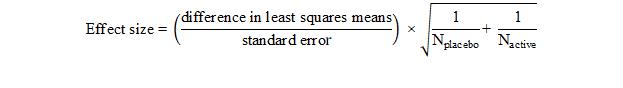
Tonix Pharmaceuticals Holding Corp. 8-K
Exhibit 99.02
Pain Relief by Targeting Nonrestorative Sleep
in Fibromyalgia: A Phase 3 Randomized
Trial of Bedtime Sublingual Cyclobenzaprine
Seth Lederman, MD1; Lesley M. Arnold, MD2; Ben Vaughn, MS3*; Jean M. Engels, MS1; Mary Kelley, MPH1; Gregory M. Sullivan, MD1
1Tonix Pharmaceuticals, Inc., Chatham, NJ, USA; 2University of Cincinnati College of Medicine, Cincinnati, OH, USA; 3Rho Inc, Chapel Hill, NC, USA
*At the time the study was conducted.
| 1 |
Supplementary Materials
Table of Contents
| Supplementary Methods | 3 |
| Supplementary Figure S1. Sensitivity analyses | 5 |
| Supplementary Figure S2. Continuous responder analysis of reduction in daily pain | 6 |
| Supplementary Figure S3. Change from baseline in FIQR Function domain score | 7 |
| Supplementary Figure S4. Change from baseline in diary-reported sleep quality | 8 |
| Supplementary Table S1. Subgroup analysis change from baseline in daily pain | 9 |
| Supplementary Table S2. Change from baseline in FIQR individual items | 10 |
| Supplementary Figure S5. Post hoc analysis | 11 |
| References | 12 |
| 2 |
Supplementary Methods
Assessments
The Patient Global Impression of Change (PGIC) scale evaluated the patient’s assessment of overall change in their fibromyalgia condition (1). A score of 1 on the PGIC indicated very much improved, 4 indicated no change, and 7 indicated very much worse. The Fibromyalgia Impact Questionnaire (Revised; FIQR) is a validated 21-item questionnaire that assessed the domains of Function (9 questions), Symptoms (10 questions), and Overall Impact (2 questions). Patient-Reported Outcomes Measurement Information System (PROMIS) Sleep Disturbance (version 8A) assessed perceptions of sleep quality, depth, and restoration; perceived difficulty in getting or staying asleep; and the adequacy and satisfaction with sleep (2). PROMIS Fatigue (version 8A) assessed the experience of fatigue and impact of fatigue on physical, mental, and social activities (2). Standardized electronic learning modules for placebo-response reduction and accurate pain reporting were administered to all participants at every in-clinic study visit prior to any patient-reported outcomes.
Statistical Analysis
A total of 4 prespecified sensitivity analyses were performed, modifying the specific elements of the primary endpoint analysis:
| (1) | Pain scores were censored on the days in which opioid pain medications were used. Before the weekly averaging, pain scores on impacted days were replaced by the score obtained on the day immediately before the first day of opioid use (and until the opioid was no longer used). |
| 3 |
| (2) | Patients that discontinued owing to withdrawal of consent or investigator decision were grouped with the lack of efficacy (LOE) and adverse event (AE) dropouts when performing the multiple imputation procedure. |
| (3) | All patients that discontinued were grouped with the LOE and AE dropouts when performing the multiple imputation procedure. |
| (4) | A tipping point analysis was performed on the values for the primary analysis. A shift parameter was applied to the TNX-102 SL arm starting at 0.25 and increasing in increments of 0.25 through either 5 or when the outcome was no longer significant. |
Effect sizes for the primary and secondary endpoints, except for PGIC, were calculated as:
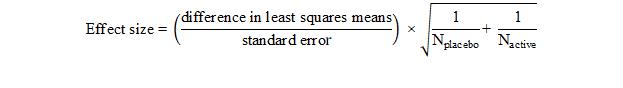
A post hoc analysis was conducted to assess the potential for unblinding among patients in the TNX-102 SL group who experienced any of the 3 common oral-sensory-related AEs that were not associated with mucosal tissue changes. The oral AEs in the preferred terms were hypoaesthesia oral (oral numbness), paraesthesia oral (oral tingling), and product taste abnormal (bitter aftertaste). In a 3-group mixed model for repeated measures approach with multiple imputation analysis, comparisons of reduction in daily pain intensity ratings were conducted comparing the entire placebo group with those who experienced any of the oral-sensory-related AEs (TNX-102 SL/oral-sensory AE subgroup) and those who did not experience any of these AEs (TNX-102 SL/no-oral-sensory AE subgroup). Additionally, the 2 TNX-102 SL subgroups, based on the presence or absence of oral sensory AEs, were compared to each other.
| 4 |
Supplementary Figure 1. Sensitivity analyses for change from baseline in weekly average of daily pain NRS intensity scores: (A) days with opioid use censored, (B) discontinuation due to withdrawal of consent and investigator decision considered missing not at random, (C) all discontinuations considered missing not at random, and (D) tipping point analysis at week 14. LS, least-squares; NRS, numeric rating scale; SL, sublingual.
| 5 |
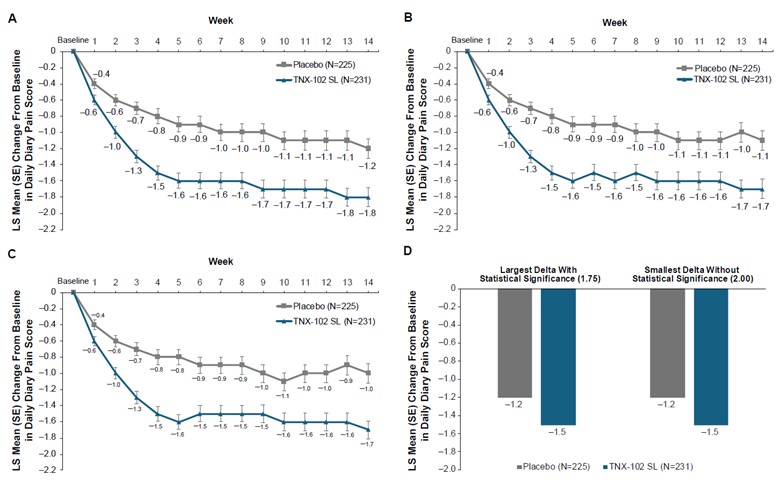
Supplementary Figure 2. Continuous responder analysis of daily pain reduction from baseline at week 14. SL, sublingual.
| 6 |
Supplementary Figure 3. LS mean (SE) change from baseline in FIQR Function domain score. No. of patients indicates patients with available data at each time point; multiple imputation was used to account for missing data. FIQR, Fibromyalgia Impact Questionnaire (Revised); LS, least-squares; SL, sublingual. *P=0.001; †P<0.01 (uncorrected).
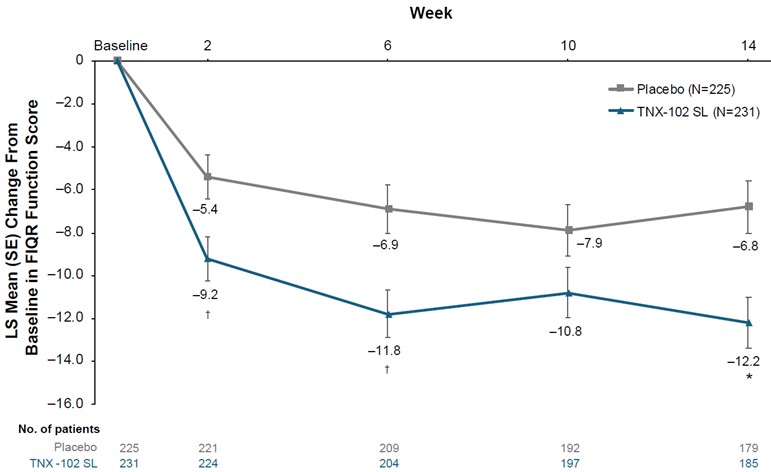
| 7 |
Supplementary Figure 4. Change from baseline weekly average of diary-reported sleep quality. No. of patients indicates patients with available data at each time point; multiple imputation was used to account for missing data. LS, least-squares; SL, sublingual. *P<0.001; †P<0.01 (uncorrected).
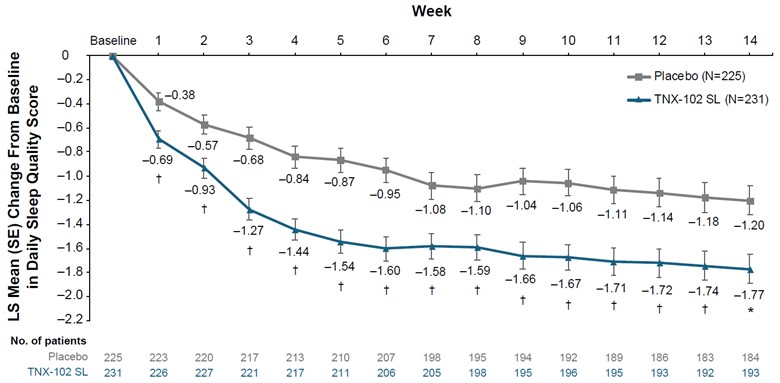
| 8 |
Supplementary Table 1. Subgroup Analysis of Mean Change From Baseline at Week 14 in Weekly Average of Daily Pain NRS Intensity Scores
| Subgroup |
TNX-102 SL |
Placebo |
LSMD (95% CI) | P value | ||
| n | LS mean (SE) change | n | LS mean (SE) change | |||
| Ethnicity | ||||||
| Hispanic/ Latino |
36 | –2.07 (0.31) | 35 | –0.92 (0.32) | –1.15 (–2.02 to –0.27) |
0.010 |
| Not Hispanic/ Latino |
195 | –1.74 (0.12) | 189 | –1.15 (0.12) | –0.59 (–0.92 to –0.26) | <0.001 |
| Sex | ||||||
| Female | 224 | –1.79 (0.12) | 211 | –1.16 (0.12) | –0.63 (–0.95 to –0.31) | <0.001 |
| Male* | 7 | –1.57 (1.07) | 8 | -0.85 (2.29) | –0.7 (NA) | |
| Race | ||||||
| White | 194 | –1.73 (0.12) | 192 | –1.04 (0.13) | –0.69 (–1.02 to –0.35) | <0.001 |
| Nonwhite | 37 | –2.12 (0.30) | 33 | –1.66 (0.31) | –0.46 (–1.31 to 0.39) | 0.289 |
LS, least-squares; LSMD, LS mean difference; NA, not available; NRS, numeric rating scale; SL, sublingual.
*Reported as observed mean (SD) change from baseline and observed mean difference between treatment groups.
| 9 |
Supplementary Table 2. Mean Change From Baseline at Week 14 in FIQR Individual Items for Symptoms and Overall Impact
| Item* |
TNX-102 SL N=185 |
Placebo N=179 |
LSMD | LSMD 95% CI |
| Rate your… (last 7 days) | ||||
| Level of pain | −2.2 (0.15) | −1.2 (0.15) | –1.0 | −1.4 to –0.6 |
| Level of energy | −1.6 (0.16) | −0.9 (0.17) | –0.8 | −1.2 to –0.3 |
| Level of stiffness | −2.0 (0.17) | −1.2 (0.17) | –0.8 | −1.3 to –0.3 |
| Quality of sleep | −2.9 (0.19) | −1.5 (0.19) | –1.4 | −2.0 to –0.9 |
| Level of depression | −0.9 (0.15) | −0.2 (0.15) | –0.8 | −1.2 to –0.4 |
| Level of memory problems | −1.3 (0.16) | −0.6 (0.17) | –0.8 | −1.2 to –0.3 |
| Level of anxiety | −1.2 (0.17) | −0.4 (0.17) | –0.8 | −1.2 to –0.3 |
| Level of tenderness to touch | −2.1 (0.18) | −1.3 (0.18) | –0.8 | −1.3 to –0.3 |
| Level of balance problems | −1.1 (0.15) | −0.6 (0.15) | –0.5 | −0.9 to –0.1 |
| Level of sensory sensitivity† | −1.8 (0.18) | −1.3 (0.18) | –0.6 | −1.0 to –0.1 |
| Over the last 7 days, fibromyalgia… | ||||
| Prevented accomplishing goals | −2.4 (0.18) | −1.6 (0.18) | –0.8 | −1.3 to –0.3 |
| Completely overwhelmed me | −2.1 (0.18) | −1.4 (0.18) | –0.7 | −1.2 to –0.2 |
FIQR, Fibromyalgia Impact Questionnaire (Revised); LS, least-squares; LSMD, LS mean difference; SL, sublingual.
*Reported as LS mean (SE) change from baseline.
†To loud noises, bright lights, odors, and cold.
| 10 |
Supplementary Figure 5. Post hoc analysis for the change from baseline in weekly average of daily pain NRS intensity score in patients with or without oral sensory AEs. *P<0.001; †P<0.01; ‡P<0.05 vs placebo. AE, adverse events; LS, least-squares; NRS, numeric rating scale; SL, sublingual.
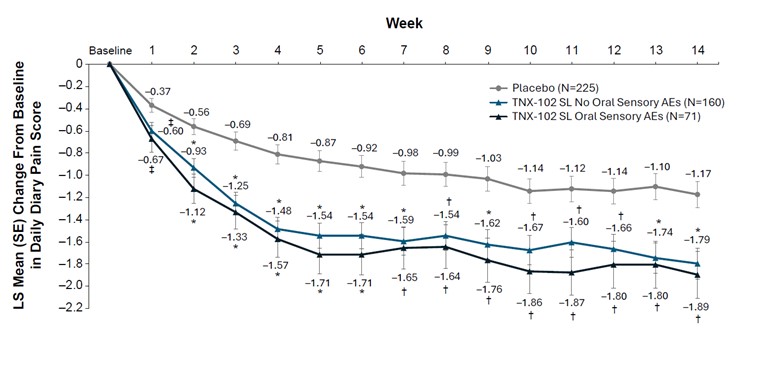
| 11 |
Supplementary Materials References
| 1. | Rampakakis E, Ste-Marie PA, Sampalis JS, et al. Real-life assessment of the validity of Patient Global Impression of Change in fibromyalgia. RMD Open 2015;1:e000146. |
| 2. | Cella D, Riley W, Stone A, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. J Clin Epidemiol 2010;63:1179-94. |
| 12 |