Nasar, Ph.D., M.P.H. WVC April 23, 2025 PO6066 April 23, 2025 (Doc 1583) © 2025 Tonix Pharmaceuticals Holding Corp.

Certain statements in this presentation regarding strategic plans, expectations and objectives for future operations or results are “forward - looking statements” as defined by the Private Securities Litigation Reform Act of 1995. These statements may be identified by the use of forward - looking words such as “anticipate,” “believe,” “forecast,” “estimate” and “intend,” among others. These forward - looking statements are based on Tonix’s current expectations and actual results could differ materially. There are a number of factors that could cause actual events to differ materially from those indicated by such forward - looking statements. These factors include, but are not limited to, the risks related to failure to obtain FDA clearances or approvals and noncompliance with FDA regulations; risks related to the failure to successfully market any of our products; risks related to the timing and progress of clinical development of our product candidates; our need for additional financing; uncertainties of patent protection and litigation; uncertainties of government or third party payor reimbursement; limited research and development efforts and dependence upon third parties; and substantial competition. As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products. The forward - looking statements in this presentation are made as of the date of this presentation, even if subsequently made available by Tonix on its website or otherwise. Tonix does not undertake an obligation to update or revise any forward - looking statement, except as required by law. Investors should read the risk factors set forth in the Annual Report on Form 10 - K for the year ended December 31, 2024, as filed with the Securities and Exchange Commission (the “SEC”) on March 18, 2025, and periodic reports and current reports filed with the SEC on or after the date thereof. All of Tonix's forward - looking statements are expressly qualified by all such risk factors and other cautionary statements. 2 © 2025 Tonix Pharmaceuticals Holding Corp.

TALK OVERVIEW 3 © 2025 Tonix Pharmaceuticals Holding Corp. 1) Background 2) TNX - 801 attenuation in vitro and in vivo 3) TNX - 801 immunogenicity and efficacy in animal models *TNX - 801 is in the pre - IND stage of development and has not been approved for any indication.
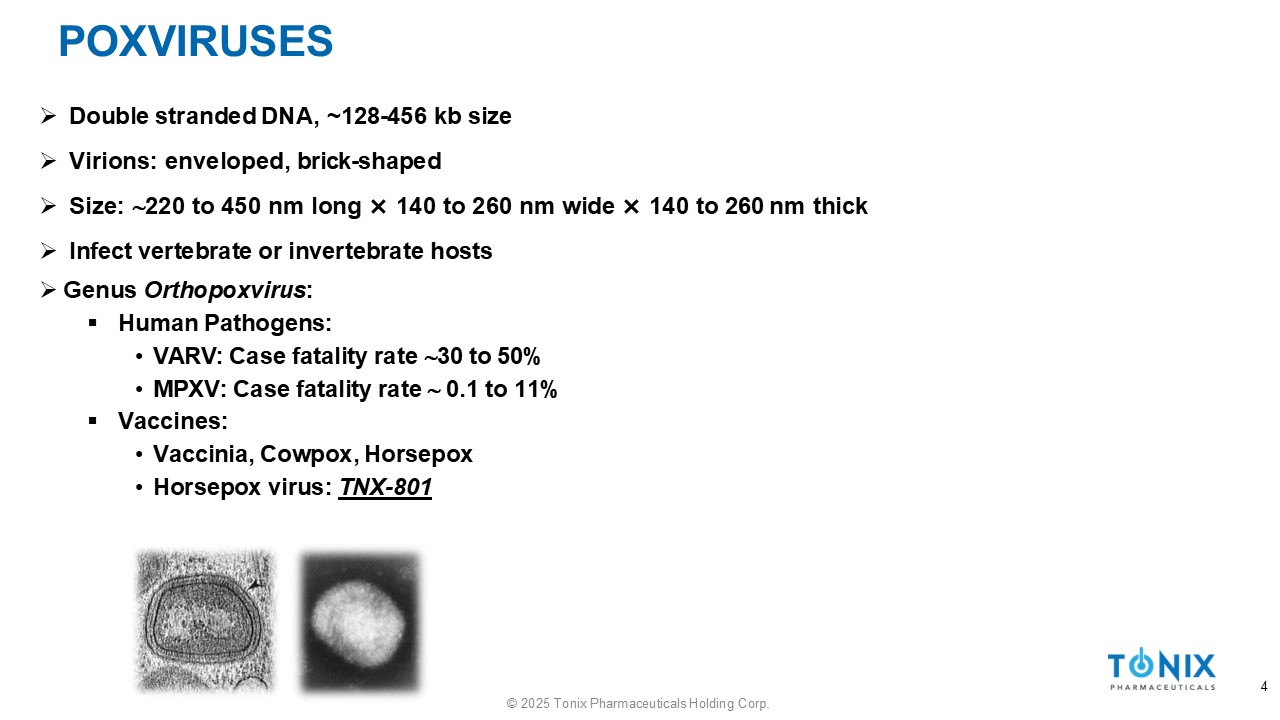
POXVIRUSES » Double stranded DNA, ~128 - 456 kb size » Virions: enveloped, brick - shaped » Size: 220 to 450 nm long î 140 to 260 nm wide î 140 to 260 nm thick » Infect vertebrate or invertebrate hosts » Genus Orthopoxvirus : ▪ Human Pathogens: • VARV: Case fatality rate 30 to 50% • MPXV: Case fatality rate 0.1 to 11% ▪ Vaccines: • Vaccinia, Cowpox, Horsepox • Horsepox virus: TNX - 801 4 © 2025 Tonix Pharmaceuticals Holding Corp.
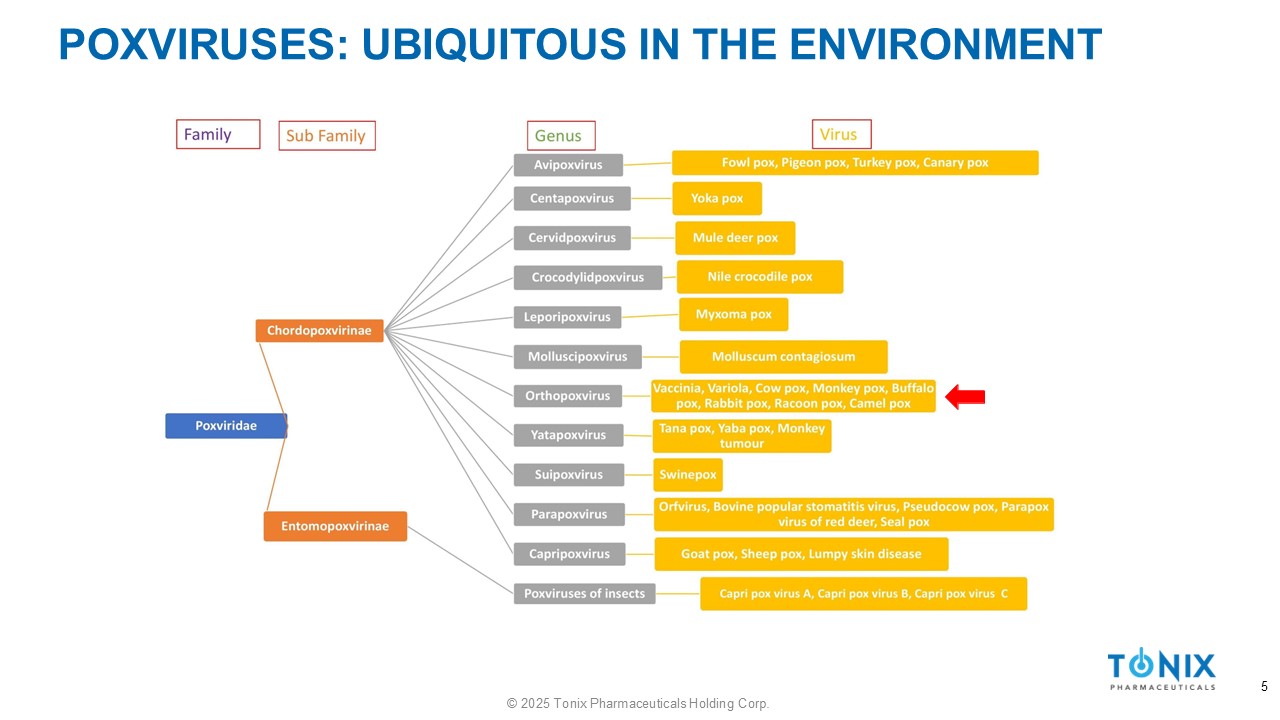
POXVIRUSES: UBIQUITOUS IN THE ENVIRONMENT 5 © 2025 Tonix Pharmaceuticals Holding Corp.
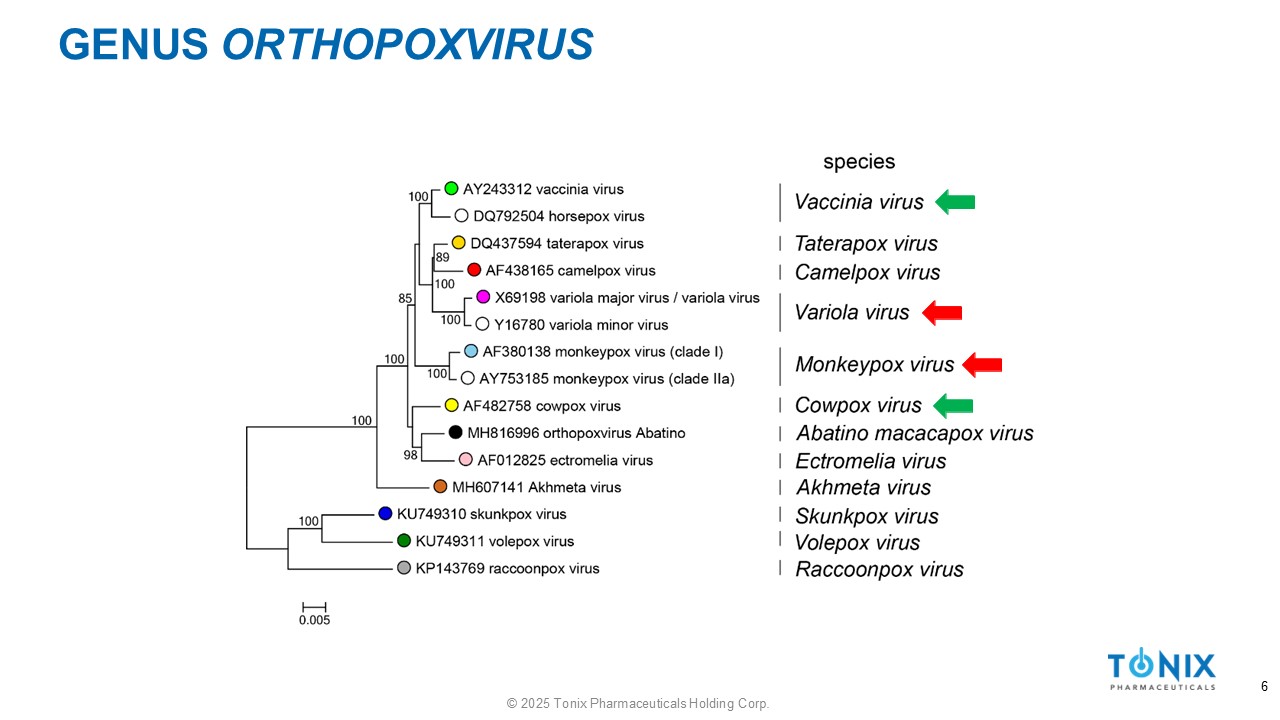
GENUS ORTHOPOXVIRUS 6 © 2025 Tonix Pharmaceuticals Holding Corp.
MONKEYPOX VIRUS (MPOX) 7 © 2025 Tonix Pharmaceuticals Holding Corp. » Endemic in Central and West Africa » Two Clades: 1) Clade I (DRC) 2) Clade IIa (West Africa) and IIb (Nigeria) » Human Case Fatality Rate: ▪ Clade I – 11% ▪ Clade IIa – 3% ▪ Clade IIb – <0.1% » Clade IIb – 2022 Outbreak ▪ 122 Countries ▪ 100,000 Confirmed Cases
VARIOLA VIRUS (SMALLPOX) 8 © 2025 Tonix Pharmaceuticals Holding Corp. » Oldest written record – 3,500 years » Oldest sequences – 1,400 years » Human Case Fatality Rate: 30% » 20 th century – 250 to 500 million deaths » Eradication: 1980

EDWARD JENNER - SMALLPOX VACCINE (1796) » Jenner observed milkmaids were protected from smallpox, reasoned that infection with an illness similar to smallpox but less deadly could protect one against smallpox ▪ “Cowpox” was the name of a disease in cows that could transfer to humans and cause sores ▪ Jenner “vaccinated” (from vacca , Latin for “cow”) a patient with pustule matter from “cowpox” sores on a milkmaid’s hands; that patient remained healthy when challenged with smallpox virus » Jenner suspected that the agent causing cowpox, which he called vaccinia originated in horses and had been transferred from horses to cows’ udders by dirty hands The College of Physicians of Philadelphia. Accessed July 15, 2021. https:/ /w w w.historyofvaccines.org 9 © 2025 Tonix Pharmaceuticals Holding Corp.
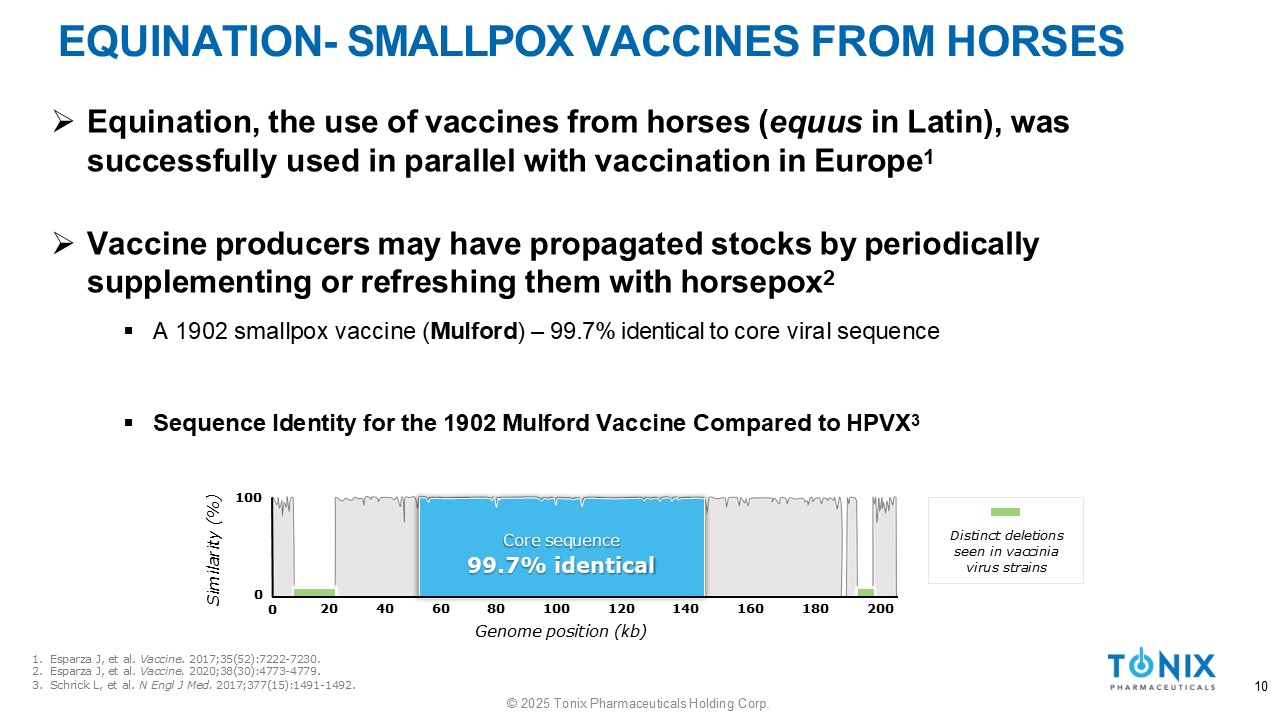
EQUINATION - SMALLPOX VACCINES FROM HORSES » Equination, the use of vaccines from horses ( equus in Latin), was successfully used in parallel with vaccination in Europe 1 » Vaccine producers may have propagated stocks by periodically supplementing or refreshing them with horsepox 2 ▪ A 1902 smallpox vaccine ( Mulford ) – 99.7% identical to core viral sequence ▪ Sequence Identity for the 1902 Mulford Vaccine Compared to HPVX 3 Similarity (%) 100 20 40 60 Core sequence 99.7% identical 80 100 120 Genome position (kb) 140 160 180 200 0 0 Distinct deletions seen in vaccinia virus strains 1. Esparza J, et al. Vaccine . 2017;35(52):7222 - 7230. 2. Esparza J, et al. Vaccine. 2020;38(30):4773 - 4779. 3. Schrick L, et al. N Engl J Med. 2017;377(15):1491 - 1492. 10 © 2025 Tonix Pharmaceuticals Holding Corp.
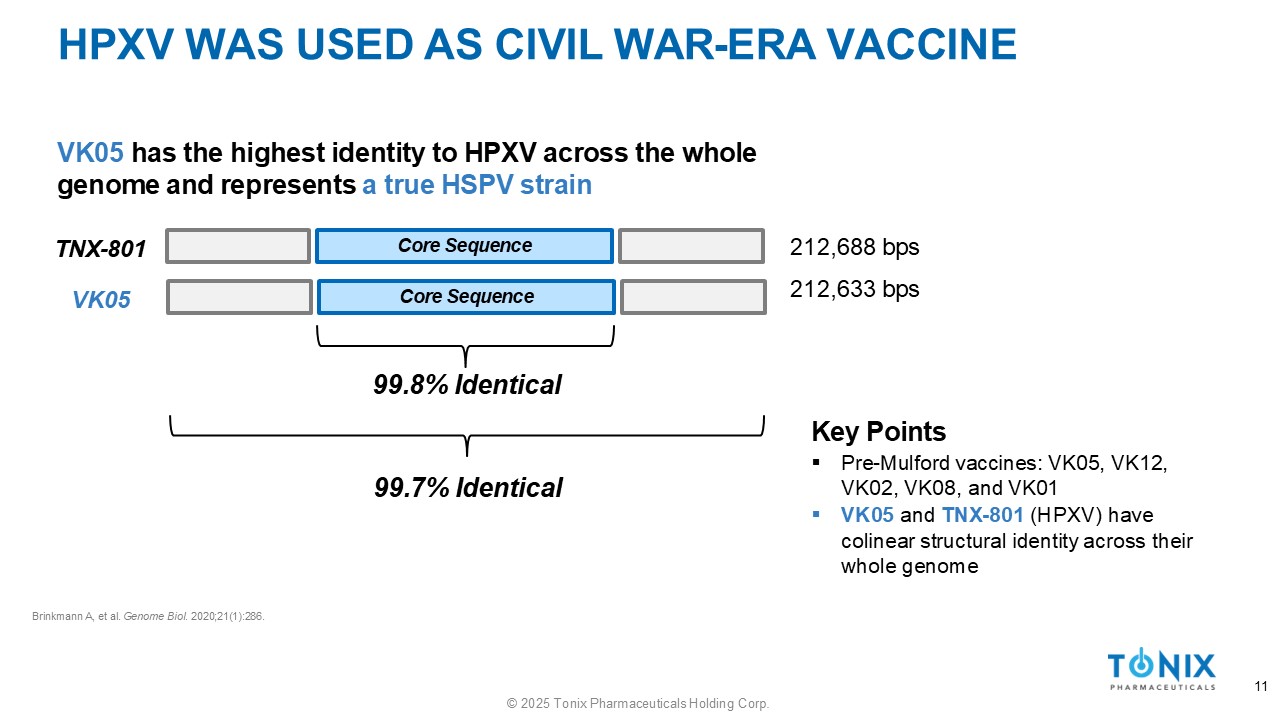
HPXV WAS USED AS CIVIL WAR - ERA VACCINE TNX - 801 VK05 has the highest identity to HPXV across the whole genome and represents a true HSPV strain 99.8% Identical Brinkmann A, et al. Genome Biol . 2020;21(1):286. 99.7% Identical 212,688 bps 212,633 bps Key Points ▪ Pre - Mulford vaccines: VK05, VK12, VK02, VK08, and VK01 ▪ VK05 and TNX - 801 (HPXV) have colinear structural identity across their whole genome Core Sequence Core Sequence VK05 11 © 2025 Tonix Pharmaceuticals Holding Corp.

SMALLPOX VACCINES 1 Downie AW. 1939. Br J Exp Pathol 20:158 – 176. 12 © 2025 Tonix Pharmaceuticals Holding Corp. » Vaccine: Cowpox origin » Serial passaging: Humans, cows, and horses (143 years) » Vaccine: Vaccinia Virus (1939) closely related to cowpox but serologically distinct 1 » Multiple Vaccinia virus - based vaccines developed » Smallpox eradication
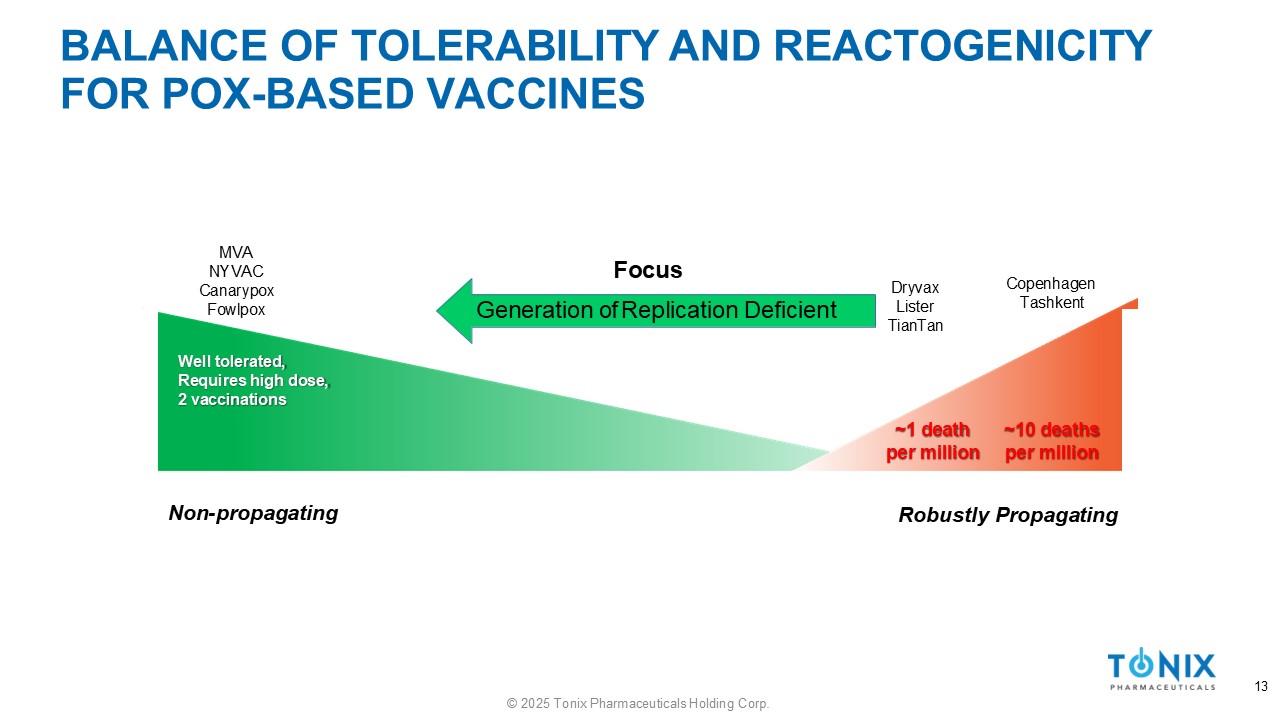
BALANCE OF TOLERABILITY AND REACTOGENICITY FOR POX - BASED VACCINES Well tolerated, Requires high dose, 2 vaccinations ~1 death per million ~10 deaths per million Non - propagating 13 © 2025 Tonix Pharmaceuticals Holding Corp. Robustly Propagating Dryvax Lister TianTan Copenhagen Tashkent MVA NYVAC Canarypox Fowlpox Focus Generation of Replication Deficient
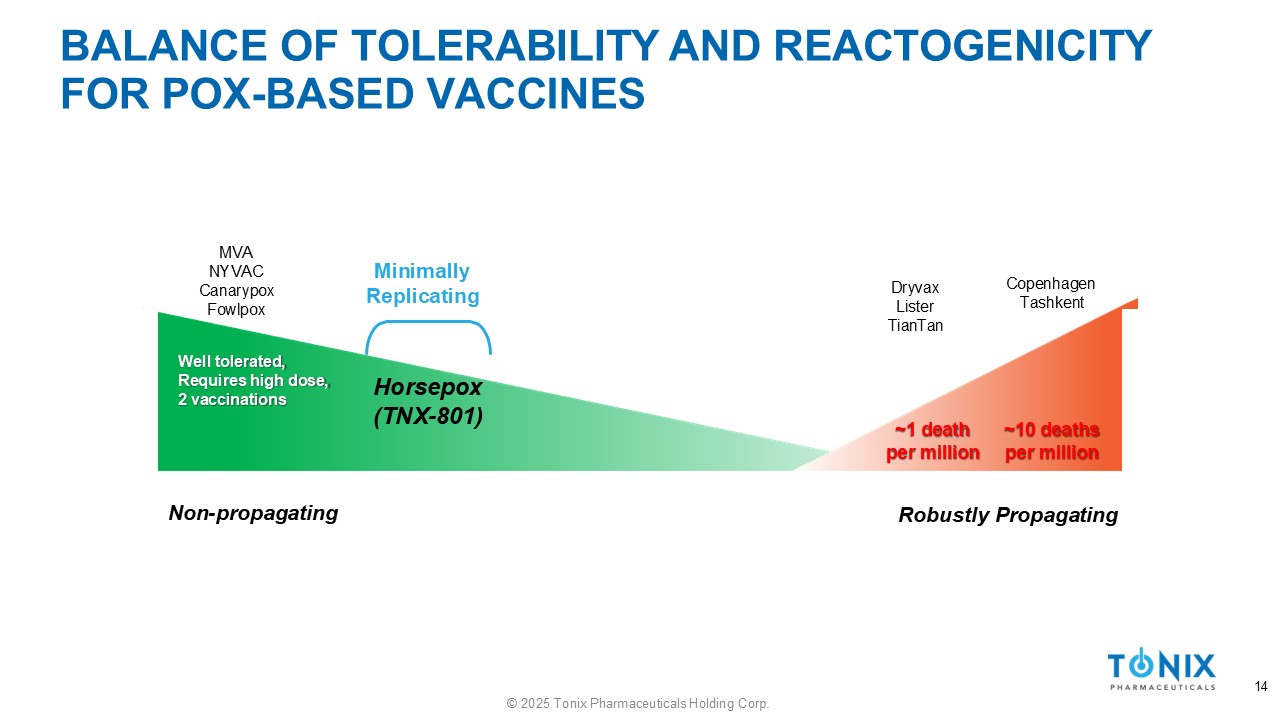
BALANCE OF TOLERABILITY AND REACTOGENICITY FOR POX - BASED VACCINES Well tolerated, Requires high dose, 2 vaccinations ~1 death per million ~10 deaths per million Non - propagating 14 © 2025 Tonix Pharmaceuticals Holding Corp. Robustly Propagating Dryvax Lister TianTan Copenhagen Tashkent MVA NYVAC Canarypox Fowlpox Minimally Replicating Horsepox (TNX - 801)
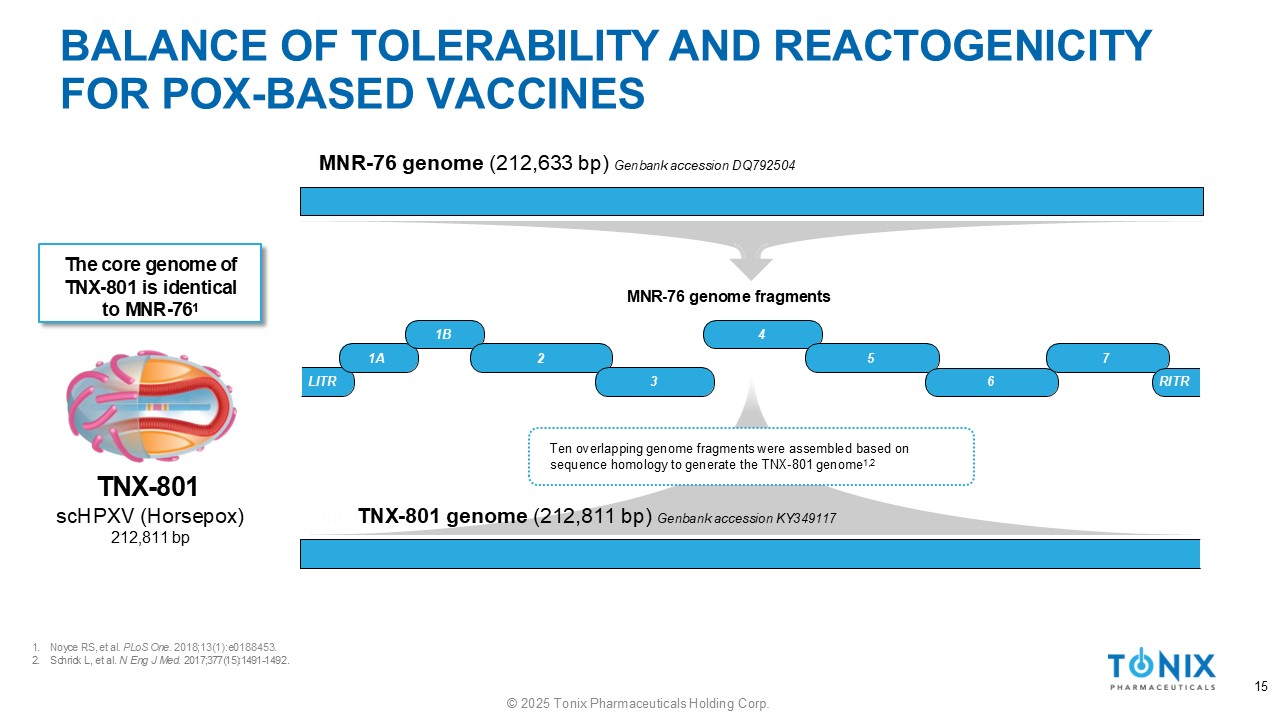
BALANCE OF TOLERABILITY AND REACTOGENICITY FOR POX - BASED VACCINES 1. Noyce RS, et al. PLoS One. 2018;13(1):e0188453. 2. Schrick L, et al. N Eng J Med. 2017;377(15):1491 - 1492. TNX - 801 scHPXV (Horsepox) 212,811 bp LITR 1A 1B 4 2 3 5 6 7 RITR MNR - 76 genome fragments MNR - 76 genome (212,633 bp) Genbank accession DQ792504 The core genome of TNX - 801 is identical to MNR - 76 1 TNX - 801 genome (212,811 bp) Genbank accession KY349117 Ten overlapping genome fragments were assembled based on sequence homology to generate the TNX - 801 genome 1,2 15 © 2025 Tonix Pharmaceuticals Holding Corp.

4 PRONG APPROACH TO MPOX/SMALLPOX VACCINE (TNX - 801) 16 © 2025 Tonix Pharmaceuticals Holding Corp. 1) Well - tolerated 2) Single dose 3) Durable 4) Protection against mpox disease (lesions)
TNX - 801 ATTENUATION IN VITRO AND IN VIVO 17 © 2025 Tonix Pharmaceuticals Holding Corp.
IN VITRO ATTENUATION OF TNX - 801 18 © 2025 Tonix Pharmaceuticals Holding Corp. » Investigate attenuation of TNX - 801 in vitro relative to VACV strains ▪ Positive Control: VACV - Western Reserve (WR) , VACV - International Health Department (IHD) ▪ Older vaccines used in smallpox eradication: 1) VACV - Lister (Lis) 2) VACV - New York City Board of Health (NYCBH) ▪ New Vaccine: TNX - 801 ▪ Non - replicating control: MVA » In vitro Assays: 1) Plaque phenotype – BSC - 40 and Vero - E6 2) Replication Kinetics ▪ Immortalized non - human primate cell lines ▪ Human primary cells from two main route of poxvirus transmission • Dermal and respiratory tracts
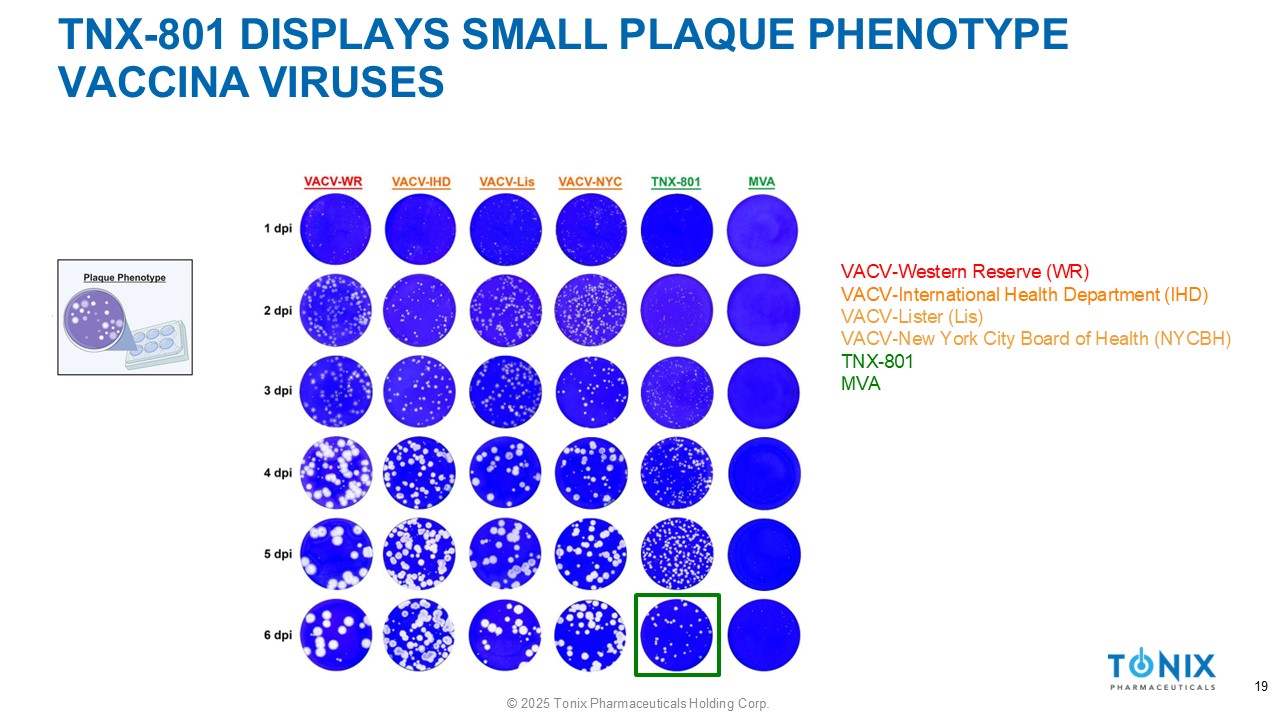
TNX - 801 DISPLAYS SMALL PLAQUE PHENOTYPE VACCINA VIRUSES VACV - Western Reserve (WR) VACV - International Health Department (IHD) VACV - Lister (Lis) VACV - New York City Board of Health (NYCBH) TNX - 801 MVA 19 © 2025 Tonix Pharmaceuticals Holding Corp.
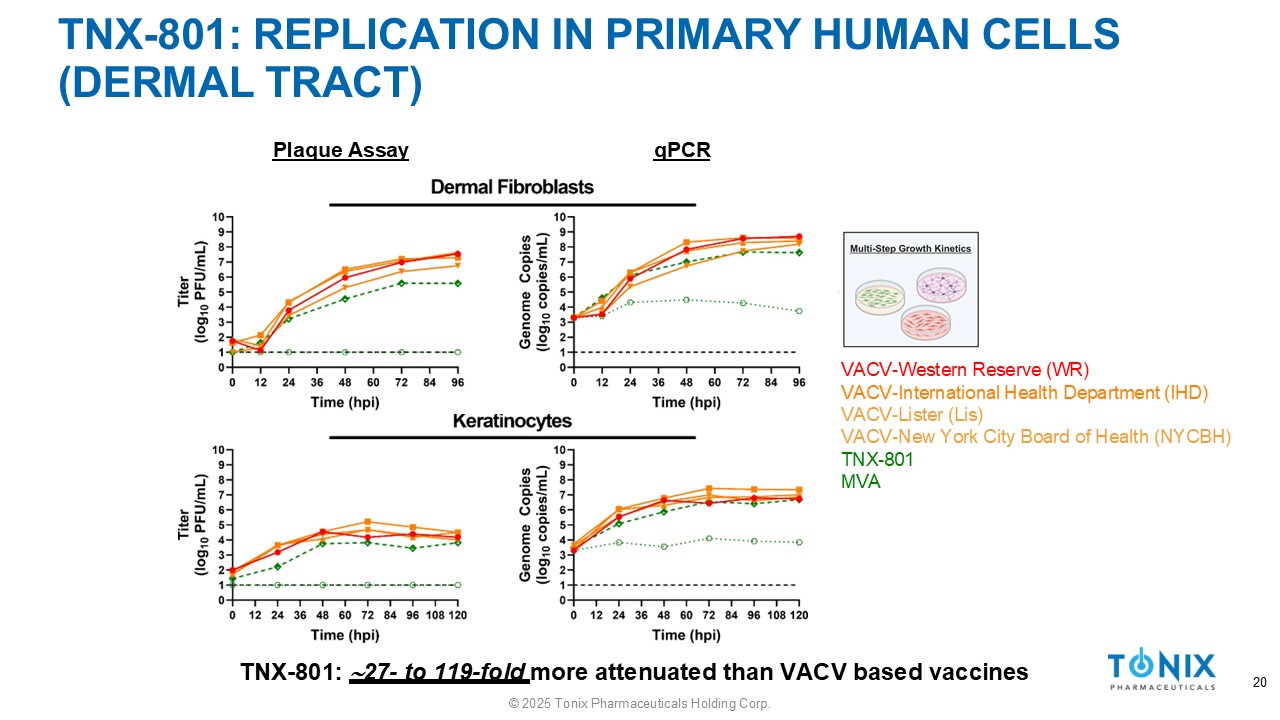
TNX - 801: REPLICATION IN PRIMARY HUMAN CELLS (DERMAL TRACT) Plaque Assay qPCR VACV - Western Reserve (WR) VACV - International Health Department (IHD) VACV - Lister (Lis) VACV - New York City Board of Health (NYCBH) TNX - 801 MVA TNX - 801: 27 - to 119 - fold more attenuated than VACV based vaccines © 2025 Tonix Pharmaceuticals Holding Corp. 20
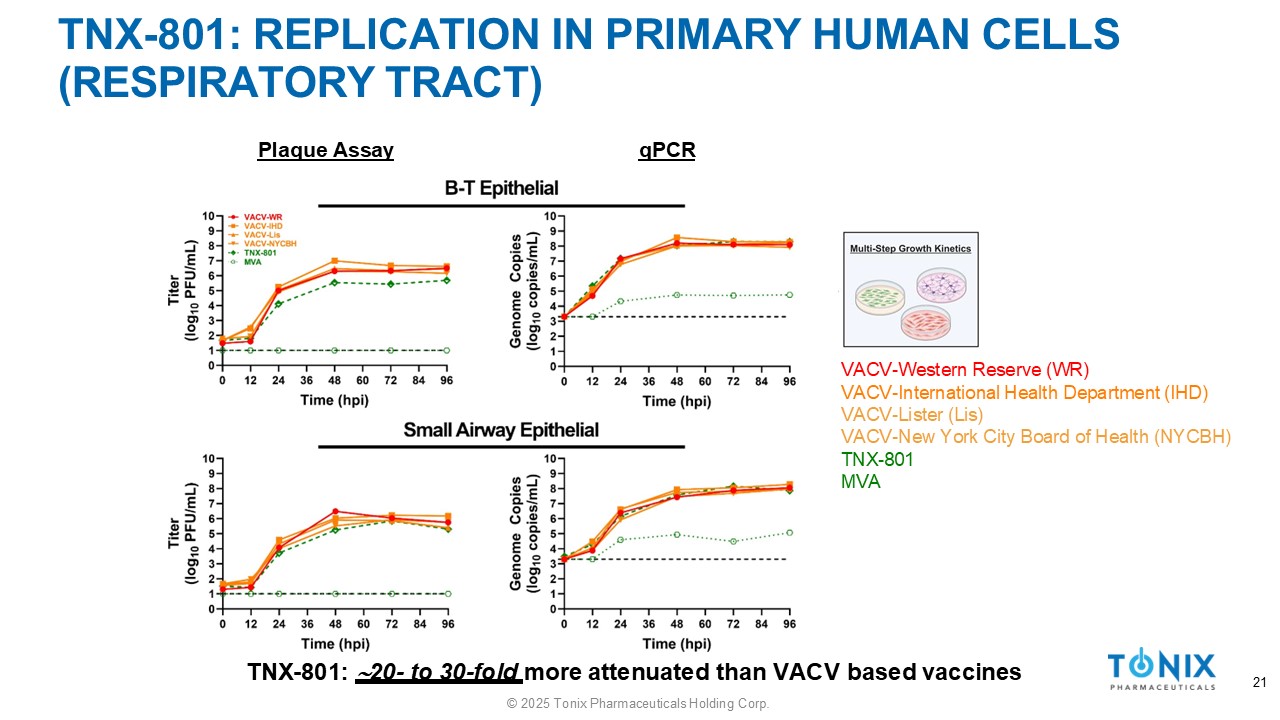
TNX - 801: REPLICATION IN PRIMARY HUMAN CELLS (RESPIRATORY TRACT) 21 Plaque Assay qPCR TNX - 801: 20 - to 30 - fold more attenuated than VACV based vaccines VACV - Western Reserve (WR) VACV - International Health Department (IHD) VACV - Lister (Lis) VACV - New York City Board of Health (NYCBH) TNX - 801 MVA © 2025 Tonix Pharmaceuticals Holding Corp.
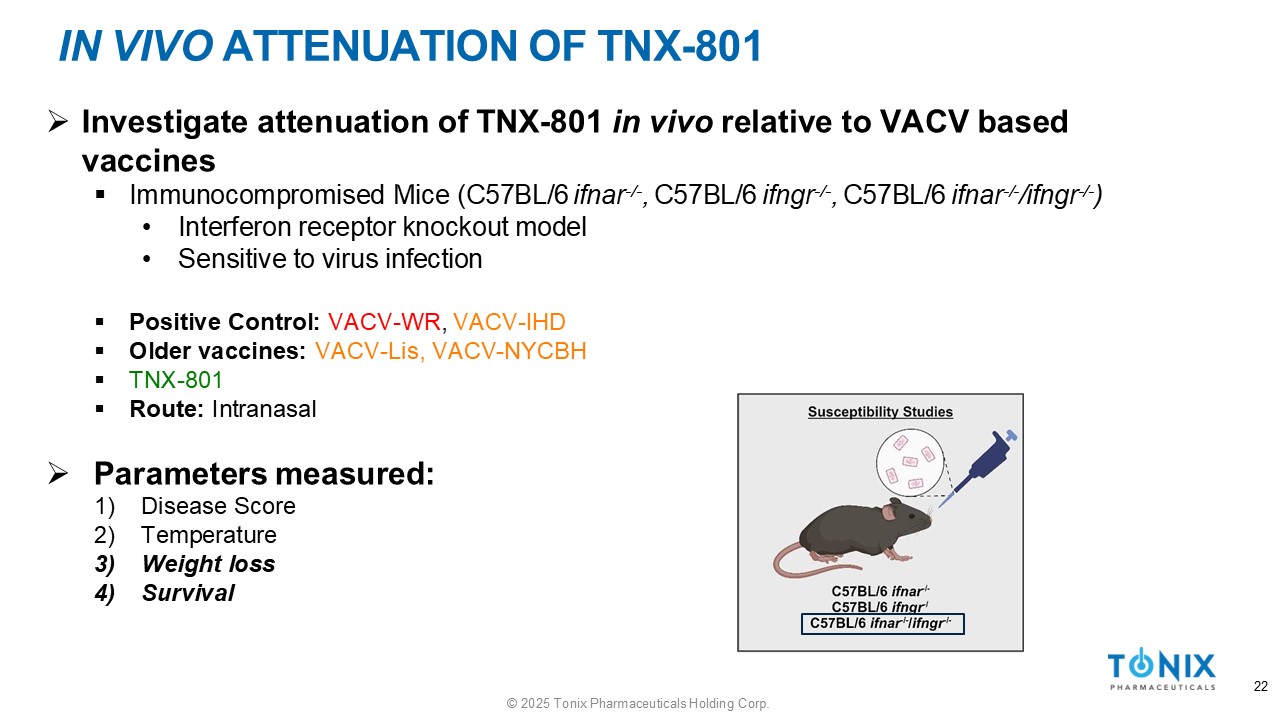
IN VIVO ATTENUATION OF TNX - 801 » Investigate attenuation of TNX - 801 in vivo relative to VACV based vaccines ▪ Immunocompromised Mice (C57BL/6 ifnar - / - , C57BL/6 ifngr - / - , C57BL/6 ifnar - / - /ifngr - / - ) • Interferon receptor knockout model • Sensitive to virus infection ▪ Positive Control: VACV - WR , VACV - IHD ▪ Older vaccines: VACV - Lis, VACV - NYCBH ▪ TNX - 801 ▪ Route: Intranasal » Parameters measured: 1) Disease Score 2) Temperature 3) Weight loss 4) Survival 22 © 2025 Tonix Pharmaceuticals Holding Corp.
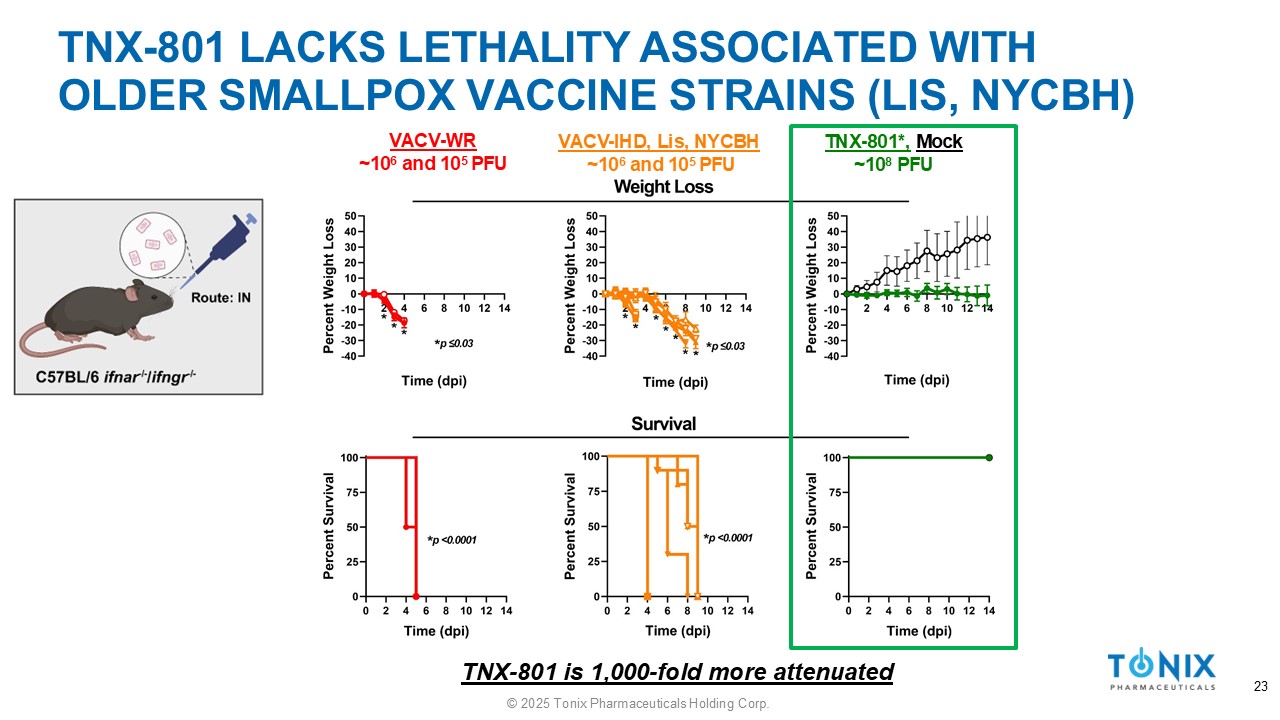
TNX - 801 LACKS LETHALITY ASSOCIATED WITH OLDER SMALLPOX VACCINE STRAINS (LIS, NYCBH) TNX - 801*, Mock ~10 8 PFU VACV - IHD, Lis, NYCBH ~10 6 and 10 5 PFU VACV - WR ~10 6 and 10 5 PFU TNX - 801 is 1,000 - fold more attenuated 23 © 2025 Tonix Pharmaceuticals Holding Corp.
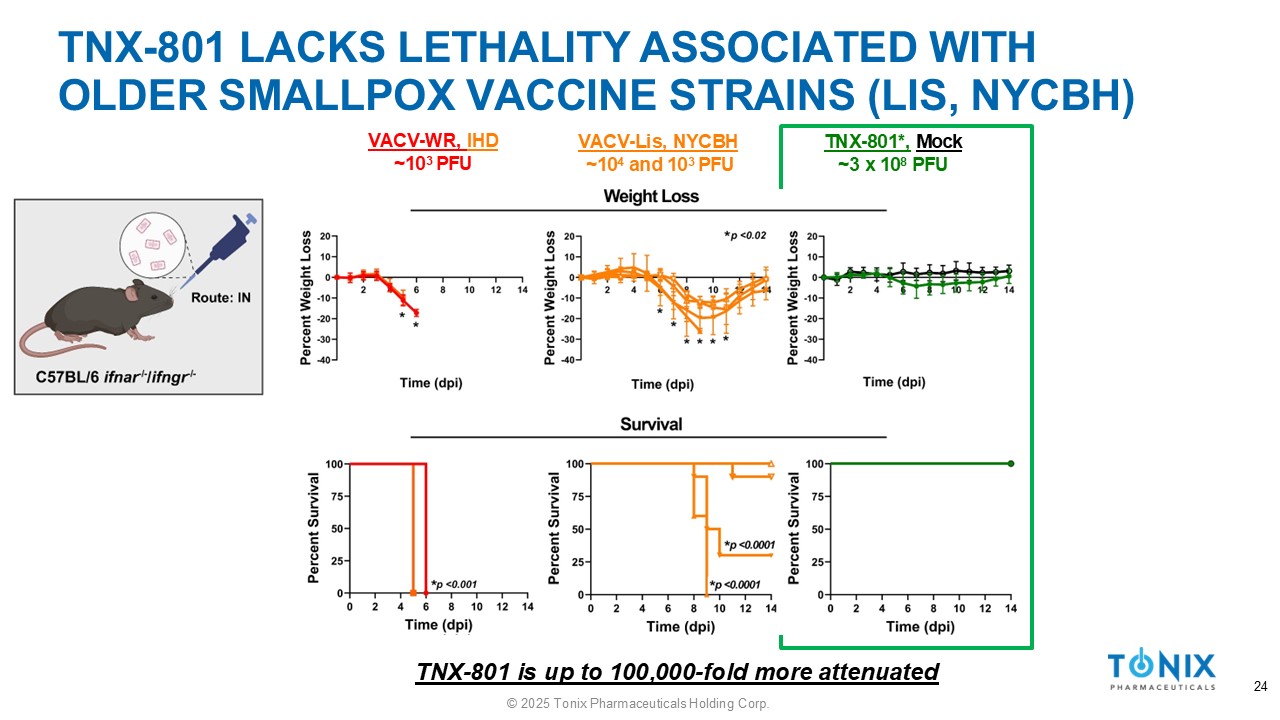
TNX - 801 LACKS LETHALITY ASSOCIATED WITH OLDER SMALLPOX VACCINE STRAINS (LIS, NYCBH) TNX - 801*, Mock ~3 x 10 8 PFU VACV - Lis, NYCBH ~10 4 and 10 3 PFU VACV - WR, IHD ~10 3 PFU TNX - 801 is up to 100,000 - fold more attenuated 24 © 2025 Tonix Pharmaceuticals Holding Corp.
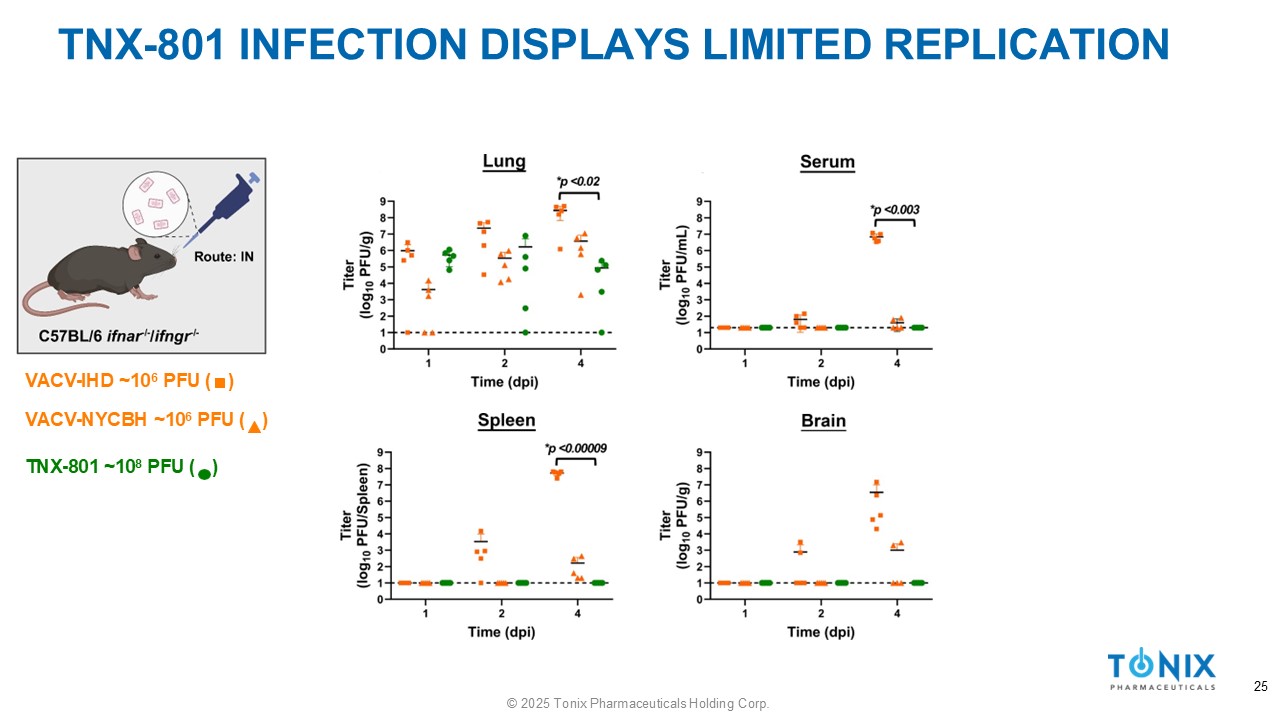
TNX - 801 INFECTION DISPLAYS LIMITED REPLICATION VACV - IHD ~10 6 PFU ( ) VACV - NYCBH ~10 6 PFU ( ) TNX - 801 ~10 8 PFU ( ) 25 © 2025 Tonix Pharmaceuticals Holding Corp.
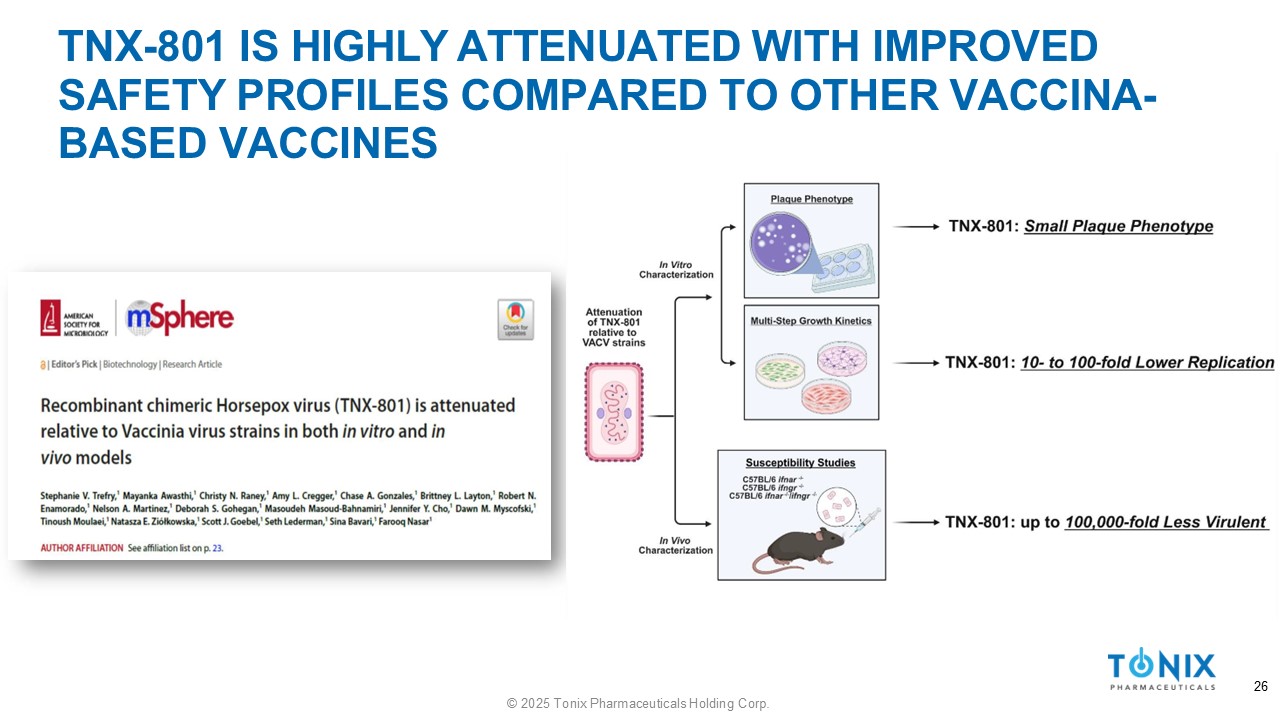
TNX - 801 IS HIGHLY ATTENUATED WITH IMPROVED SAFETY PROFILES COMPARED TO OTHER VACCINA - BASED VACCINES 26 © 2025 Tonix Pharmaceuticals Holding Corp.
TNX - 801 IMMUNOGENICITY AND EFFICACY IN ANIMAL MODELS 1) 2) 3) 27 © 2025 Tonix Pharmaceuticals Holding Corp.
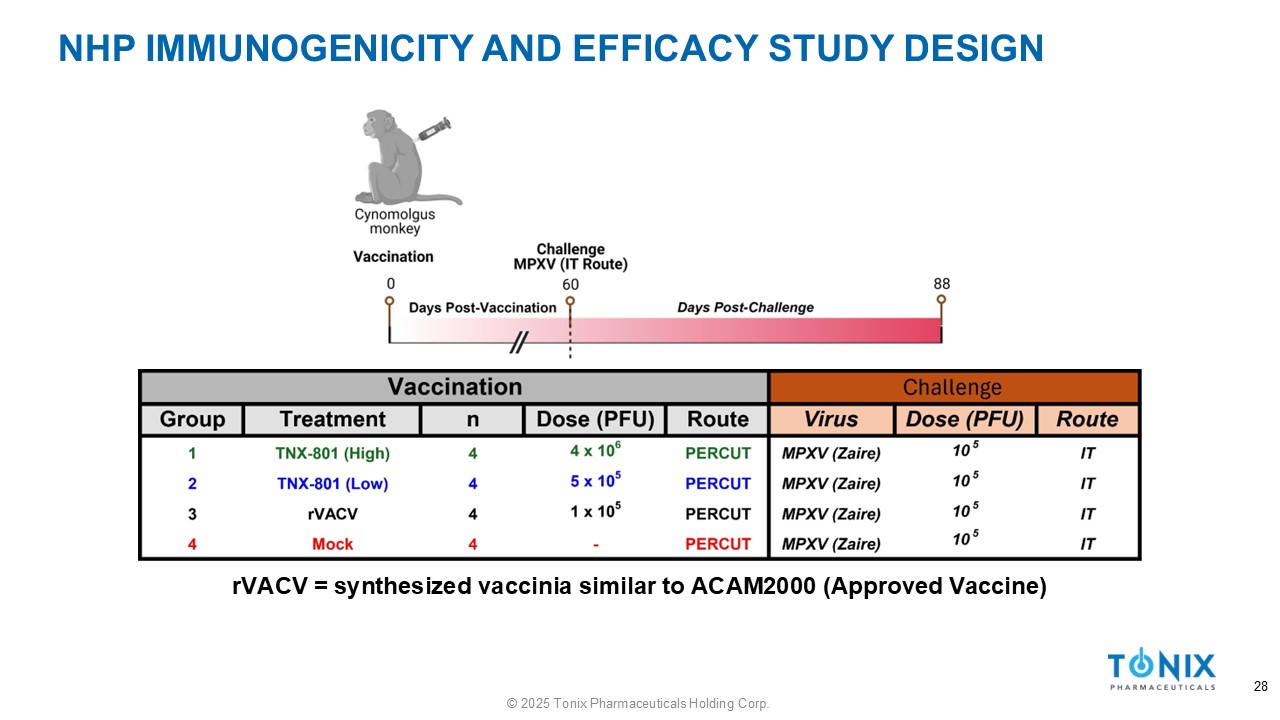
NHP IMMUNOGENICITY AND EFFICACY STUDY DESIGN rVACV = synthesized vaccinia similar to ACAM2000 (Approved Vaccine) 28 © 2025 Tonix Pharmaceuticals Holding Corp.
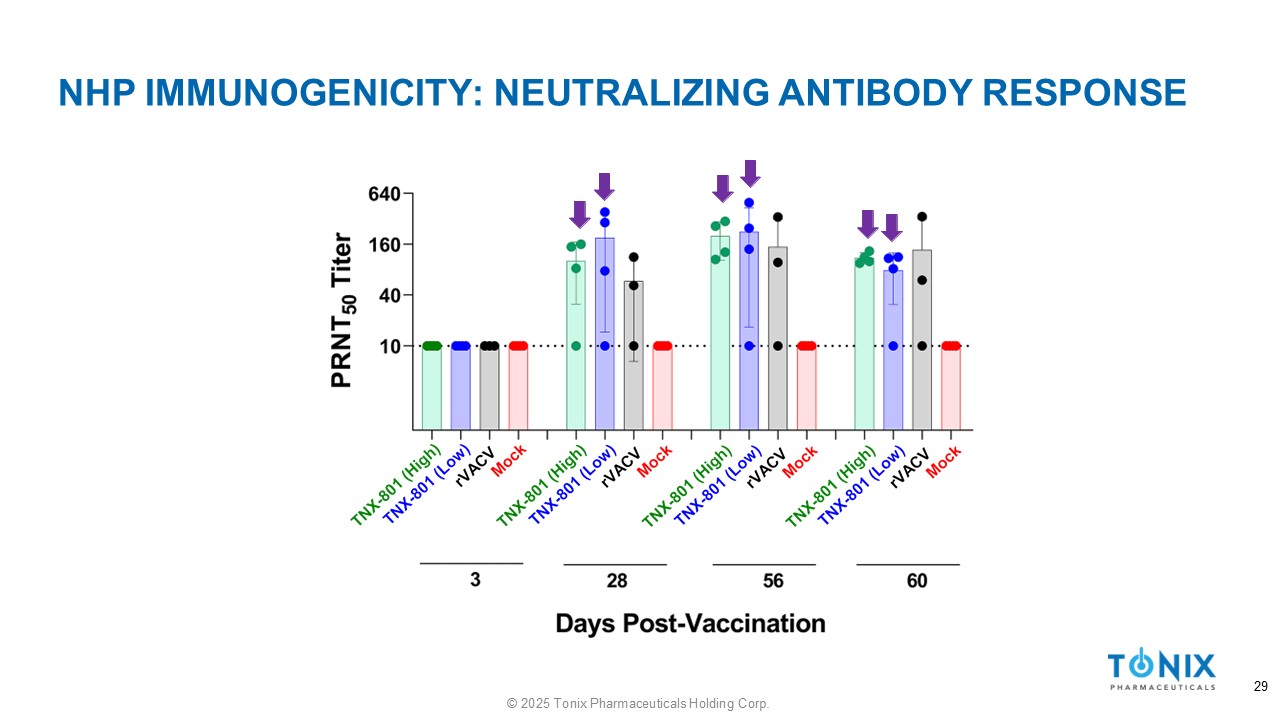
NHP IMMUNOGENICITY: NEUTRALIZING ANTIBODY RESPONSE 29 © 2025 Tonix Pharmaceuticals Holding Corp.
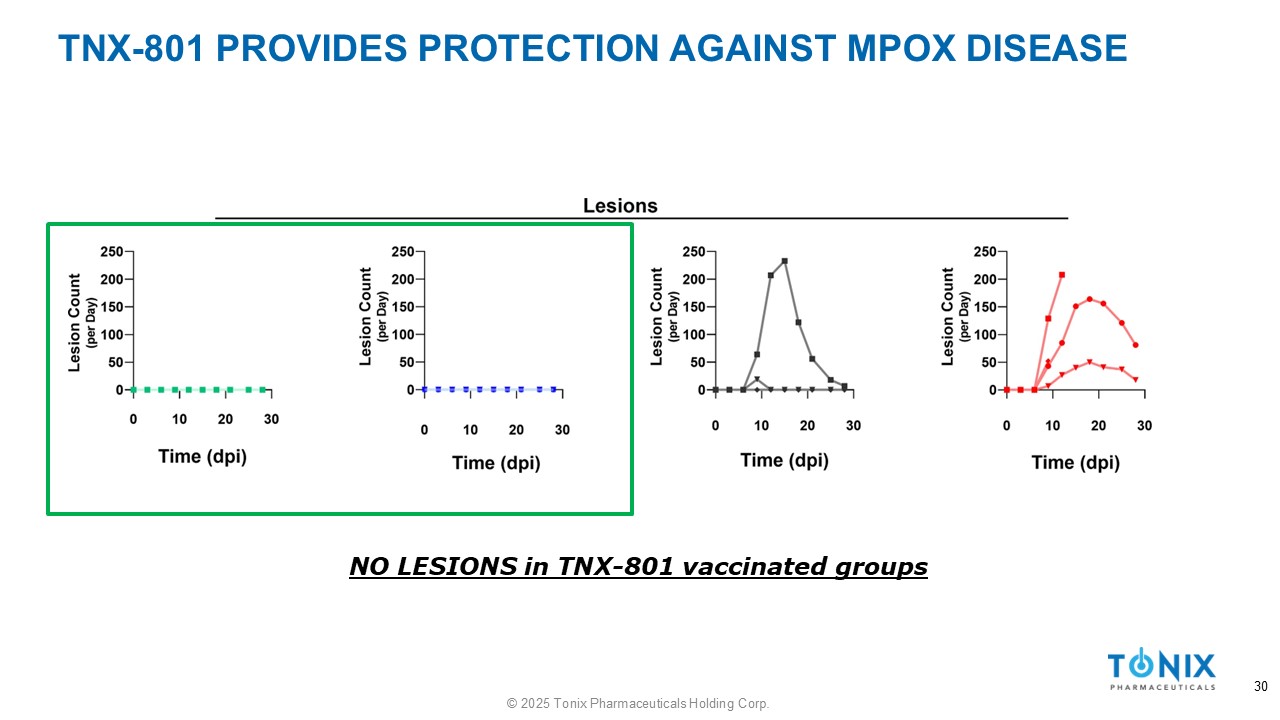
TNX - 801 PROVIDES PROTECTION AGAINST MPOX DISEASE NO LESIONS in TNX - 801 vaccinated groups 30 © 2025 Tonix Pharmaceuticals Holding Corp.
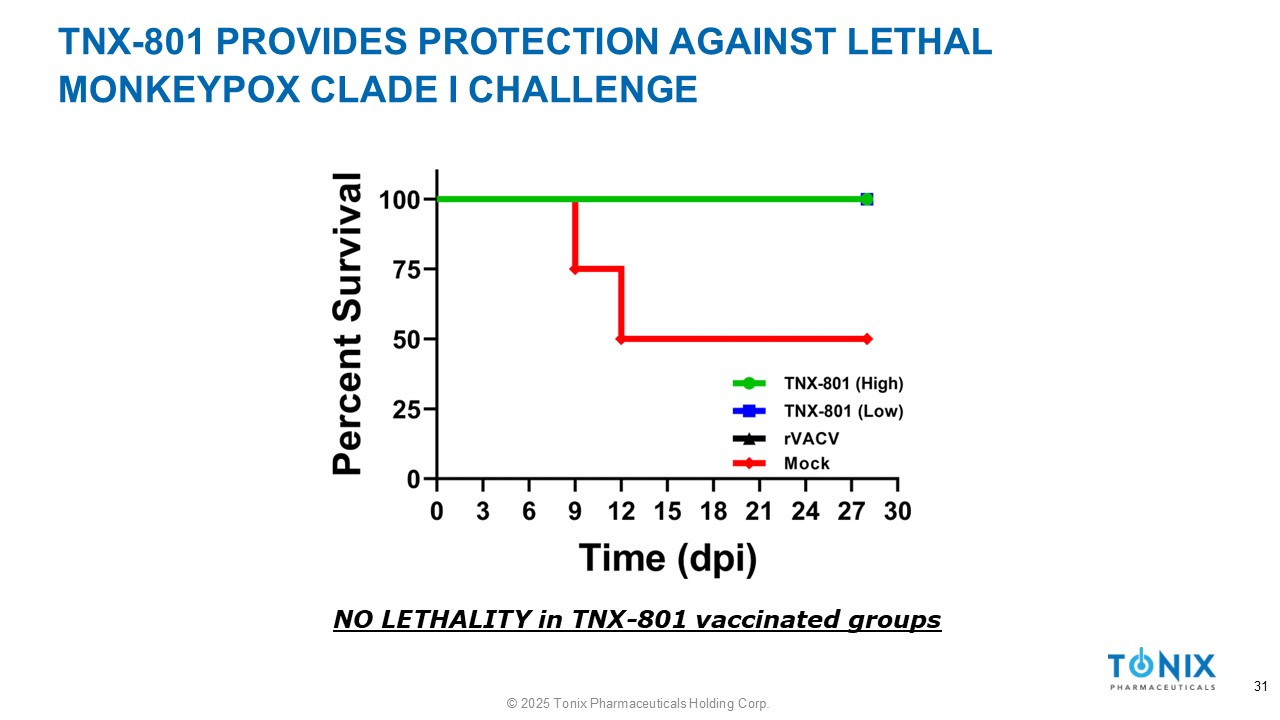
TNX - 801 PROVIDES PROTECTION AGAINST LETHAL MONKEYPOX CLADE I CHALLENGE NO LETHALITY in TNX - 801 vaccinated groups 31 © 2025 Tonix Pharmaceuticals Holding Corp.
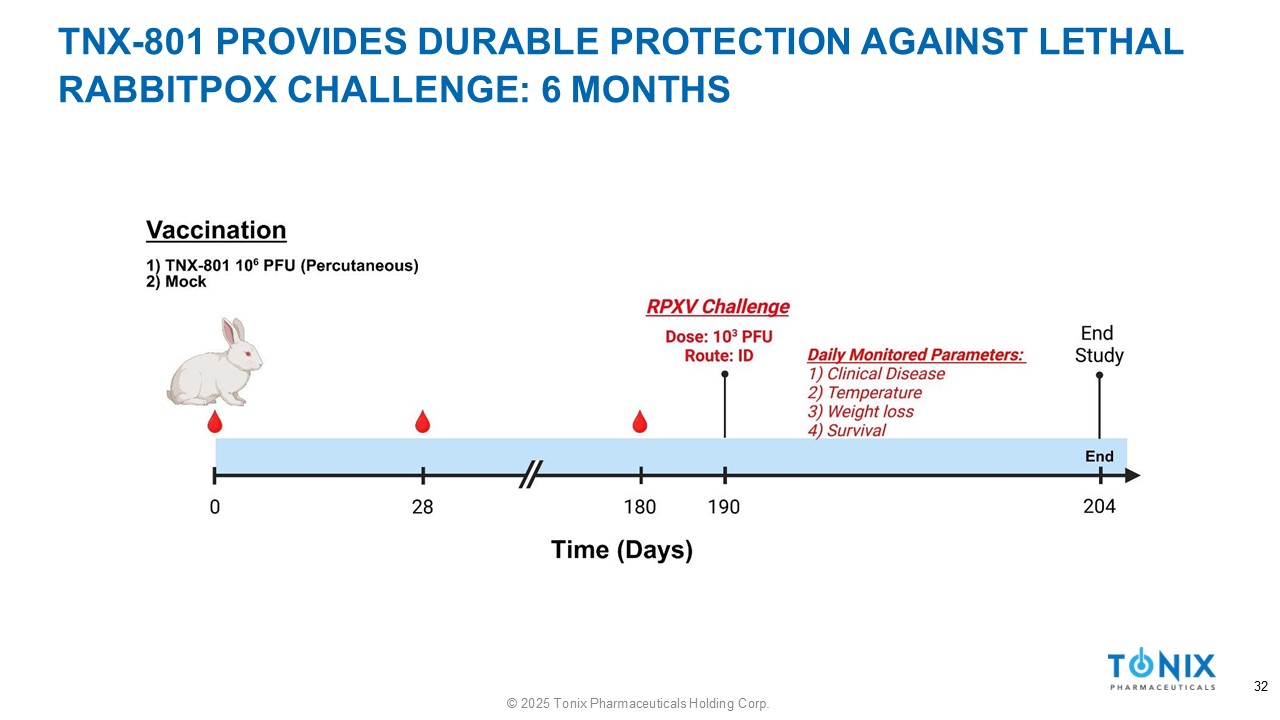
TNX - 801 PROVIDES DURABLE PROTECTION AGAINST LETHAL RABBITPOX CHALLENGE: 6 MONTHS 32 © 2025 Tonix Pharmaceuticals Holding Corp.
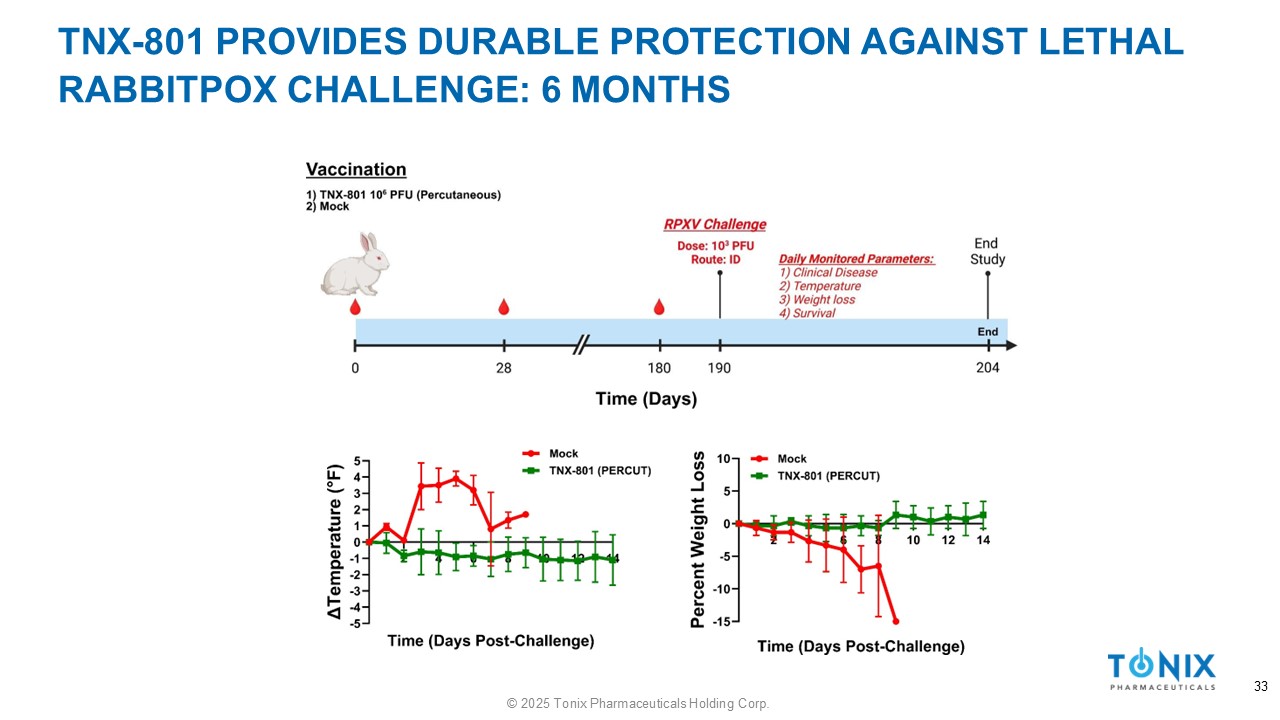
TNX - 801 PROVIDES DURABLE PROTECTION AGAINST LETHAL RABBITPOX CHALLENGE: 6 MONTHS 33 © 2025 Tonix Pharmaceuticals Holding Corp.
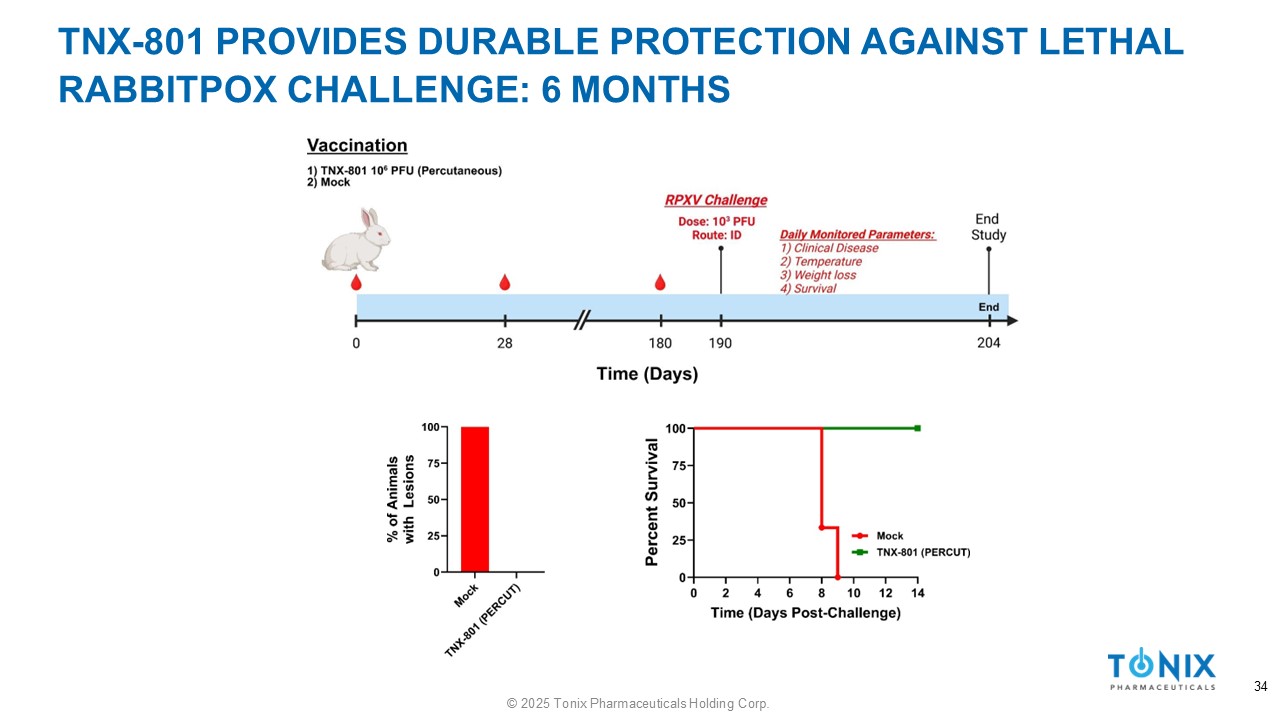
TNX - 801 PROVIDES DURABLE PROTECTION AGAINST LETHAL RABBITPOX CHALLENGE: 6 MONTHS 34 © 2025 Tonix Pharmaceuticals Holding Corp.
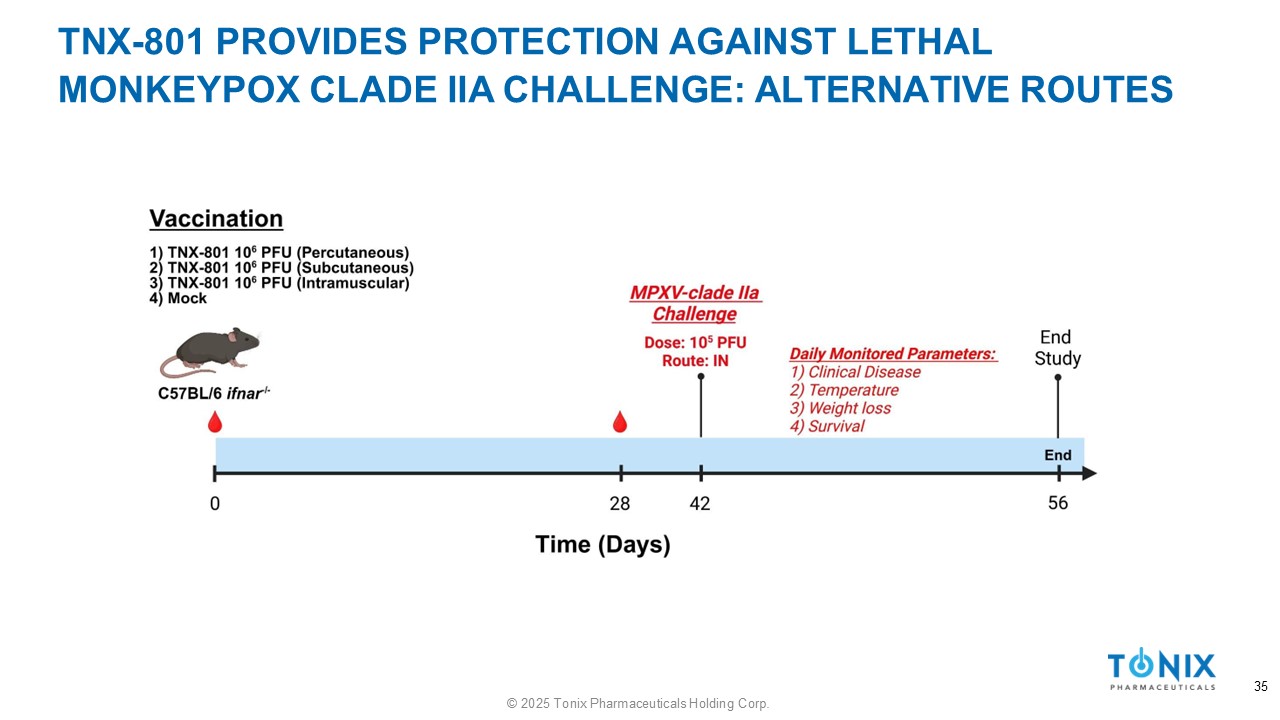
TNX - 801 PROVIDES PROTECTION AGAINST LETHAL MONKEYPOX CLADE IIA CHALLENGE: ALTERNATIVE ROUTES 35 © 2025 Tonix Pharmaceuticals Holding Corp.
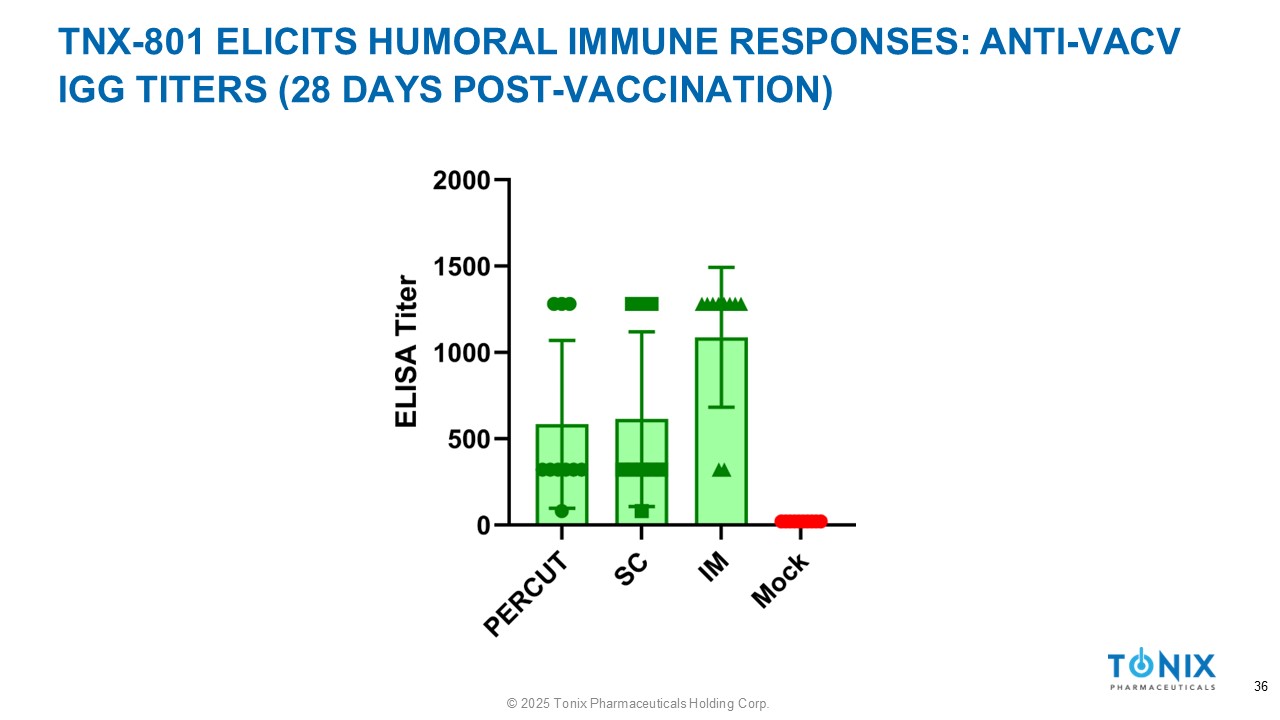
TNX - 801 ELICITS HUMORAL IMMUNE RESPONSES: ANTI - VACV IGG TITERS (28 DAYS POST - VACCINATION) 36 © 2025 Tonix Pharmaceuticals Holding Corp.
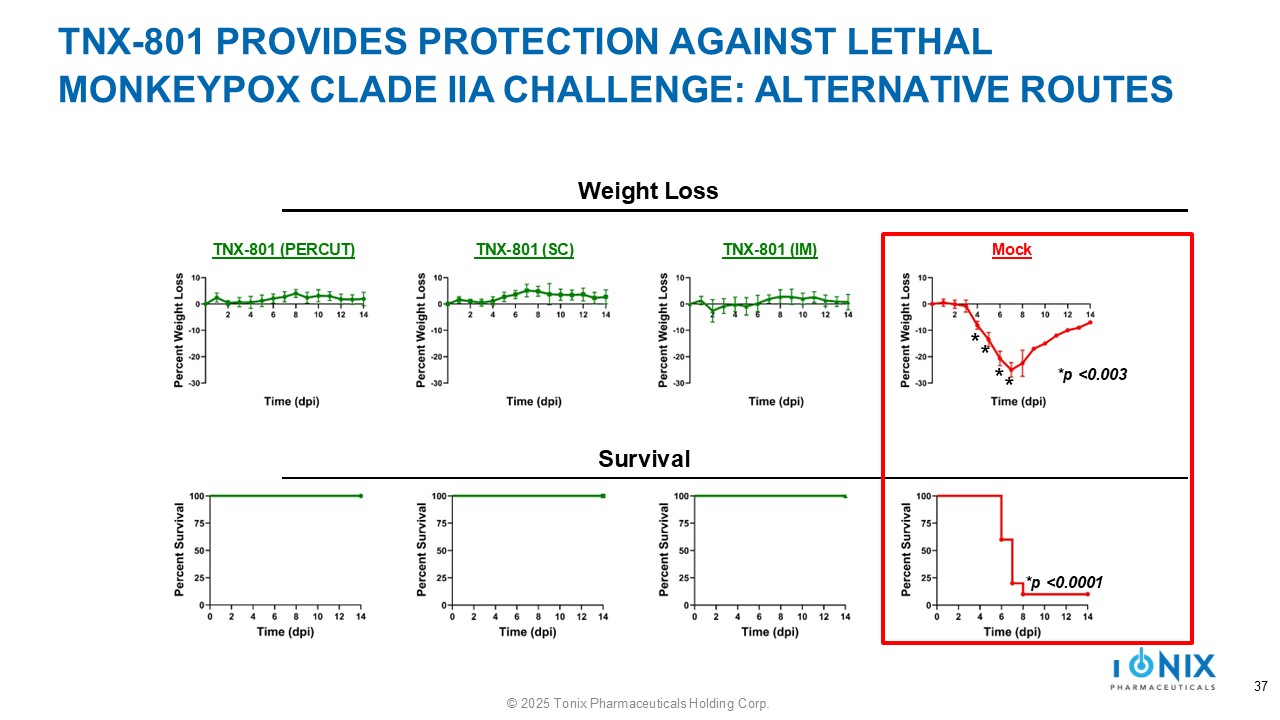
TNX - 801 PROVIDES PROTECTION AGAINST LETHAL MONKEYPOX CLADE IIA CHALLENGE: ALTERNATIVE ROUTES TNX - 801 (PERCUT) TNX - 801 (SC) TNX - 801 (IM) Mock Weight Loss Survival *p <0.0001 *p <0.003 * * * * 37 © 2025 Tonix Pharmaceuticals Holding Corp.
TNX - 801 SAFETY 38 © 2025 Tonix Pharmaceuticals Holding Corp. » In vitro : ▪ Small plaque phenotype ▪ Up to 100 - fold lower replication than VACV strains ▪ Primary cells from dermal and respiratory tracts » In vivo : ▪ Well tolerated in mice, rabbits, hamsters, and NHPs ▪ Minimal or no disease in immunocompromised murine models ▪ up to 100,000 - fold more attenuated than VACV - based vaccines ▪ Minimally replicates at site of delivery
TNX - 801 IMMUNOGENICITY AND EFFICACY (SINGLE DOSE) 39 © 2025 Tonix Pharmaceuticals Holding Corp. » Evaluated in multiple animal models ▪ Mouse, Rabbits, and NHPs » Elicits IgG and/or neutralizing responses ▪ Various route percutaneous, subcutaneous, intramuscular ▪ Microneedle delivery » Provides 100% protection against lesions ▪ Rabbit and NHP models » Provides 100% protection against lethal challenge ▪ Models: Mouse, Rabbit, and NHP ▪ Viruses: VACV, RPXV, MPXV clade Ia and IIa

4 PRONG APPROACH TO MPOX/SMALLPOX VACCINE (TNX - 801) 40 © 2025 Tonix Pharmaceuticals Holding Corp. 1) Well - tolerated 2) Single dose 3) Durable 4) Protection against mpox disease (lesions)
ACKNOWLEDGEMENTS 41 © 2025 Tonix Pharmaceuticals Holding Corp. » University of Alberta ▪ Ryan Noyce ▪ David Evans » Southern Research » Tonix Pharmaceuticals ▪ Scott Goebel ▪ Tinoush Moulaei ▪ Natasza Ziółkowska ▪ Siobhan Fogarty ▪ Helen Stillwell ▪ Bruce Daugherty ▪ Sina Bavari ▪ Seth Lederman » Tonix Pharmaceuticals ▪ Stephanie Trefry ▪ Mayanka Awasthi ▪ Christy Raney ▪ Amy Cregger ▪ Robert Enamorado ▪ Nelson Martinez ▪ Deborah Gohegan ▪ Zeil Rosenberg