
TONIX PHARMACEUTICALS HOLDING CORP. 8-K
Exhibit 99.01

INVESTOR PRESENTATION NASDAQ: TNXP © 2022 Tonix Pharmaceuticals Holding Corp. Version P03 53 June 1 May 9 , 2022 (Doc 10 30 )

Cautionary Note on Forward - Looking Statements 2 © 2022 Tonix Pharmaceuticals Holding Corp. Certain statements in this presentation regarding strategic plans, expectations and objectives for future operations or results are “forward - looking statements” as defined by the Private Securities Litigation Reform Act of 1995. These statements may be identified by the use of forward - looking words such as “anticipate,” “believe,” “forecast,” “estimate” and “intend,” among others. These forward - looking statements are based on Tonix’s current expectations and actual results could differ materially. There are a number of factors that could cause actual events to differ materially from those indicated by such forward - looking statements. These factors include, but are not limited to, the risks related to failure to obtain FDA clearances or approvals and noncompliance with FDA regulations; delays and uncertainties caused by the global COVID - 19 pandemic; risks related to the timing and progress of clinical development of our product candidates; our need for additional financing; uncertainties of patent protection and litigation; uncertainties of government or third party payor reimbursement; limited research and development efforts and dependence upon third parties; and substantial competition. As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products. The forward - looking statements in this presentation are made as of the date of this presentation, even if subsequently made available by Tonix on its website or otherwise. Tonix does not undertake an obligation to update or revise any forward - looking statement, except as required by law. Investors should read the risk factors set forth in the Annual Report on Form 10 - K for the year ended December 31, 2021, as filed with the Securities and Exchange Commission (the “SEC”) on March 14, 2022, and periodic reports and current reports filed with the SEC on or after the date thereof. All of Tonix's forward - looking statements are expressly qualified by all such risk factors and other cautionary statements.

What we do OUR MISSION ADVANCING THE SCIENCE AND UNDERSTANDING OF DISEASES by developing innovative therapies that improve population health by focusing on unmet needs in patient care OUR STRATEGY Using our integrated development engine, we advance innovative programs across multiple therapeutic areas into the clinic while maximizing asset potential 3 © 2022 Tonix Pharmaceuticals Holding Corp.
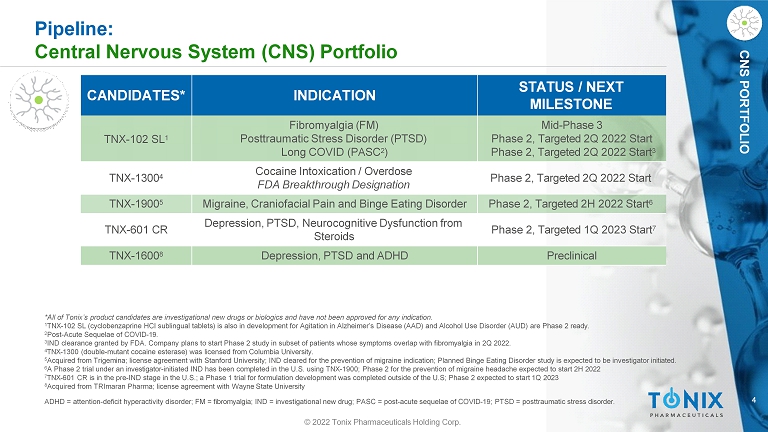
CNS PORTFOLIO Pipeline: Central Nervous System (CNS) Portfolio CANDIDATES* INDICATION STATUS / NEXT MILESTONE TNX - 102 SL 1 Fibromyalgia (FM) Posttraumatic Stress Disorder (PTSD) Long COVID (PASC 2 ) Mid - Phase 3 Phase 2, Targeted 2Q 2022 Start Phase 2, Targeted 2Q 2022 Start 3 TNX - 1300 4 Cocaine Intoxication / Overdose FDA Breakthrough Designation Phase 2, Targeted 2Q 2022 Start TNX - 1900 5 Migraine, Craniofacial Pain and Binge Eating Disorder Phase 2, Targeted 2H 2022 Start 6 TNX - 601 CR Depression, PTSD, Neurocognitive Dysfunction from Steroids Phase 2, Targeted 1Q 2023 Start 7 TNX - 1600 8 Depression, PTSD and ADHD Preclinical 4 ADHD = attention - deficit hyperactivity disorder; FM = fibromyalgia; IND = investigational new drug; PASC = post - acute sequelae of COVID - 19; PTSD = posttraumatic stress disorder. © 2022 Tonix Pharmaceuticals Holding Corp. *All of Tonix’s product candidates are investigational new drugs or biologics and have not been approved for any indication. 1 TNX - 102 SL (cyclobenzaprine HCl sublingual tablets) is also in development for Agitation in Alzheimer’s Disease (AAD) and Alcohol Use Disorder (AUD) are Phase 2 ready. 2 Post - Acute Sequelae of COVID - 19. 3 IND clearance granted by FDA. Company plans to start Phase 2 study in subset of patients whose symptoms overlap with fibromyalgia in 2Q 2022. 4 TNX - 1300 (double - mutant cocaine esterase) was licensed from Columbia University. 5 Acquired from Trigemina; license agreement with Stanford University; IND cleared for the prevention of migraine indication; Planned Binge Eating Disorder study is expected to be investigator initiated. 6 A Phase 2 trial under an investigator - initiated IND has been completed in the U.S. using TNX - 1900; Phase 2 for the prevention of migraine headache expected to start 2H 2022 7 TNX - 601 CR is in the pre - IND stage in the U.S.; a Phase 1 trial for formulation development was completed outside of the U.S; Phase 2 expected to start 1Q 2023 8 Acquired from TRImaran Pharma; license agreement with Wayne State University
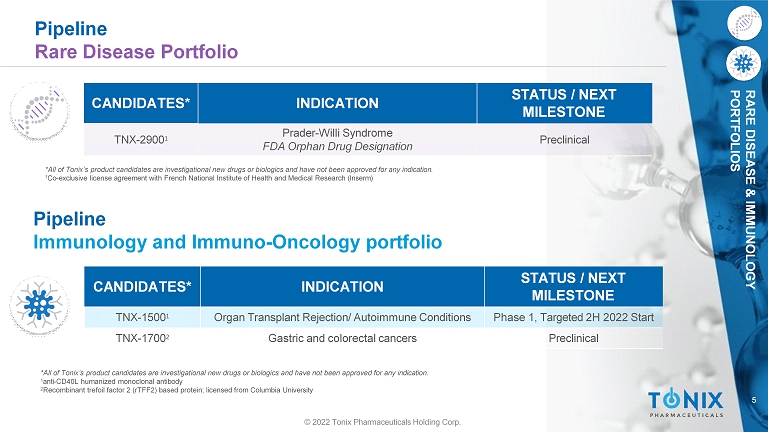
5 RARE DISEASE & IMMUNOLOGY PORTFOLIOS Pipeline Rare Disease Portfolio CANDIDATES* INDICATION STATUS / NEXT MILESTONE TNX - 2900 1 Prader - Willi Syndrome FDA Orphan Drug Designation Preclinical *All of Tonix’s product candidates are investigational new drugs or biologics and have not been approved for any indication. 1 Co - exclusive license agreement with French National Institute of Health and Medical Research (Inserm) CANDIDATES* INDICATION STATUS / NEXT MILESTONE TNX - 1500 1 Organ Transplant Rejection/ Autoimmune Conditions Phase 1, Targeted 2H 2022 Start TNX - 1700 2 Gastric and colorectal cancers Preclinical © 2022 Tonix Pharmaceuticals Holding Corp. Pipeline Immunology and Immuno - Oncology portfolio *All of Tonix’s product candidates are investigational new drugs or biologics and have not been approved for any indication. 1 anti - CD40L humanized monoclonal antibody 2 Recombinant trefoil factor 2 (rTFF2) based protein; licensed from Columbia University
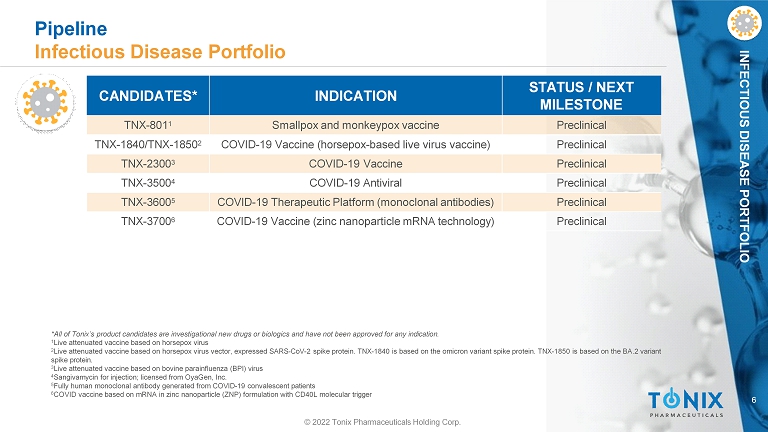
6 INFECTIOUS DISEASE PORTFOLIO *All of Tonix’s product candidates are investigational new drugs or biologics and have not been approved for any indication. 1 Live attenuated vaccine based on horsepox virus 2 Live attenuated vaccine based on horsepox virus vector, expressed SARS - CoV - 2 spike protein. TNX - 1840 is based on the omicron variant spike protein. TNX - 1850 is based on the BA.2 variant spike protein. 3 Live attenuated vaccine based on bovine parainfluenza (BPI) virus 4 Sangivamycin for injection; licensed from OyaGen, Inc. 5 Fully human monoclonal antibody generated from COVID - 19 convalescent patients 6 COVID vaccine based on mRNA in zinc nanoparticle (ZNP) formulation with CD40L molecular trigger CANDIDATES* INDICATION STATUS / NEXT MILESTONE TNX - 801 1 Smallpox and monkeypox vaccine Preclinical TNX - 1840/TNX - 1850 2 COVID - 19 Vaccine (horsepox - based live virus vaccine) Preclinical TNX - 2300 3 COVID - 19 Vaccine Preclinical TNX - 3500 4 COVID - 19 Antiviral Preclinical TNX - 3600 5 COVID - 19 Therapeutic Platform (monoclonal antibodies) Preclinical TNX - 3700 6 COVID - 19 Vaccine (zinc nanoparticle mRNA technology) Preclinical © 2022 Tonix Pharmaceuticals Holding Corp. Pipeline Infectious Disease Portfolio

CNS: KEY CANDIDATES © 2022 Tonix Pharmaceuticals Holding Corp.
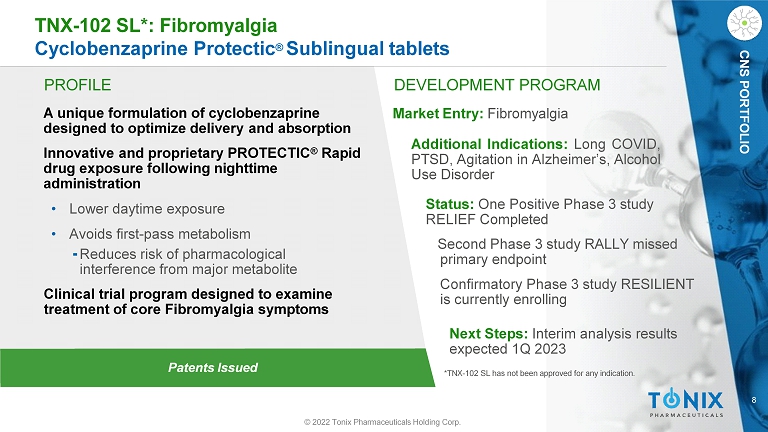
8 Patents Issued TNX - 102 SL*: Fibromyalgia Cyclobenzaprine Protectic ® Sublingual tablets © 2022 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO PROFILE A unique formulation of cyclobenzaprine designed to optimize delivery and absorption Innovative and proprietary PROTECTIC ® Rapid drug exposure following nighttime administration • Lower daytime exposure • Avoids first - pass metabolism ⁃ Reduces risk of pharmacological interference from major metabolite Clinical trial program designed to examine treatment of core Fibromyalgia symptoms DEVELOPMENT PROGRAM Market Entry: Fibromyalgia Additional Indications : Long COVID, PTSD, Agitation in Alzheimer’s, Alcohol Use Disorder Status: One Positive Phase 3 study RELIEF Completed Second Phase 3 study RALLY missed primary endpoint Confirmatory Phase 3 study RESILIENT is currently enrolling Next Steps: Interim analysis results expected 1Q 2023 *TNX - 102 SL has not been approved for any indication.
9 TNX - 102 SL: Fibromyalgia Program Update © 2022 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Phase 3 Study, RESILIENT (F307), will compare TNX - 102 SL 5.6 mg and placebo • First patient enrolled in April 2022 • Interim Analysis results expected 1Q 2023 • Parallel design, double - blind, randomized placebo - controlled study, all U.S. sites • Primary endpoint is pain at week 14 analyzed by MMRM with MI • Projecting adverse event related discontinuations to decrease towards rates in RELIEF and PTSD Studies Phase 3 Study, RALLY (F306), comparison of TNX - 102 SL 5.6 mg and placebo • As expected from interim results published July 2021, RALLY Study missed primary endpoint • Unexpected ~80% increase in adverse event - related discontinuations in both drug and placebo arms • Multiple imputation approach on 'Missing Data' attenuated statistical significance of efficacy endpoints’ • TNX - 102 SL was generally well tolerated with overall adverse event profile comparable to prior studies; no new safety signals observed

10 Patents Issued TNX - 102 SL*: Long COVID (PASC) Cyclobenzaprine Protectic ® Sublingual Tablets PROFILE Long COVID or Post - acute Sequelae of COVID - 19 (PASC 1 ) • Symptoms can include fatigue, sleep disorders, pain, fevers, shortness of breath, cognitive impairment described as “brain fog”, gastrointestinal symptoms, anxiety, and depression 2 • Can persist for months and can range in severity from mild to incapacitating • Occurs in 30% of recovered COVID - 19 patients • Typically associated with moderate or severe COVID - 19, Long COVID can occur after mild COVID - 19 or even after asymptomatic SARS - CoV - 2 infection To address the urgent need for PASC therapies, Congress awarded the National Institutes of Health $1.15 billion to study Long COVID. 3 DEVELOPMENT PROGRAM Market Entry : Long COVID (PASC) Status: Clinical – IND clearance granted Next Steps: Start Phase 2 study for treating subset of Long COVID patients whose symptoms overlap with fibromyalgia in 2Q 2022 1 Feb. 24, 2021 - White House COVID - 19 Response Team press briefing; Feb 25, 2021 - policy brief from the World Health Organization on long COVID 2 Nalbandian, Ani, et al. "Post - acute COVID - 19 syndrome." Nature Medicine (2021): 1 - 15. 3 The NIH provision of Title III Health and Human Services, Division M - - Coronavirus Response and Relief Supplemental Appropriations Act, 2021, of H.R. 133, The Consolidated Appropriations Act of 2021. The bill was enacted into law on 27 December 2020, becoming Public Law 116 - 260. © 2022 Tonix Pharmaceuticals Holding Corp. *TNX - 102 SL has not been approved for any indication. CNS PORTFOLIO Additional Indications: Fibromyalgia, PTSD, Agitation in Alzheimer’s, Alcohol Use Disorder
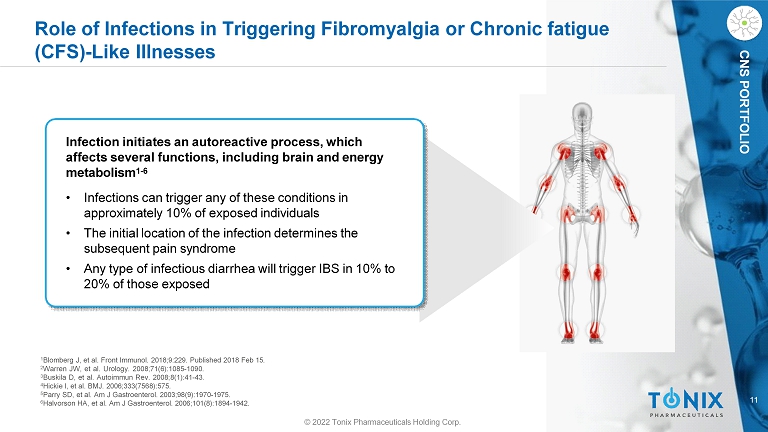
11 CNS PORTFOLIO Role of Infections in Triggering Fibromyalgia or Chronic fatigue (CFS) - Like Illnesses © 2022 Tonix Pharmaceuticals Holding Corp. Infection initiates an autoreactive process, which affects several functions, including brain and energy metabolism 1 - 6 • Infections can trigger any of these conditions in approximately 10% of exposed individuals • The initial location of the infection determines the subsequent pain syndrome • Any type of infectious diarrhea will trigger IBS in 10% to 20% of those exposed 1 Blomberg J, et al . Front Immunol . 2018 ; 9 : 229 . Published 2018 Feb 15 . 2 Warren JW, et al . Urology . 2008 ; 71 ( 6 ) : 1085 - 1090 . 3 Buskila D, et al . Autoimmun Rev . 2008 ; 8 ( 1 ) : 41 - 43 . 4 Hickie I, et al . BMJ . 2006 ; 333 ( 7568 ) : 575 . 5 Parry SD, et al . Am J Gastroenterol . 2003 ; 98 ( 9 ) : 1970 - 1975 . 6 Halvorson HA, et al . Am J Gastroenterol . 2006 ; 101 ( 8 ) : 1894 - 1942 .
12 CNS PORTFOLIO TNX - 102 SL: Long COVID a.k.a Post - Acute Sequelae of SARS - CoV - 2 Infection (PASC) • Long COVID is a heterogeneous condition that displays elements of nociplastic pain in many individuals, who experience otherwise unexplained 1 - 2 : • Symptoms (multi - site pain, fatigue, sleep disorders and cognitive dysfunction) overlap with the key symptoms of fibromyalgia • The primary outcome measure for fibromyalgia - type Long COVID will be decrease in multi - site pain measured by a daily diary Multisite pain Memory issues Fatigue Sleep disturbances © 2022 Tonix Pharmaceuticals Holding Corp. 1 Bierle DM, et al. Central Sensitization Phenotypes in Post Acute Sequelae of SARS - CoV - 2 Infection (PASC): Defining the Post COVID Syndrome. J Prim Care Community Health 2021;12:21501327211030826. doi: 10.1177/21501327211030826. 2 Moghimi, N. et al. The Neurological Manifestations of Post - Acute Sequelae of SARS - CoV - 2 infection Curr Neurol Neurosci Rep. 2021;21(9):44. doi: 10.1007/s11910 - 02101130 - 1.
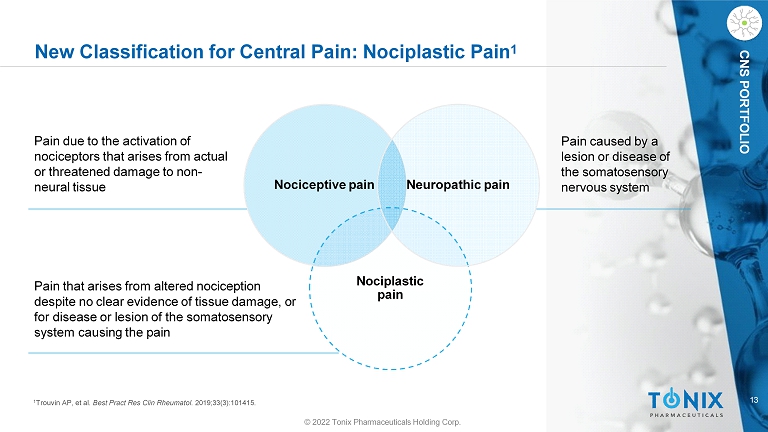
13 CNS PORTFOLIO New Classification for Central Pain: Nociplastic Pain 1 © 2022 Tonix Pharmaceuticals Holding Corp. Nociceptive pain Nociplastic pain Neuropathic pain Pain due to the activation of nociceptors that arises from actual or threatened damage to non - neural tissue Pain that arises from altered nociception despite no clear evidence of tissue damage, or for disease or lesion of the somatosensory system causing the pain Pain caused by a lesion or disease of the somatosensory nervous system 1 Trouvin AP, et al. Best Pract Res Clin Rheumatol . 2019;33(3):101415.
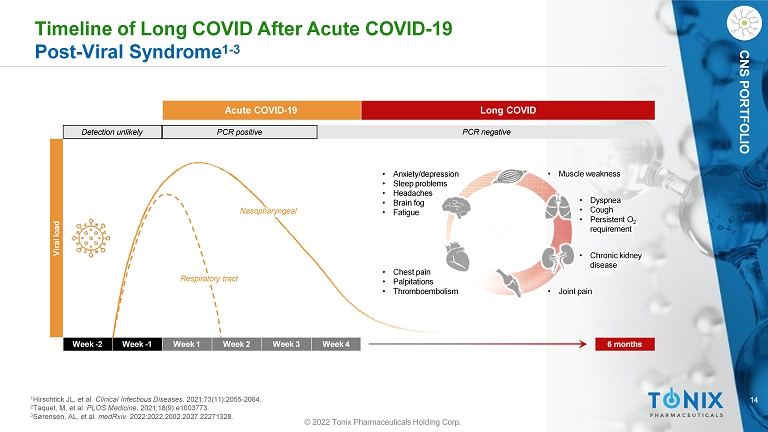
14 CNS PORTFOLIO Timeline of Long COVID After Acute COVID - 19 Post - Viral Syndrome 1 - 3 Week - 2 Week - 1 Week 1 Week 2 Week 3 Week 4 6 months Detection unlikely PCR positive PCR negative Acute COVID - 19 Long COVID Viral load Nasopharyngeal Respiratory tract • Chest pain • Palpitations • Thromboembolism • Dyspnea • Cough • Persistent O 2 requirement • Anxiety/depression • Sleep problems • Headaches • Brain fog • Fatigue • Chronic kidney disease • Muscle weakness • Joint pain 1 Hirschtick JL, et al. Clinical Infectious Diseases . 2021;73(11):2055 - 2064. 2 Taquet, M, et al. PLOS Medicine . 2021;18(9):e1003773. 3 Sørensen, AL, et al. medRxiv . 2022:2022.2002.2027.22271328. © 2022 Tonix Pharmaceuticals Holding Corp.
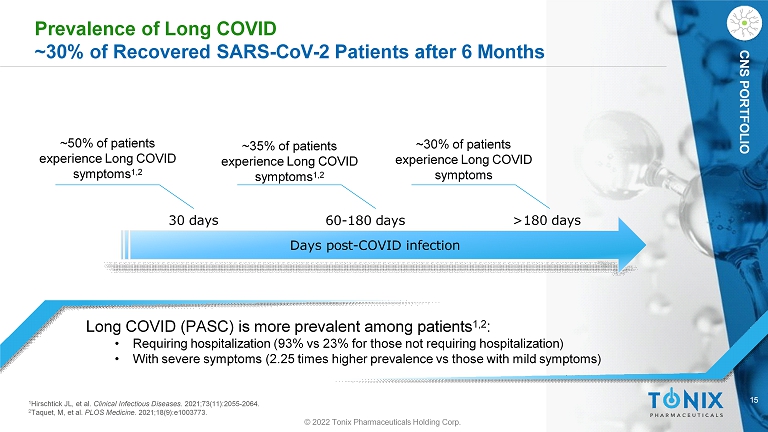
15 CNS PORTFOLIO Prevalence of Long COVID ~30% of Recovered SARS - CoV - 2 Patients after 6 Months Long COVID (PASC) is more prevalent among patients 1,2 : • Requiring hospitalization (93% vs 23% for those not requiring hospitalization) • With severe symptoms (2.25 times higher prevalence vs those with mild symptoms) ~50% of patients experience Long COVID symptoms 1,2 30 days 60 - 180 days Days post - COVID infection >180 days ~35% of patients experience Long COVID symptoms 1,2 ~30% of patients experience Long COVID symptoms 1 Hirschtick JL, et al. Clinical Infectious Diseases . 2021;73(11):2055 - 2064. 2 Taquet, M, et al. PLOS Medicine . 2021;18(9):e1003773. © 2022 Tonix Pharmaceuticals Holding Corp.
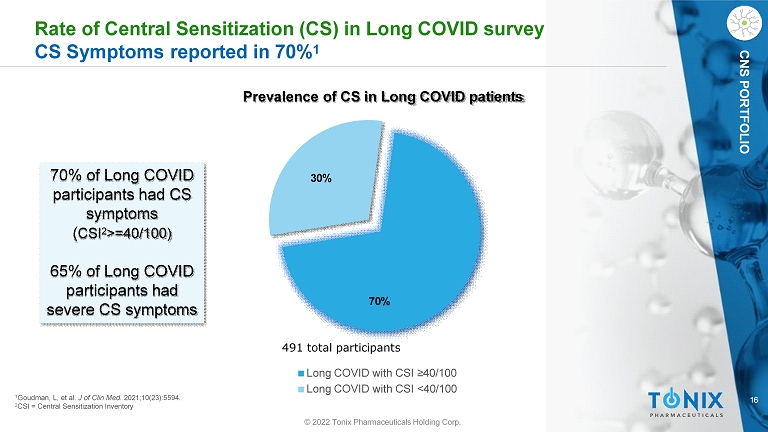
16 CNS PORTFOLIO Rate of Central Sensitization (CS) in Long COVID survey CS Symptoms reported in 70% 1 70% 30% 491 total participants Long COVID with CSI ≥40/100 Long COVID with CSI <40/100 1 Goudman, L, et al. J of Clin Med . 2021;10(23):5594. 2 CSI = Central Sensitization Inventory 70% of Long COVID participants had CS symptoms ( CSI 2 >=40/100) 65% of Long COVID participants had severe CS symptoms Prevalence of CS in Long COVID patients © 2022 Tonix Pharmaceuticals Holding Corp.
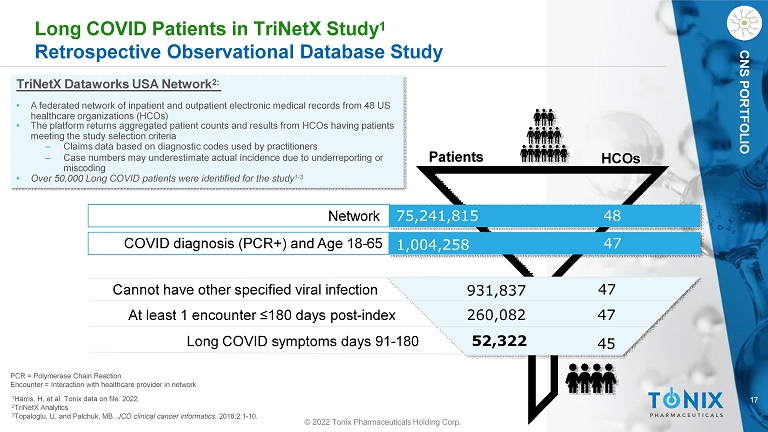
17 CNS PORTFOLIO Long COVID Patients in TriNetX Study 1 Retrospective Observational Database Study TriNetX Dataworks USA Network 2: • A federated network of inpatient and outpatient electronic medical records from 48 US healthcare organizations (HCOs) • The platform returns aggregated patient counts and results from HCOs having patients meeting the study selection criteria ‒ Claims data based on diagnostic codes used by practitioners ‒ Case numbers may underestimate actual incidence due to underreporting or miscoding • Over 50,000 Long COVID patients were identified for the study 1 - 3 52,322 260,082 931,837 47 47 45 48 47 1,004,258 HCOs Patients Network 75,241,815 © 2022 Tonix Pharmaceuticals Holding Corp. COVID diagnosis (PCR+) and Age 18 - 65 Cannot have other specified viral infection At least 1 encounter ≤180 days post - index Long COVID symptoms days 91 - 180 PCR = Polymerase Chain Reaction Encounter = Interaction with healthcare provider in network 1 Harris, H, et al. Tonix data on file. 2022. 2 TriNetX Analytics 3 Topaloglu, U, and Palchuk, MB. JCO clinical cancer informatics . 2018;2:1 - 10.
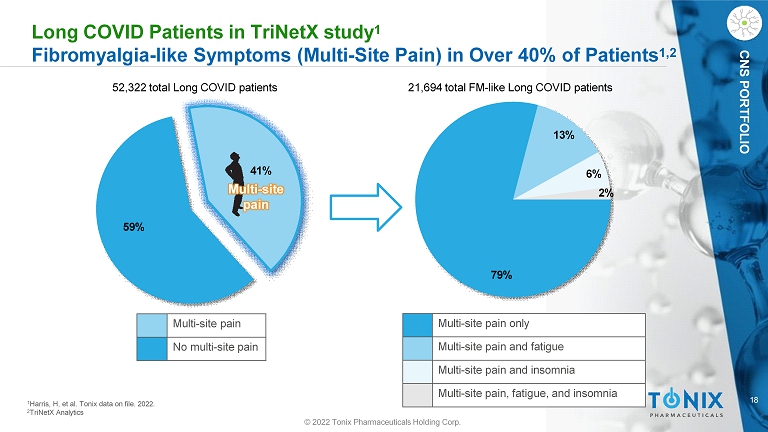
18 CNS PORTFOLIO Long COVID Patients in TriNetX study 1 Fibromyalgia - like Symptoms (Multi - Site Pain) in Over 40% of Patients 1,2 79% 13% 6% 2% 52,322 total Long COVID patients 21,694 total FM - like Long COVID patients 1 Harris, H, et al. Tonix data on file. 2022. 2 TriNetX Analytics 59% 41% Multi - site pain Multi - site pain No multi - site pain Multi - site pain only Multi - site pain and fatigue Multi - site pain and insomnia Multi - site pain, fatigue, and insomnia © 2022 Tonix Pharmaceuticals Holding Corp.
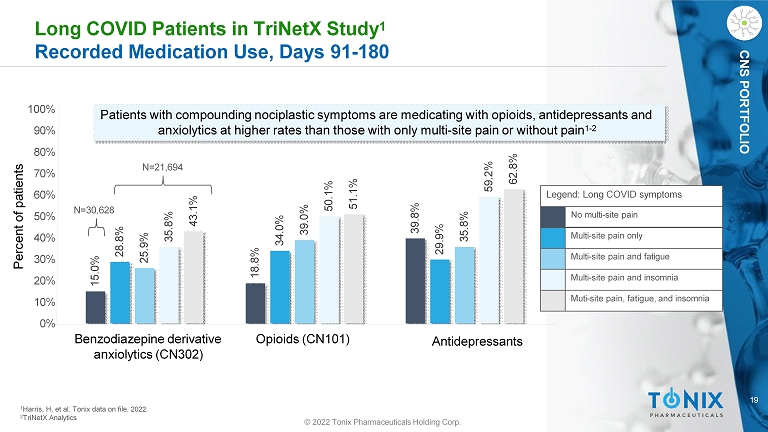
19 © 2022 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Long COVID Patients in TriNetX Study 1 Recorded Medication Use, Days 91 - 180 15.0% 18.8% 39.8% 28.8% 34.0% 29.9% 25.9% 39.0% 35.8% 35.8% 50.1% 59.2% 43.1% 51.1% 62.8% 100% 90% 80% 70% 60% 50% 40% 30% 20% 10% 0% Patients with compounding nociplastic symptoms are medicating with opioids, antidepressants and anxiolytics at higher rates than those with only multi - site pain or without pain 1 - 2 1 Harris, H, et al. Tonix data on file. 2022. 2 TriNetX Analytics Percent of patients Benzodiazepine derivative anxiolytics (CN302) Opioids (CN101) Antidepressants Legend: Long COVID symptoms No multi - site pain Multi - site pain only Multi - site pain and fatigue Multi - site pain and insomnia Muti - site pain, fatigue, and insomnia N=21,694 N=30,628
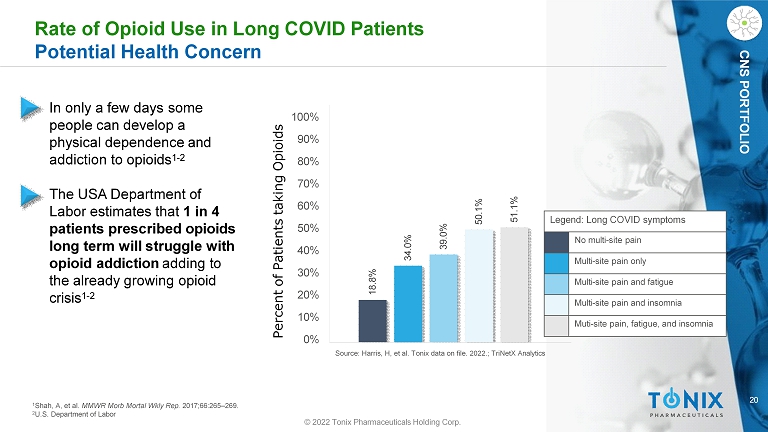
20 CNS PORTFOLIO 18.8% 34.0% 39.0% 50.1% 51.1% 100% 90% 80% 70% 60% 50% 40% 30% 20% 10% 0% Percent of Patients taking Opioids Rate of Opioid Use in Long COVID Patients Potential Health Concern • In only a few days some people can develop a physical dependence and addiction to opioids 1 - 2 • The USA Department of Labor estimates that 1 in 4 patients prescribed opioids long term will struggle with opioid addiction adding to the already growing opioid crisis 1 - 2 1 Shah, A, et al. MMWR Morb Mortal Wkly Rep. 2017;66:265 – 269. 2 U.S. Department of Labor Source: Harris, H, et al. Tonix data on file. 2022.; TriNetX Analytics Legend: Long COVID symptoms No multi - site pain Multi - site pain only Multi - site pain and fatigue Multi - site pain and insomnia Muti - site pain, fatigue, and insomnia © 2022 Tonix Pharmaceuticals Holding Corp.
21 CNS PORTFOLIO Significant Financial Impact of Long COVID for Households and Economies 25% of Long COVID patients are unable to return to work 1 Over 250,000 Quality Adjusted Life - Years (QUALYS) will be lost due to Long COVID in the UK 2 $23.3 billion is estimated to be paid by the UK government to avoid QUALY losses due to Long COVID 2 © 2022 Tonix Pharmaceuticals Holding Corp. 1 Davis, HE, et al. eClinicalMedicine . 2021;38. 2 Martin, C, et al. PloS one . 2021;16(12):e0260843 - e0260843.
22 CNS PORTFOLIO Long COVID Presidential Memorandum President Biden – April 5, 2022 1 © 2022 Tonix Pharmaceuticals Holding Corp. Policy • Commits to redoubling efforts to address the long - term effects of COVID - 19 Organizing Government Wide Response • Harnesses the full potential of the Federal Government, in coordination with public - and private - sector partners, to mount a full and effective response National Research Action Plane • Coordinates efforts across the public and private sectors • Orders establishment of the first - ever interagency national research agenda to, among other things, foster development of new treatments based on a better understanding of the pathophysiological mechanisms of the SARS - CoV - 2 virus Previously, Congress awarded NIH $ 1 . 15 billion to study Long COVID . 2 • Funded among other things the RECOVER Initiative implemented by the National Institutes of Health. 1 April 5, 2022 President Biden. “Memorandum on Addressing the Long - Term Effects of COVID - 19 - www.whitehouse.gov/briefing - room/presidential - actions/2022/04/05/memorandum - on - addressing - the - long - term - effects - of - covid - 19/ 2 The NIH provision of Title III Health and Human Services, Division M - - Coronavirus Response and Relief Supplemental Appropriations Act, 2021, of H.R. 133, The Consolidated Appropriations Act of 2021. The bill was enacted into law on 27 December 2020, becoming Public Law 116 - 260.
23 CNS PORTFOLIO Lo ng COVID and Vaccination Recent Reports 1 © 2022 Tonix Pharmaceuticals Holding Corp. Vaccination may not change risk of Long COVID after Breakthrough COVID - 19 • A retrospective cohort study of 10,024 breakthrough infection in the US showed no benefit of vaccination in decreasing Long COVID after breakthrough infection 1 ‒ Vaccination has benefits in decreased symptoms of acute breakthrough COVID • A UK study (different vaccines than are used in US) showed a ~50% reduction in Long COVID after breakthrough COVID 2 Herd immunity concept may not apply to COVID - 19 • Dr. Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases (NIAID) has written 3 − “‘Classical’ herd immunity, leading to disease eradication or elimination, almost certainly is an unattainable goal” − Prior discussion about COVID not disrupting most people’s lives was focused on herd immunity − For other viruses, herd immunity occurs when “natural infection with a pathogen” reaches a “community circulation [that] is reduced below the level of significant public health threat.” 1 Taquet, M et al. (2022) “Six - month sequelae of post - vaccination SARS - CoV - 2 infection: A retrospective cohort study of 10,024 breakthrough infections. “Brain, Behavior, and Immunity,” 103, 154 - 162, https://doi.org/10.1016/j.bbi.2022.04.013. 2 Antonelli, M et al. (2022) “Risk factors and disease profile of post - vaccination SARS - CoV - 2 infection in UK users of the COVID Symptom Study app: a prospective, community - based, nested, case - control study,” Lancet Infectious Diseases, 22(1) 43 - 55, https://doi.org/10.1016/S1473 - 3099(21)00460 - 6. 3 David M Morens, DM, Folkers, GK and Fauci, AS. “The Concept of Classical Herd Immunity May Not Apply to COVID - 19”, The Journal of Infectious Diseases , 2022;, jiac109, https://doi.org/10.1093/infdis/jiac109
24 CNS PORTFOLIO Opportunities to Expand TNX - 102 SL to Other Indications Growing recognition that there are many disorders where sleep disturbances may have a role in the pathophysiology (cardiovascular, metabolic, neurologic) • Sleep quality plays a homeostatic role in several disorders Psychiatric Disorders • Stress Disorders (PTSD) • Mood Disorders (Depression) • Anxiety Disorders • Addiction (Alcohol Use Disorder) Chronic Pain States • Chronic wide - spread pain (fibromyalgia) • Osteoarthritis Role of sleep disturbance more established in common psychiatric and neurological/pain disorders • Recognized as a core symptom of many of these disorders • Traditional sleep medications, which increase sleep quantity, may not provide benefit (benzodiazepines in major depression) or are contraindicated Psychiatric Symptoms of Neurological Disorders © 2022 Tonix Pharmaceuticals Holding Corp. • Agitation in Alzheimer’s • Psychosis in Parkinson’s, Alzheimer’s and other dementias

25 CNS PORTFOLIO Patents Issued TNX 102 SL*: Posttraumatic Stress disorder (PTSD) Cyclobenzaprine Protectic® Sublingual Tablets © 2022 Tonix Pharmaceuticals Holding Corp. PROFILE PTSD is a serious chronic psychiatric illness • Defined as maladaptive prolonged stress response which occurs after experiencing severely injurious traumatic event(s) Affects approximately 12 million Americans adults 1,2 Large unmet clinical need and limited effective therapies available • Advances in pharmacological treatments beyond the currently approved SSRIs (e.g., Zoloft® (sertraline), Paxil® (paroxetine)) are needed 3 DEVELOPMENT PROGRAM Market Entry: PTSD Additional Indications : Fibromyalgia, Long COVID, Agitation in Alzheimer’s, Alcohol Use Disorder Status: One Phase 2 study (AtEase) completed Two Phase 3 studies (HONOR, RECOVERY) conducted Next Steps: 2Q 2022 Initiate Phase 2 Trial in Kenya 1 Goldstein RB, et al. The epidemiology of DSM - 5 posttraumatic stress disorder in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions - III. Soc Psychiatry Psychiatr Epidemiol. 2016;51(8):1137 - 1148. 2 Pietrzak RH, et al. Prevalence and Axis I comorbidity of full and partial posttraumatic stress disorder in the United States: results from Wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. J Anxiety Disord. 2011;25(3):456 - 465. *TNX - 102 SL has not been approved for any indication. 3 Cain, C. K., et al. Targeting memory processes with drugs to prevent or cure PTSD. Expert Opin Investig Drugs. 2012; 21(9), 1323 - 1350
26 © 2022 Tonix Pharmaceuticals Holding Corp. DEVELOPMENT PROGRAM Patents Issued TNX - 1300*: Cocaine Intoxication Cocaine Esterase (CocE) PROFILE Cocaine is the main cause for drug - related ED visits 1 Cocaine use can cause irreversible structural damage to the heart and accelerate cardiovascular disease 2 • In one survey of 94 long - term cocaine users, 71 % had some form of cardiovascular disease 3 CocE is a recombinant protein that degrades cocaine in the bloodstream • Rapidly reverses physiologic effects of cocaine • Drops plasma exposure by 90% in 2 minutes Market Entry: Cocaine Intoxication Additional Indications: Cocaine Overdose Status: Phase 2 Open Label Next Steps: 2Q 2022 Initiate Trial 1 Havakuk O et al. J Am Coll Cardiol . 2017;70:101 - 113. 2 Phillips K et al. Am J Cardiovasc Drugs . 2009;9:177 - 196. 3 Maceira AM et al. J Cardiovasc Magn Reson . 2014;16:26. ED = emergency department. FDA Breakthrough Therapy Designation *TNX - 1300 has not been approved for any indication. CNS PORTFOLIO
27 Patents Issued TNX - 601 CR*: Depression Tianeptine Oxalate and Naloxone © 2022 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO PROFILE A novel, oral, controlled release once - daily tablet Mechanistically different from traditional monoaminergic treatments for depression Indirectly modulates the glutamatergic system • No direct binding to NMDA, AMPA, or kainate receptors Naloxone added to deter parenteral abuse Treatment effect of tianeptine in depression is well - established DEVELOPMENT PROGRAM Market Entry: Major Depressive Disorder Additional Indications: PTSD, Neurocognitive Disorder From Corticosteroids Status: pre - IND Next Steps: 1Q 2023 Initiate Phase 2 Trial AMPA=α - amino - 3 - hydroxy - 5 - methyl - 4 - isoxazolepropionic acid; MAOI=monoamine oxidase inhibitors; NMDA=N - methyl - D - aspartate. *TNX - 601 CR is in the pre - IND stage of development and has not been approved for any indication.
28 DEVELOPMENT PROGRAM Patents Issued TNX - 1900*: Migraine Intranasal Potentiated Oxytocin (OT) with Magnesium © 2022 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO PROFILE Intranasal OT has potential utility in treating migraine 1 • Intranasal OT reaches the trigeminal ganglion • Preclinical evidence of OT blocking CGRP release and suppressing pain • Association of low OT levels during and preceding migraine episodes • Novel non - CGRP antagonist approach to treatment Magnesium is known to potentiate the binding of OT to its receptor 2,3 One billion individuals worldwide suffer from migraines Market Entry: Chronic Migraine Additional Indications: Acute Migraine, Craniofacial Pain, Insulin Resistance, Binge Eating Disorder Status: Clinical – IND cleared for prevention of migraine headache 4 Next Steps: 2H 2022 Initiate Phase 2 Trial and Investigator Initiated Phase 2 Trial in Binge Eating Disorder 1 Tzabazis A, et al. Oxytocin and Migraine Headache. Headache. 2017 May;57 Suppl 2:64 - 75. doi: 10.1111/head.13082. PMID: 28485846. 2 Antoni FA, Chadio SE. Essential role of magnesium in oxytocin - receptor affinity and ligand specificity. Biochem J. 1989 Jan 15;257(2):611 - 4. doi: 10.1042/bj2570611. PMID: 2539090; PMCID: PMC1135623. 3 Meyerowitz, J.G., et al. The oxytocin signaling complex reveals a molecular switch for cation dependence. Nat Struct Mol Biol (2022). ( https://doi.org/10.1038/s41594 - 022 - 00728 - 4) 4 A Phase 2 trial under an investigator - initiated IND has been completed in the U.S. using TNX - 1900 *TNX - 1900 has not been approved for any indication. CGRP = calcitonin gene - related peptide.
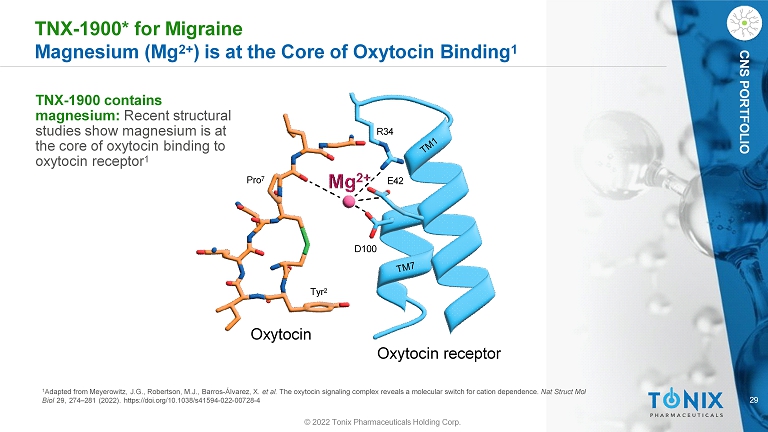
29 CNS PORTFOLIO TNX - 1900* for Migraine Magnesium (Mg 2+ ) is at the Core of Oxytocin Binding 1 1 Adapted from Meyerowitz, J.G., Robertson, M.J., Barros - Álvarez, X. et al. The oxytocin signaling complex reveals a molecular switch for cation dependence. Nat Struct Mol Biol 29, 274 – 281 (2022). https://doi.org/10.1038/s41594 - 022 - 00728 - 4 R34 E42 D100 © 2022 Tonix Pharmaceuticals Holding Corp. Tyr 2 Pro 7 Oxytocin Oxytocin receptor TNX - 1900 contains magnesium: Recent structural studies show magnesium is at the core of oxytocin binding to oxytocin receptor 1

RARE DISEASE: KEY CANDIDATES © 2022 Tonix Pharmaceuticals Holding Corp.
31 RARE DISEASE PORTFOLIO Patents Issued TNX - 2900*: Prader - Willi Syndrome Intranasal Potentiated Oxytocin (OT) with Magnesium © 2022 Tonix Pharmaceuticals Holding Corp. PROFILE Prader - Willi Syndrome is the most common genetic cause of life - threatening childhood obesity • Rare disease occurring in 1 in 15,000 births Symptoms include lack of suckling as infants, poor muscle strength, and constant hunger (hyperphagia) • In animal models, OT has improved suckling and suppressed hunger ‒ Tonix’s patented potentiated OT formulation is believed to increase specificity for OT receptors relative to off - target vasopressin receptors DEVELOPMENT PROGRAM Market Entry: Prader - Willi Syndrome Additional Indications: Rare Hyperphagia Conditions Status: Preclinical, granted orphan drug designation by FDA Next Steps: pre - IND Meeting to seek agreement on development plans *TNX - 2900 is in the pre - IND stage of development and has not been approved for any indication.

IMMUNOLOGY: KEY CANDIDATES © 2022 Tonix Pharmaceuticals Holding Corp.
33 IMMUNOLOGY PORTFOLIO TNX - 1500 ( ߙ ߙ - CD40L mAb): Prophylaxis of Transplant Rejection Potential Treatment for Autoimmune Conditions Pre - IND Candidate Significant Unmet Need © 2022 Tonix Pharmaceuticals Holding Corp. Targeted as a first - line monotherapy for autoimmunity and add - on therapy for preventing and treating organ transplant rejection • Distinct mechanism of action (MOA) — TNX - 1500 blocks T cell helper function New molecular entity, biologic • US Patient Protection and Affordable Care Act provides 12 years of exclusivity for biologics Patent applications directed to composition of matter • Expected patent protection through 2039 Clinical evidence for anti - CD40L mAbs in the treatment of systemic lupus erythematosus (SLE) and allogeneic kidney transplant • Several studies have shown anti - CD40L to be active in the treatment of human SLE 1 - 3 and transplant rejection 4,5 1 Huang W, et al. Arthritis Rheum . 2002;46(6):1554 - 1562. 2 Boumpas DT, et al. Arthritis Rheum . 2003;48(3):719 - 727. 3 Grammer AC, et al. J Clin Invest . 2003;112(10):1506 - 1520. 4 Kawai T, et al. Nat Med . 2000;6(2):114. 5 Koyama I, et al. Transplantation . 2004;77(3):460 - 462.
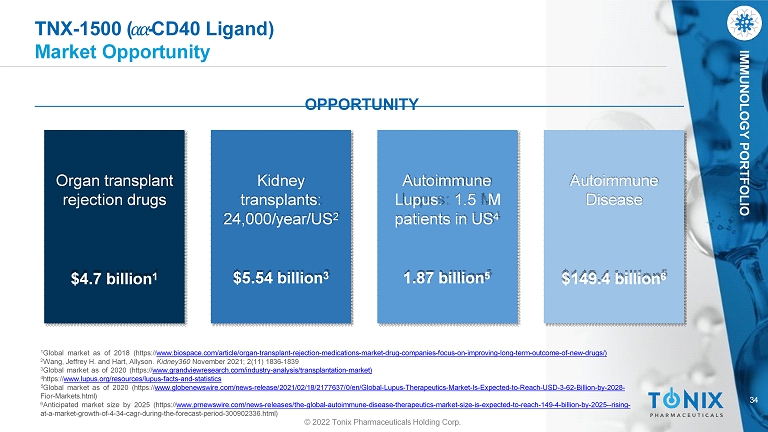
34 IMMUNOLOGY PORTFOLIO TNX - 1500 ( ߙ ߙ - CD40 Ligand) Market Opportunity Autoimmune Disease $149.4 billion 6 OPPORTUNITY Kidney transplants: 24,000/year/US 2 $5.54 billion 3 Autoimmune Lupus : 1 . 5 M patients in US 4 1.87 billion 5 Organ transplant rejection drugs $4.7 billion 1 © 2022 Tonix Pharmaceuticals Holding Corp. 1 Global market as of 2018 (https:// www.biospace.com/article/organ - transplant - rejection - medications - market - drug - companies - focus - on - improving - long - term - outcome - of - new - drugs/) 2 Wang, Jeffrey H. and Hart, Allyson. Kidney360 November 2021; 2(11) 1836 - 1839 3 Global market as of 2020 (https:// www.grandviewresearch.com/industry - analysis/transplantation - market) 4 https:// www.lupus.org/resources/lupus - facts - and - statistics 5 Global market as of 2020 (https:// www.globenewswire.com/news - release/2021/02/18/2177637/0/en/Global - Lupus - Therapeutics - Market - Is - Expected - to - Reach - USD - 3 - 62 - Billio n - by - 2028 - Fior - Markets.html) 6 Anticipated market size by 2025 (https:// www.prnewswire.com/news - releases/the - global - autoimmune - disease - therapeutics - market - size - is - expected - to - reach - 149 - 4 - billion - by - 2025 -- rising - at - a - market - growth - of - 4 - 34 - cagr - during - the - forecast - period - 300902336.html)
35 IMMUNOLOGY PORTFOLIO About CD40L (also called CD154) CD40L is a transiently expressed T cell surface molecule and is also called CD154 1 - 4 ‒ Predominantly expressed by T cells and interacts with CD40 on B cells and macrophages Mediates T cell helper function 1 - 4 ‒ Activates B cells for humoral (antibody - mediated) immune response ‒ Activates macrophages and dendritic cells ‒ Provides T cell help to activated CD8+ T cells X - linked hyper - IgM syndrome is caused by a defective CD40L gene 5 - 6 ‒ Lack of T helper function with only IgM serum antibodies but no IgG or IgE because T cells are required for B cell isotype switching ‒ If maintained on gamma globulin, patients are otherwise healthy Member of the TNFα superfamily 4 ‒ TNFα and RANKL are other family members and are drug targets for approved products 2 Lederman S, et al. J Immunol . 1992;149(12):3817 - 3826. 3 Lederman S, et al. J Immunol . 1994;152(5):2163 - 2171. © 2022 Tonix Pharmaceuticals Holding Corp. 1 Lederman S, et al. J Exp Med . 1992;175(4):1091 - 1101. 4 Covey LR, et al. Mol Immunol . 1994;31(6):471 - 484. 5 Ramesh N, et al. Int Immunol . 1993;5(7):769 - 773. 6 Callard RE, et al. J Immunol . 1994;153(7):3295 - 3306.
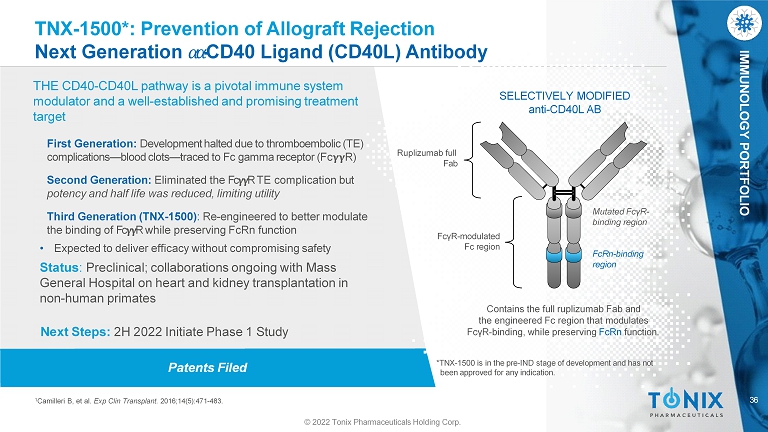
IMMUNOLOGY PORTFOLIO Patents Filed TNX - 1500*: Prevention of Allograft Rejection Next Generation ߙ ߙ - CD40 Ligand (CD40L) Antibody THE CD40 - CD40L pathway is a pivotal immune system modulator and a well - established and promising treatment target SELECTIVELY MODIFIED anti - CD40L AB Ruplizumab full Fab Contains the full ruplizumab Fab and the engineered Fc region that modulates FcγR - binding, while preserving FcRn function. Mutated FcγR - binding region 36 1 Camilleri B, et al. Exp Clin Transplant. 2016;14(5):471 - 483. © 2022 Tonix Pharmaceuticals Holding Corp. FcRn - binding region FcγR - modulated Fc region First Generation: Development halted due to thromboembolic (TE) complications — blood clots — traced to Fc gamma receptor (Fc R) Second Generation: Eliminated the Fc R TE complication but potency and half life was reduced, limiting utility Third Generation (TNX - 1500) : Re - engineered to better modulate the binding of Fc R while preserving FcRn function • Expected to deliver efficacy without compromising safety Status : Preclinical; collaborations ongoing with Mass General Hospital on heart and kidney transplantation in non - human primates Next Steps: 2H 2022 Initiate Phase 1 Study *TNX - 1500 is in the pre - IND stage of development and has not been approved for any indication.
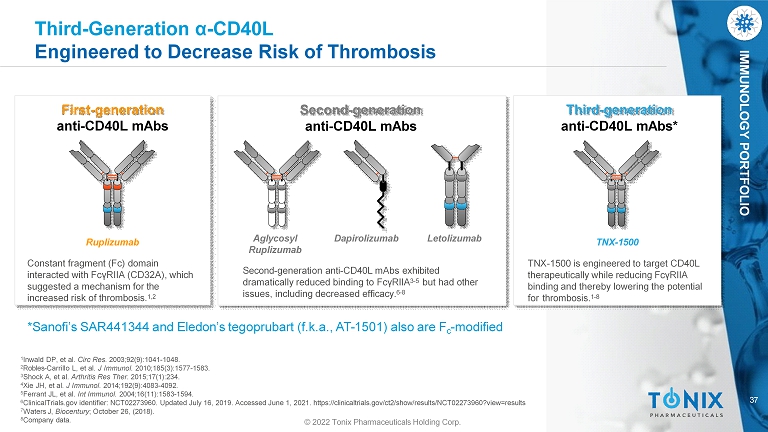
37 © 2022 Tonix Pharmaceuticals Holding Corp. IMMUNOLOGY PORTFOLIO Third - Generation α - CD40L Engineered to Decrease Risk of Thrombosis First - generation anti - CD40L mAbs Ruplizumab Constant fragment (Fc) domain interacted with FcγRIIA (CD32A), which suggested a mechanism for the increased risk of thrombosis. 1,2 Second - generation anti - CD40L mAbs Second - generation anti - CD40L mAbs exhibited dramatically reduced binding to FcγRIIA 3 - 5 but had other issues, including decreased efficacy. 6 - 8 Dapirolizumab Letolizumab Aglycosyl Ruplizumab Third - generation anti - CD40L mAbs* TNX - 1500 TNX - 1500 is engineered to target CD40L therapeutically while reducing FcγRIIA binding and thereby lowering the potential for thrombosis. 1 - 8 *Sanofi’s SAR441344 and Eledon’s tegoprubart (f.k.a., AT - 1501) also are F c - modified 1 Inwald DP, et al. Circ Res . 2003;92(9):1041 - 1048. 2 Robles - Carrillo L, et al. J Immunol . 2010;185(3):1577 - 1583. 3 Shock A, et al. Arthritis Res Ther . 2015;17(1):234. 4 Xie JH, et al. J Immunol . 2014;192(9):4083 - 4092. 5 Ferrant JL, et al. Int Immunol . 2004;16(11):1583 - 1594. 6 ClinicalTrials.gov identifier: NCT02273960. Updated July 16, 2019. Accessed June 1, 2021. https://clinicaltrials.gov/ct2/show/results/NCT02273960?view=results 7 Waters J, Biocentury ; October 26, (2018). 8 Company data.
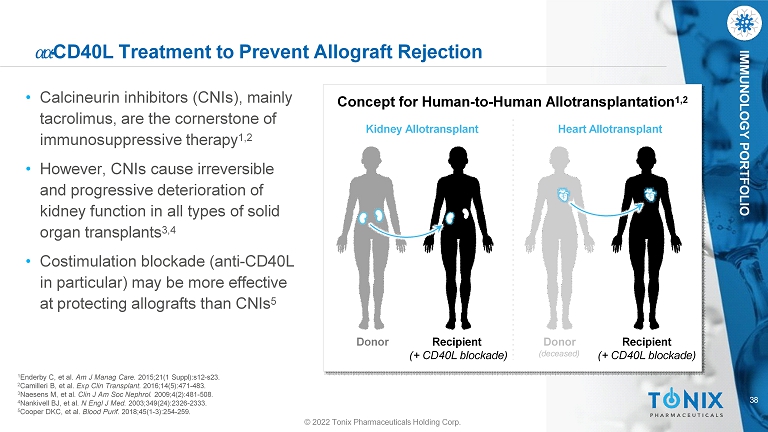
38 IMMUNOLOGY PORTFOLIO ߙ ߙ - CD40L Treatment to Prevent Allograft Rejection • Calcineurin inhibitors (CNIs), mainly tacrolimus, are the cornerstone of immunosuppressive therapy 1,2 • However, CNIs cause irreversible and progressive deterioration of kidney function in all types of solid organ transplants 3,4 • Costimulation blockade (anti - CD40L in particular) may be more effective at protecting allografts than CNIs 5 1 Enderby C, et al. Am J Manag Care. 2015;21(1 Suppl):s12 - s23. 2 Camilleri B, et al. Exp Clin Transplant. 2016;14(5):471 - 483. 3 Naesens M, et al. Clin J Am Soc Nephrol. 2009;4(2):481 - 508. 4 Nankivell BJ, et al. N Engl J Med. 2003;349(24):2326 - 2333. 5 Cooper DKC, et al. Blood Purif. 2018;45(1 - 3):254 - 259. Donor Recipient (+ CD40L blockade) Concept for Human - to - Human Allotransplantation 1,2 Kidney Allotransplant Heart Allotransplant Donor (deceased) © 2022 Tonix Pharmaceuticals Holding Corp. Recipient (+ CD40L blockade)
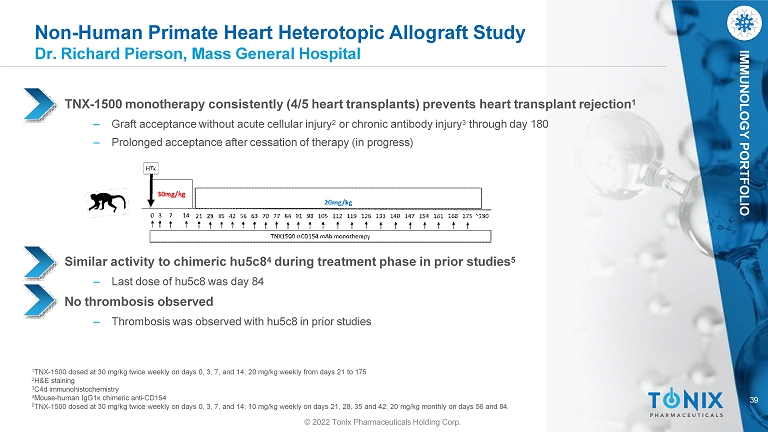
39 IMMUNOLOGY PORTFOLIO Non - Human Primate Heart Heterotopic Allograft Study Dr. Richard Pierson, Mass General Hospital TNX - 1500 monotherapy consistently (4/5 heart transplants) prevents heart transplant rejection 1 ‒ Graft acceptance without acute cellular injury 2 or chronic antibody injury 3 through day 180 ‒ Prolonged acceptance after cessation of therapy (in progress) Similar activity to chimeric hu5c8 4 during treatment phase in prior studies 5 ‒ Last dose of hu5c8 was day 84 No thrombosis observed ‒ Thrombosis was observed with hu5c8 in prior studies 1 TNX - 1500 dosed at 30 mg/kg twice weekly on days 0, 3, 7, and 14; 20 mg/kg weekly from days 21 to 175 2 H&E staining 3 C4d immunohistochemistry 4 Mouse - human IgG1κ chimeric anti - CD154 5 TNX - 1500 dosed at 30 mg/kg twice weekly on days 0, 3, 7, and 14; 10 mg/kg weekly on days 21, 28, 35 and 42; 20 mg/kg monthly on days 56 and 84. © 2022 Tonix Pharmaceuticals Holding Corp.
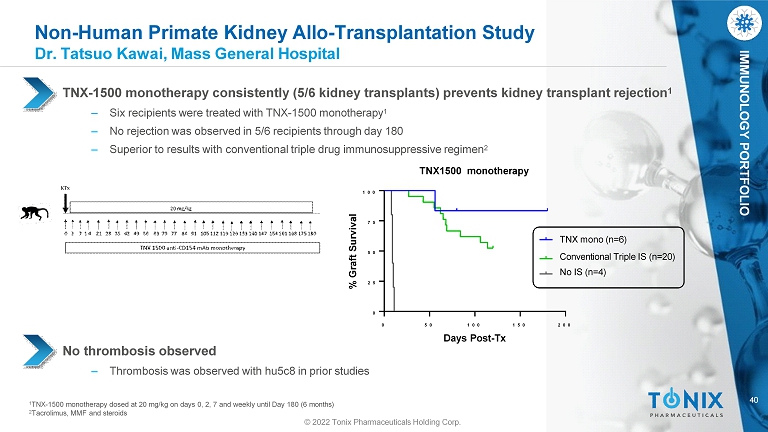
40 IMMUNOLOGY PORTFOLIO Non - Human Primate Kidney Allo - Transplantation Study Dr. Tatsuo Kawai, Mass General Hospital No thrombosis observed ‒ Thrombosis was observed with hu5c8 in prior studies 1 TNX - 1500 monotherapy dosed at 20 mg/kg on days 0, 2, 7 and weekly until Day 180 (6 months) 2 Tacrolimus, MMF and steroids 0 5 0 1 0 0 1 5 0 2 0 0 0 2 5 5 0 7 5 1 0 0 TNX - 1500 monotherapy consistently (5/6 kidney transplants) prevents kidney transplant rejection 1 ‒ Six recipients were treated with TNX - 1500 monotherapy 1 ‒ No rejection was observed in 5/6 recipients through day 180 ‒ Superior to results with conventional triple drug immunosuppressive regimen 2 TNX1500 monotherapy Days Post - Tx % Graft Survival TNX mono (n=6) Conventional Triple IS (n=20) No IS (n=4) © 2022 Tonix Pharmaceuticals Holding Corp.
41 Tolerance Induction with Donor Bone Marrow Transplantation © 2022 Tonix Pharmaceuticals Holding Corp. Induction of “mixed chimerism” induces allograft tolerance ‒ Long - lasting, durable tolerance — specifically to donor tissues ‒ Initial protocols required that the recipient’s mature T cells be severely depleted Tolerance induction via “mixed chimerism” allows long - term kidney transplant survival in humans without maintenance immunosuppression 1 - 2 ‒ Combined kidney and bone marrow transplantation (CKBMT) Non - myeloablative conditioning for induction of mixed chimerism is being developed ‒ Mixed chimerism and tolerance can be induced even without complete T cell depletion using costimulatory pathway blockade using anti - CD40L mAb and/or CTLA - 4 - Ig ‒ Prof. Tatsuo Kawai showed addition of CD40L blockade to the conditioning regimen facilitates induction of mixed chimerism and renal allograft tolerance 3 1 Kawai T, et al. N Engl J Med . 2008;358(4):353 - 361. 2 Kawai T, et al. Am J Transplant . 2014;14(7):1599 - 1611. 3 Kawai, T et al. Am J Transplant . 2004;4(9):1391 - 1398. IMMUNOLOGY PORTFOLIO
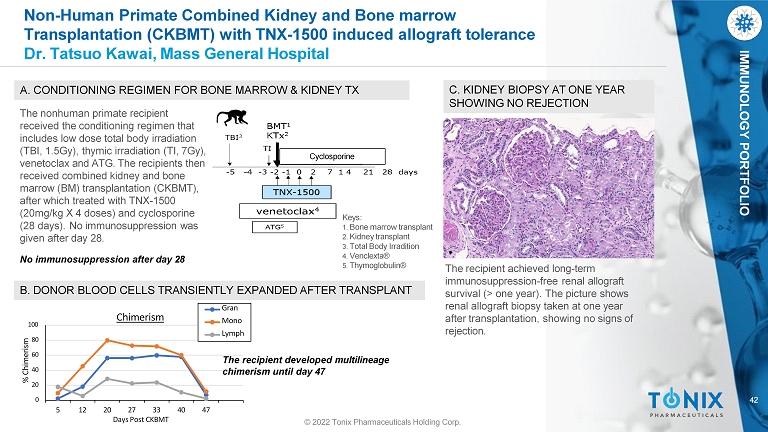
42 © 2022 Tonix Pharmaceuticals Holding Corp. IMMUNOLOGY PORTFOLIO 0 20 40 60 80 100 5 12 20 40 47 % Chimerism 27 33 Days Post CKBMT Chimerism Gran Mono Lymph Keys: 1. Bone marrow transplant 2. Kidney transplant 3. Total Body Irradition 4. Venclexta® 5. Thymoglobulin® A. CONDITIONING REGIMEN FOR BONE MARROW & KIDNEY TX B. DONOR BLOOD CELLS TRANSIENTLY EXPANDED AFTER TRANSPLANT C. KIDNEY BIOPSY AT ONE YEAR SHOWING NO REJECTION Cyclosporine No immunosuppression after day 28 The nonhuman primate recipient received the conditioning regimen that includes low dose total body irradiation (TBI, 1.5Gy), thymic irradiation (TI, 7Gy), venetoclax and ATG. The recipients then received combined kidney and bone marrow (BM) transplantation (CKBMT), after which treated with TNX - 1500 (20mg/kg X 4 doses) and cyclosporine (28 days). No immunosuppression was given after day 28. The recipient achieved long - term immunosuppression - free renal allograft survival (> one year). The picture shows renal allograft biopsy taken at one year after transplantation, showing no signs of rejection. The recipient developed multilineage chimerism until day 47 Non - Human Primate Combined Kidney and Bone marrow Transplantation (CKBMT) with TNX - 1500 induced allograft tolerance Dr. Tatsuo Kawai, Mass General Hospital
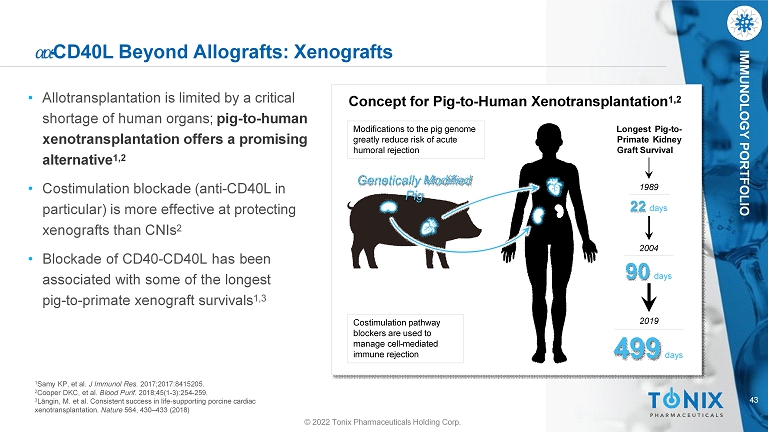
43 IMMUNOLOGY PORTFOLIO ߙ ߙ - CD40L Beyond Allografts: Xenografts • Allotransplantation is limited by a critical shortage of human organs ; pig - to - human xenotransplantation offers a promising alternative 1,2 • Costimulation blockade (anti - CD40L in particular) is more effective at protecting xenografts than CNIs 2 • Blockade of CD 40 - CD 40 L has been associated with some of the longest pig - to - primate xenograft survivals 1 , 3 1 Samy KP, et al. J Immunol Res. 2017;2017:8415205. 2 Cooper DKC, et al. Blood Purif. 2018;45(1 - 3):254 - 259. 3 Längin, M. et al. Consistent success in life - supporting porcine cardiac xenotransplantation. Nature 564, 430 – 433 (2018) Genetically Modified Pig Costimulation pathway blockers are used to manage cell - mediated immune rejection Concept for Pig - to - Human Xenotransplantation 1,2 Modifications to the pig genome greatly reduce risk of acute humoral rejection Longest Pig - to - Primate Kidney Graft Survival 1989 22 days 2004 90 days 499 days 2019 © 2022 Tonix Pharmaceuticals Holding Corp.
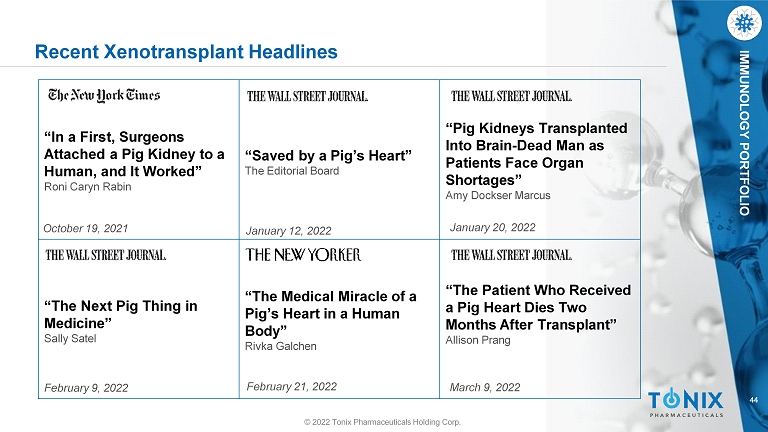
44 IMMUNOLOGY PORTFOLIO Recent Xenotransplant Headlines “In a First, Surgeons Attached a Pig Kidney to a Human, and It Worked” Roni Caryn Rabin October 19, 2021 “Saved by a Pig’s Heart” The Editorial Board January 12, 2022 “Pig Kidneys Transplanted Into Brain - Dead Man as Patients Face Organ Shortages” Amy Dockser Marcus January 20, 2022 “The Next Pig Thing in Medicine” Sally Satel February 9, 2022 “The Medical Miracle of a Pig’s Heart in a Human Body” Rivka Galchen February 21, 2022 “The Patient Who Received a Pig Heart Dies Two Months After Transplant” Allison Prang March 9, 2022 © 2022 Tonix Pharmaceuticals Holding Corp.
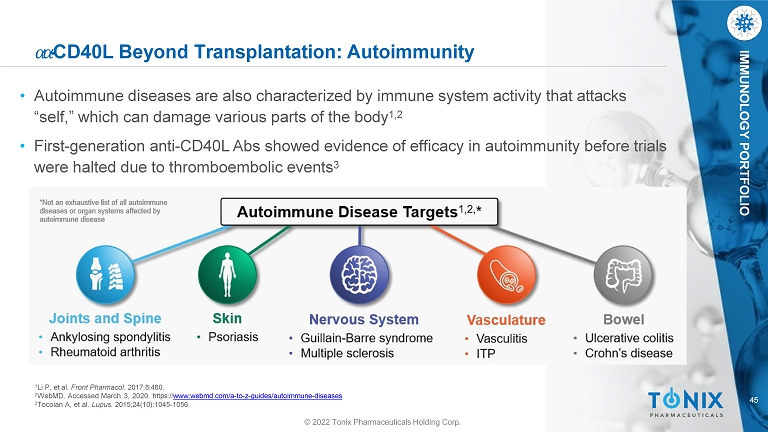
45 IMMUNOLOGY PORTFOLIO © 2022 Tonix Pharmaceuticals Holding Corp. ߙ ߙ - CD40L Beyond Transplantation: Autoimmunity • Autoimmune diseases are also characterized by immune system activity that attacks “self,” which can damage various parts of the body 1,2 • First - generation anti - CD40L Abs showed evidence of efficacy in autoimmunity before trials were halted due to thromboembolic events 3 1 Li P, et al. Front Pharmacol. 2017;8:460. 2 WebMD. Accessed March 3, 2020. https:// www.webmd.com/a - to - z - guides/autoimmune - diseases 3 Tocoian A, et al. Lupus. 2015;24(10):1045 - 1056.

46 IMMUNOLOGY PORTFOLIO TNX - 1500: Key Considerations • TNX - 1500 may be used in large markets that are not currently well served • There is a long history of use of monoclonal antibodies • Tonix has engineered a safer, potentially more efficacious molecule than previous anti - CD40L mAbs • Intellectual property is in place (composition of matter) • Manufacturing (CMC) is in progress Key milestones: Pre - IND meeting (FDA) 3Q 2022; Phase 1 2H 2022 Autoimmune disorders – Planning INDs © 2022 Tonix Pharmaceuticals Holding Corp.
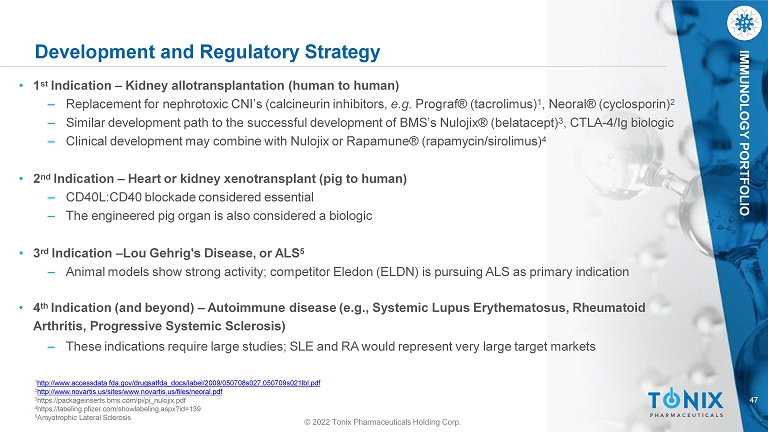
47 © 2022 Tonix Pharmaceuticals Holding Corp. IMMUNOLOGY PORTFOLIO Development and Regulatory Strategy • 1 st Indication – Kidney allotransplantation (human to human) ‒ Replacement for nephrotoxic CNI’s (calcineurin inhibitors, e.g. Prograf® (tacrolimus) 1 , Neoral® (cyclosporin) 2 ‒ Similar development path to the successful development of BMS’s Nulojix® (belatacept) 3 , CTLA - 4/Ig biologic ‒ Clinical development may combine with Nulojix or Rapamune® (rapamycin/sirolimus) 4 • 2 nd Indication – Heart or kidney xenotransplant (pig to human) ‒ CD40L:CD40 blockade considered essential ‒ The engineered pig organ is also considered a biologic • 3 rd Indication – Lou Gehrig's Disease, or ALS 5 ‒ Animal models show strong activity; competitor Eledon (ELDN) is pursuing ALS as primary indication • 4 th Indication (and beyond) – Autoimmune disease (e.g., Systemic Lupus Erythematosus, Rheumatoid Arthritis, Progressive Systemic Sclerosis) ‒ These indications require large studies; SLE and RA would represent very large target markets 1 http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/050708s027,050709s021lbl.pdf 2 http://www.novartis.us/sites/www.novartis.us/files/neoral.pdf 3 https://packageinserts.bms.com/pi/pi_nulojix.pdf 4 https://labeling.pfizer.com/showlabeling.aspx?id=139 5 Amyotrophic Lateral Sclerosis
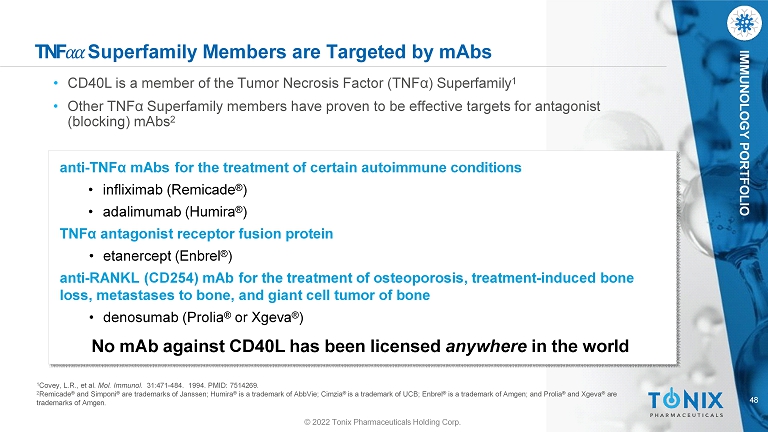
48 IMMUNOLOGY PORTFOLIO TNF ߙ ߙ Superfamily Members are Targeted by mAbs • CD40L is a member of the Tumor Necrosis Factor (TNFα) Superfamily 1 • Other TNFα Superfamily members have proven to be effective targets for antagonist (blocking) mAbs 2 anti - TNFα mAbs for the treatment of certain autoimmune conditions • infliximab (Remicade ® ) • adalimumab (Humira ® ) TNFα antagonist receptor fusion protein • etanercept (Enbrel ® ) anti - RANKL (CD254) mAb for the treatment of osteoporosis, treatment - induced bone loss, metastases to bone, and giant cell tumor of bone • denosumab (Prolia ® or Xgeva ® ) No mAb against CD40L has been licensed anywhere in the world 1 Covey, L.R., et al. Mol. Immunol. 31:471 - 484. 1994. PMID: 7514269. 2 Remicade ® and Simponi ® are trademarks of Janssen; Humira ® is a trademark of AbbVie; Cimzia ® is a trademark of UCB; Enbrel ® is a trademark of Amgen; and Prolia ® and Xgeva ® are trademarks of Amgen. © 2022 Tonix Pharmaceuticals Holding Corp.
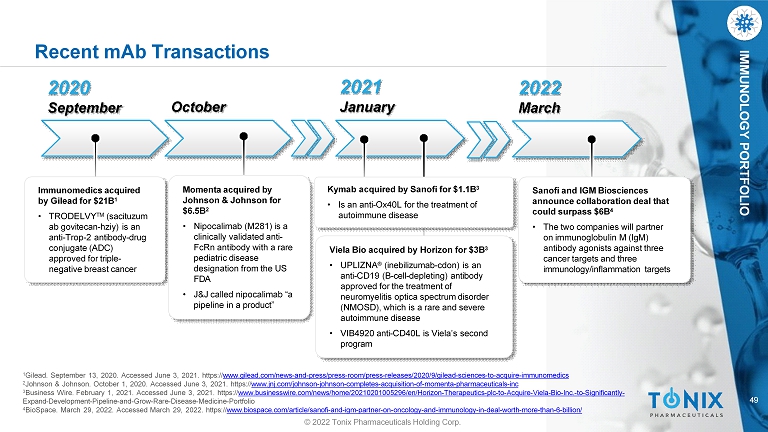
49 IMMUNOLOGY PORTFOLIO 2020 September October 2021 January Immunomedics acquired by Gilead for $21B 1 • TRODELVY TM (sacituzum ab govitecan - hziy) is an anti - Trop - 2 antibody - drug conjugate (ADC) approved for triple - negative breast cancer Momenta acquired by Johnson & Johnson for $6.5B 2 • Nipocalimab (M281) is a clinically validated anti - FcRn antibody with a rare pediatric disease designation from the US FDA • J&J called nipocalimab “a pipeline in a product” Viela Bio acquired by Horizon for $3B 3 • UPLIZNA ® (inebilizumab - cdon) is an anti - CD19 (B - cell - depleting) antibody approved for the treatment of neuromyelitis optica spectrum disorder (NMOSD), which is a rare and severe autoimmune disease • VIB4920 anti - CD40L is Viela’s second program Kymab acquired by Sanofi for $1.1B 3 • Is an anti - Ox40L for the treatment of autoimmune disease 1 Gilead. September 13, 2020. Accessed June 3, 2021. https:// www.gilead.com/news - and - press/press - room/press - releases/2020/9/gilead - sciences - to - acquire - immunomedics 2 Johnson & Johnson. October 1, 2020. Accessed June 3, 2021. https:// www.jnj.com/johnson - johnson - completes - acquisition - of - momenta - pharmaceuticals - inc 3 Business Wire. February 1, 2021. Accessed June 3, 2021. https:// www.businesswire.com/news/home/20210201005296/en/Horizon - Therapeutics - plc - to - Acquire - Viela - Bio - Inc. - to - Significantly - Expand - Development - Pipeline - and - Grow - Rare - Disease - Medicine - Portfolio 4 BioSpace. March 29, 2022. Accessed March 29, 2022. https:// www.biospace.com/article/sanofi - and - igm - partner - on - oncology - and - immunology - in - deal - worth - more - than - 6 - billion/ Recent mAb Transactions 2022 March Sanofi and IGM Biosciences announce collaboration deal that could surpass $6B 4 • The two companies will partner on immunoglobulin M (IgM) antibody agonists against three cancer targets and three immunology/inflammation targets © 2022 Tonix Pharmaceuticals Holding Corp.
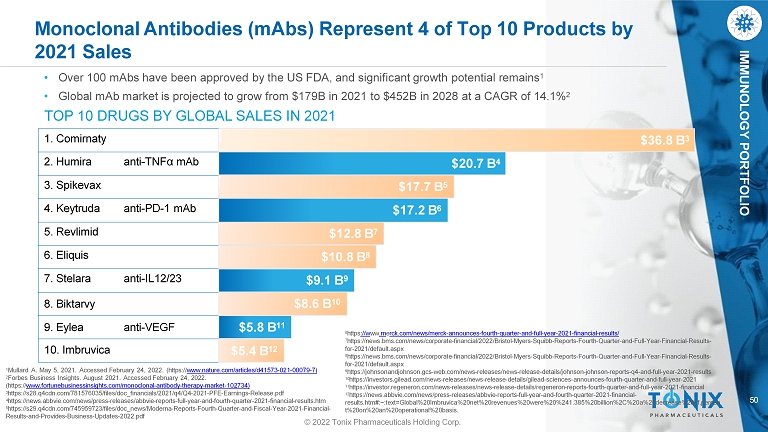
50 IMMUNOLOGY PORTFOLIO Monoclonal Antibodies (mAbs) Represent 4 of Top 10 Products by 2021 Sales 1 Mullard A. May 5, 2021. Accessed February 24, 2022. (https:// www.nature.com/articles/d41573 - 021 - 00079 - 7) 2 Forbes Business Insights. August 2021. Accessed February 24, 2022. (https:// www.fortunebusinessinsights.com/monoclonal - antibody - therapy - market - 102734) 3 https://s28.q4cdn.com/781576035/files/doc_financials/2021/q4/Q4 - 2021 - PFE - Earnings - Release.pdf 4 https://news.abbvie.com/news/press - releases/abbvie - reports - full - year - and - fourth - quarter - 2021 - financial - results.htm 5 https://s29.q4cdn.com/745959723/files/doc_news/Moderna - Reports - Fourth - Quarter - and - Fiscal - Year - 2021 - Financial - Results - and - Provides - Business - Updates - 2022.pdf 1. Comirnaty 2. Humira anti - TNFα mAb 3. Spikevax 4. Keytruda anti - PD - 1 mAb 5. Revlimid 6. Eliquis 7. Stelara anti - IL12/23 8. Biktarvy 9. Eylea anti - VEGF 10. Imbruvica • Over 100 mAbs have been approved by the US FDA, and significant growth potential remains 1 • Global mAb market is projected to grow from $179B in 2021 to $452B in 2028 at a CAGR of 14.1% 2 TOP 10 DRUGS BY GLOBAL SALES IN 2021 $5.8 B 11 $36.8 B 3 $5.4 B 12 $20.7 B 4 $17.7 B 5 $17.2 B 6 $12.8 B 7 $10.8 B 8 $9.1 B 9 $8.6 B 10 © 2022 Tonix Pharmaceuticals Holding Corp. 6 https ://w ww .m e rck.com/news/merck - announces - fourth - quarter - and - full - year - 2021 - financial - results/ 7 https://news.bms.com/news/corporate - financial/2022/Bristol - Myers - Squibb - Reports - Fourth - Quarter - and - Full - Year - Financial - Results - for - 2021/default.aspx 8 https://news.bms.com/news/corporate - financial/2022/Bristol - Myers - Squibb - Reports - Fourth - Quarter - and - Full - Year - Financial - Results - for - 2021/default.aspx 9 https://johnsonandjohnson.gcs - web.com/news - releases/news - release - details/johnson - johnson - reports - q4 - and - full - year - 2021 - results 10 https://investors.gilead.com/news - releases/news - release - details/gilead - sciences - announces - fourth - quarter - and - full - year - 2021 11 https://investor.regeneron.com/news - releases/news - release - details/regeneron - reports - fourth - quarter - and - full - year - 2021 - financial 12 https://news.abbvie.com/news/press - releases/abbvie - reports - full - year - and - fourth - quarter - 2021 - financial - results.htm#:~:text=Global%20Imbruvica%20net%20revenues%20were%20%241.385%20billion%2C%20a%20decrease%20of,percen t%20on%20an%20operational%20basis.
51 IMMUNOLOGY PORTFOLIO © 2022 Tonix Pharmaceuticals Holding Corp. TNX - 1700*: Gastric and Colorectal cancers Stabilized Recombinant Trefoil Factor 2 (rTFF2) POTENTIAL NEW CANCER TREATMENT • TNX - 1700 (rTFF2) has effects on cancer by altering the tumor micro - environment • Mechanism of action: suppresses myeloid - derived suppressor cells and activates anti - cancer CD8+ T cells • Potential synergy with anti - PD - 1 or anti - PD - L1 monoclonal antibodies (mAbs) PRECLINICAL EVIDENCE FOR INHIBITING GROWHT OF CANCER CELLS • Data showed that TFF2 - CTP augmented the efficacy of mAb anti - PD - 1 therapy. Anti - PD - 1 in combination with TFF2 - CTP showed greater anti - tumor activity in PD - L1 - overexpressing mice. LICENSED FROM COLUMBIA UNIVERSITY • Developing in partnership under sponsored research agreement Patents Filed DEVELOPMENT PROGRAM Market Entry: Gastric and colorectal cancers Status: Preclinical Next Steps: Animal studies ongoing *TNX - 1700 is in the pre - IND stage of development and has not been approved for any indication.

INFECTIOUS DISEASE: KEY CANDIDATES © 2022 Tonix Pharmaceuticals Holding Corp.

53 INFECTIOUS DISEASE PORTFOLIO © 2022 Tonix Pharmaceuticals Holding Corp. Live Virus Vaccines: Development Rationale • Control of smallpox, measles, mumps, rub ella, chickenpox and other viral conditions ‒ Prevent forward transmission • Effective in eliciting durable or long - term immunity • Economical to manufacture at scale ‒ Low dose because replication amplifies dose in vivo ‒ Single shot administration • Standard refrigeration required for shipping and storage • Live virus vaccines are the oldest vaccine technology ‒ Starting with Edward Jenner’s smallpox vaccine, the first vaccine, eradicated smallpox
54 INFECTIOUS DISEASE PORTFOLIO Patents Filed TNX - 801: Smallpox and Monkeypox Vaccine Live Virus Platform Development Program © 2022 Tonix Pharmaceuticals Holding Corp. APPLICATION OF LIVE VIRUS PLATFORM • TNX - 801 is a cloned version of horsepox 1 (without any insert) purified from cell culture • In addition to being a potential addition to the U . S . Strategic National Stockpile, TNX - 801 will support recognition of the RPV/horsepox platform ANIMAL TESTING OF TNX - 801 WITH SOUTHERN RESEARCH INSTITUTE • Non - human primate monkeypox challenge testing: positive data reported in 1Q 2020 2 DEVELOPED IN COLLABORATION WITH UNIVERSITY OF ALBERTA • Proprietary synthetic biology approach and vector system DEVELOPMENT PROGRAM Market Entry: Smallpox and Monkeypox Vaccine Status: Preclinical, Pre - IND Next Steps: Developing GMP manufacturing for TNX - 801 (horsepox); initiate Phase 1 Trial, 2H 2023 *TNX - 801 is in the pre - IND stage of development and has not been approved for any indication. 1 Noyce RS, et al. Construction of an infectious horsepox virus vaccine from chemically synthesized DNA fragments. PLoS One. 2018 Jan 19;13(1):e0188453. 2 Noyce, RS, et al. Synthetic Chimeric Horsepox Virus (scHPXV) Vaccination Protects Macaques from Monkeypox* Presented as a poster at the American Society of Microbiology BioThreats Conference - January 29, 2020, Arlington, VA. (https://content.equisolve.net/tonixpharma/media/10929ac27f4fb5f5204f5cf41d59a121.pdf)
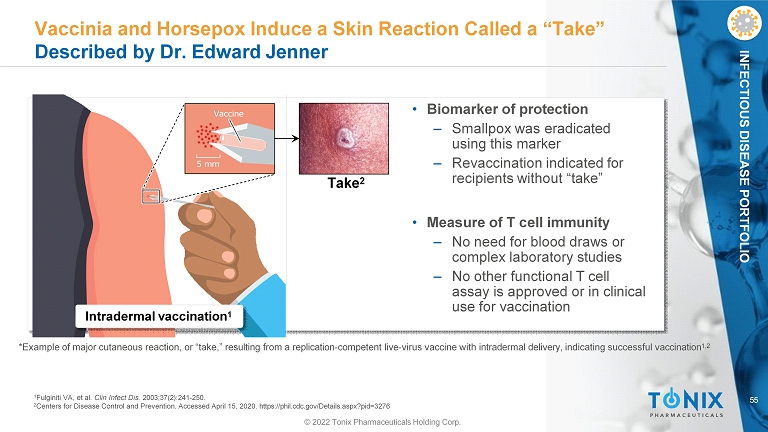
55 INFECTIOUS DISEASE PORTFOLIO Vaccinia and Horsepox Induce a Skin Reaction Called a “Take” Described by Dr. Edward Jenner *Example of major cutaneous reaction, or “take,” resulting from a replication - competent live - virus vaccine with intradermal delivery, indicating successful vaccination 1,2 5 mm Vaccine Intradermal vaccination 1 Take 2 • Biomarker of protection ‒ Smallpox was eradicated using this marker ‒ Revaccination indicated for recipients without “take” • Measure of T cell immunity ‒ No need for blood draws or complex laboratory studies ‒ No other functional T cell assay is approved or in clinical use for vaccination 1 Fulginiti VA, et al. Clin Infect Dis. 2003;37(2):241 - 250. 2 Centers for Disease Control and Prevention. Accessed April 15, 2020. https://phil.cdc.gov/Details.aspx?pid=3276 © 2022 Tonix Pharmaceuticals Holding Corp.
56 INFECTIOUS DISEASE PORTFOLIO VIRUS - BASED SCIENCE IS WELL ESTABLISHED • Streamlined development • Ability to vertically integrate development and manufacturing • Multi - dose packaging, standard cold - chain products Live Virus Recombinant Pox Vaccine (RPV) Platform Profile POTENTIALLY LONGER DURABILITY DUE TO POX - ENGINEERED ARCHITECTURE • Live virus vaccines present unique “danger signals” resulting in strong immune response PROGRAMMABLE VECTOR DESIGN FOR USE IN DIFFERENT DISEASE MODELS • Large capacity for expressing inserted genes • Wide range of clinical applications: pandemic, biodefense, infectious disease, smallpox, oncology © 2022 Tonix Pharmaceuticals Holding Corp.
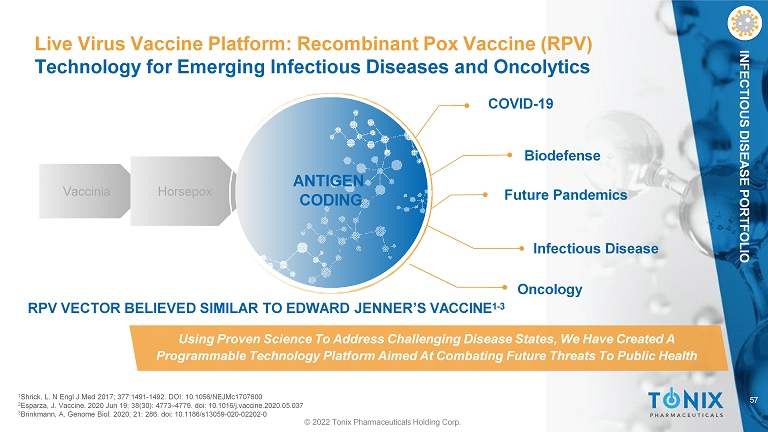
57 INFECTIOUS DISEASE PORTFOLIO Live Virus Vaccine Platform: Recombinant Pox Vaccine (RPV) Technology for Emerging Infectious Diseases and Oncolytics Future Pandemics COVID - 19 Biodefense Vaccinia Horsepox ANTIGEN CODING Infectious Disease Oncology RPV VECTOR BELIEVED SIMILAR TO EDWARD JENNER’S VACCINE 1 - 3 Using Proven Science To Address Challenging Disease States, We Have Created A Programmable Technology Platform Aimed At Combating Future Threats To Public Health © 2022 Tonix Pharmaceuticals Holding Corp. 1 Shrick, L. N Engl J Med 2017; 377:1491 - 1492. DOI: 10.1056/NEJMc1707600 2 Esparza, J. Vaccine. 2020 Jun 19; 38(30): 4773 – 4779. doi: 10.1016/j.vaccine.2020.05.037 3 Brinkmann, A. Genome Biol. 2020; 21: 286. doi: 10.1186/s13059 - 020 - 02202 - 0
58 INFECTIOUS DISEASE PORTFOLIO 4 Keeton R et al., “T cell responses to SARS - CoV02 spike cross - recognize omicron.” Nature Jan 31, 2022 . ( www.nature.com/articles/s41586 - 022 - 04460 - 3) © 2022 Tonix Pharmaceuticals Holding Corp. COVID - 19: Entering Endemic Phase in the US • Delta and Omicron variant waves are waning in most parts of the US ‒ Leaving a path of morbidity and mortality, including “breakthrough” infection and disease among vaccinated and convalescent • U.S. states are rolling back state pandemic restrictions ‒ CDC continues mask recommendation and recently increased the frequency of booster recommendations to every 3 months for individuals with weak immunity 1 ‒ California plans to treat COVID as endemic by June, 2022 2 • Vaccines : new focus on SARS - CoV - 2 variants Omicron and BA.2 3 ‒ Omicron has out - competed the original Wuhan strain, which has become rare ‒ Omicron substantially evades antibody immunity to earlier variants, but is recognized by T cell immunity to earlier variants from vaccination or prior COVID 4 ‒ Next generation vaccines are focusing on Omicron and its potential successor, BA.2 1 Achenbach, J. “Americans are tired of the pandemic. But disease experts preach caution - and endure a ‘kill the messenger moment’. Washington Post Feb 17, 2022. ( www.washingtonpost.com/health/2022/02/17/mask - mandates - opposition/) 2 Beachum L and Suliman A, “California unveils plan to become first state to treat coronavirus as ‘endemic’ risk.” Washington Post Feb 18, 2022. ( www.washingtonpost.com/nation/2022/02/18/california - covid - newsom - endemic - smarter - plan/) 3 Bernstein L. “There’s a new version of omicron but so far it doesn’t appear to be more dangerous.” Washington Post Jan 24, 2022 ( www.washingtonpost.com/health/2022/01/24/covid - omicron - ba2/)

59 INFECTIOUS DISEASE PORTFOLIO could - reshape - us - policy?) © 2022 Tonix Pharmaceuticals Holding Corp. COVID - 19: The Missing Pieces • Vaccines : early vaccines slowed pandemic, but are showing limitations ‒ Short term protection – requirement for boosters with mRNA vaccines; ‒ Increasing focus on preventing hospitalization and death • Anti - viral drugs : Veklury® (remdesivir), Paxlovid Œ (nirmatrelvir 1 ), and Lagevrio® (molnupiravir) are available ‒ Pfizer’s Paxlovid looks promising; Merck’s Lagevrio did not show benefit in 2 nd cohort 2 • Anti - SARS - CoV - 2 monoclonal antibodies : increasing adoption; concern about variants ‒ Of the original EUA mAbs, only Vir/GSK’s XEVURDY® (sotrovimab) was considered active against the omicron variant of SARS - CoV - 2 but is not considered active against BA.2 and is not longer distributed in 8 US states 3 ‒ Lilly’s bebtelovimab, active against omicron, recently received EUA for treatment of mild or moderate COVID 4 • Tests : unmet need to determine COVID immunity 3 • Long COVID : no approved treatment for ‘Long Covid’ 1 PAXLOVID Œ (nirmatrelvir plus ritonavir) 2 Merck Says Its Covid Pill Is Less Effective in a Final Analysis - The New York Times (nytimes.com) 3 Brennan, Z. Endpoints , March 28, 2022 US halts use of GSK/Vir monoclonal in 8 states as FDA says it can't defeat new Omicron subvariant. endpts.com/us - halts - use - of - gsk - vir - monoclonal - in - 8 - states - as - fda - says - it - cant - defeat - new - omicron - subvariant/ 4 Redfield R and Siegel S. “A test to determine COVID immunity could reshape US policy.” The Hill. Feb 17, 2022: (https://thehill.com/opinion/healthcare/594522 - a - test - to - determine - covid - immunity -

60 © 2022 Tonix Pharmaceuticals Holding Corp. INFECTIOUS DISEASE PORTFOLIO COVID - 19 Vaccines: Where We Are Today Durability of protection ‒ mRNA vaccinated people lose protection, starting at 4 - 6 months 1 ‒ High rates of “breakthrough” COVID during Delta and Omicron waves ‒ Booster vaccinations with mRNA vaccines recommended at 4 - 6 months Effect on forward transmission (spread of infection to others) ‒ Concerns about whether vaccinated people can be infectious to others Detecting vaccine failure ‒ Need a strategy for identifying individuals at risk after vaccination No recognized, clinical applicable biomarker of vaccine protection ‒ Best proxy is neutralizing antibodies, which are hard to measure Current and future variants (e.g., Delta, Omicron variants) ‒ Less protection from existing vaccines ‒ Unknown effectiveness for future variants 1 www.cdc.gov/media/releases/2021/s0818 - covid - 19 - booster - shots.html

61 INFECTIOUS DISEASE PORTFOLIO © 2022 Tonix Pharmaceuticals Holding Corp. COVID - 19 Vaccines: Where Do We Go From Here? mRNA vaccines have slowed pandemic, but may not be a long - term solution ‒ Vaccinated people lost protection and showed high rates of “breakthrough” COVID during Delta and Omicron waves ‒ COVID is becoming endemic in the US; vaccination of entire world every 6 months not practical Operation Warp Speed (OWS) identified 4 types of vaccines: 1. RNA/DNA – Pfizer 1 and Moderna 2 are fully approved by the FDA 2. Subunit – NovaVax submitted EUA; Sanofi/GSK have announced data showing protection from hospitalization and death 3. Non - replicating – J&J has EUA; AstraZeneca widely used ex - US 4. Live Virus Vaccines – none were ultimately adopted by OWS Live Virus Vaccines ‒ Merck was developing two programs: VSV and Measles, but they were not included in OWS and were abandoned in January 2021 3 1 COMIRNATY is the brand name for the Pfizer - BioNTech COVID - 19 vaccine 2 https ://w ww .fd a .gov/news - events/press - announcements/coronavirus - covid - 19 - update - fda - takes - key - action - approving - second - covid - 19 - vaccine 3 https ://w ww .m e rck.com/news/merck - discontinues - development - of - sars - cov - 2 - covid - 19 - vaccine - candidates - continues - development - of - two - investigational - therapeutic - candidates/
62 INFECTIOUS DISEASE PORTFOLIO Patents Filed TNX - 1840 and TNX - 1850*: COVID - 19 Vaccine Live Virus Platform Development Program © 2022 Tonix Pharmaceuticals Holding Corp. • First version TNX - 1800 encodes spike protein from SARS - CoV - 2, Wuhan strain • Planned new versions TNX - 1840 and TNX - 1850 encode spike protein from SARS - CoV - 2, omicron and BA.2 strains, respectively 1 ANIMAL TESTING OF TNX - 1800 WITH SOUTHERN RESEARCH INSTITUTE • Non - human primate immune response: positive results reported in 4Q 2020 • Non - human primate CoV - 2 challenge testing: positive data reported in 1Q 2021 DEVELOPED IN COLLABORATION WITH UNIVERSITY OF ALBERTA • Proprietary synthetic biology approach and vector system APPLICATION OF LIVE VIRUS PLATFORM DEVELOPMENT PROGRAM Market Entry: COVID - 19 Vaccine Additional Indications: Future Pandemic, Infectious Disease, Smallpox, Cancer Status: Preclinical Next Steps : Developing TNX - 1840 (omicron) and TNX - 1850 (BA . 2 ) versions ; initiate Phase 1 Trial, 2 H 2023 *TNX - 1840 and TNX - 1850 are in the pre - IND stage of development and has not been approved for any indication. 1 Brennan, Z. Endpoints March 2, 2022 (https://endpts.com/weaker - omicron - variant - is - great - news - for - the - world - but - bad - news - for - covid - related - clinical - trials/)
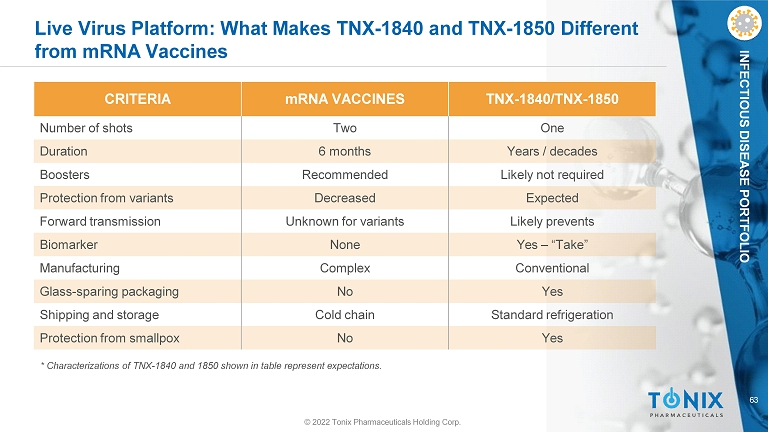
63 INFECTIOUS DISEASE PORTFOLIO © 2022 Tonix Pharmaceuticals Holding Corp. Live Virus Platform: What Makes TNX - 1840 and TNX - 1850 Different from mRNA Vaccines CRITERIA mRNA VACCINES TNX - 1840/TNX - 1850 Number of shots Two One Duration 6 months Years / decades Boosters Recommended Likely not required Protection from variants Decreased Expected Forward transmission Unknown for variants Likely prevents Biomarker None Yes – “Take” Manufacturing Complex Conventional Glass - sparing packaging No Yes Shipping and storage Cold chain Standard refrigeration Protection from smallpox No Yes * Characterizations of TNX - 1840 and 1850 shown in table represent expectations.
64 INFECTIOUS DISEASE PORTFOLIO Patents Filed TNX - 2300*: COVID - 19 Vaccine Live Virus Vaccine Based on Bovine Parainfluenza (BPI) Virus © 2022 Tonix Pharmaceuticals Holding Corp. • Previously has been shown to be an effective antigen delivery vector in humans, notably well tolerated in infants and children • Vector is well suited for mucosal immunization using a nasal atomizer, but it can also be delivered parenterally ANIMAL TESTING OF TNX - 2300 ONGOING • Non - human primate immune response: positive results reported in 4Q 2020 • Non - human primate CoV - 2 challenge testing: positive data reported in 1Q 2021 DEVELOPED IN COLLABORATION WITH KANSAS STATE UNIVERSITY (KSU) • Uses a novel live attenuated vaccine vector platform, BPI, and the CD40 - ligand to stimulate T cell immunity LIVE VIRUS VACCINE 1 - 5 DEVELOPMENT PROGRAM Market Entry: COVID - 19 Vaccine Additional Indications: Future Pandemic, Infectious Diseases Status: Preclinical Next Steps: Animal studies with KSU to test the effect of co - expression of the CD40 - ligand, also known as CD154 or 5c8 antigen, to stimulate T cell immunity. *TNX - 2300 is in the pre - IND stage of development and has not been approved for any indication. 1 Halle, AA et al. J Gen. Virology (2003) 84:2153 – 2162; 2 Halle, AA et al. J Virology (2000) 74 (24): 11626 – 11635; 3 Karron RA et al. J Inf Dis (1995) 171: 1107 - 14; 4 Karron RA et al. Vaccine (2012) 30: 3975 – 3981; 5 Schmidt AC et al. J Virology (2001) 75(10): 4594 – 4603

65 INFECTIOUS DISEASE PORTFOLIO © 2022 Tonix Pharmaceuticals Holding Corp. Live Virus RPV P latform & COVID - 19 Vaccine Internal Development & Manufacturing Capabilities Infectious Disease R&D Center (RDC) – Frederick, MD • Function : Accelerated development of vaccines and antiviral drugs against COVID - 19, its variants and other infectious diseases • Description : ~48,000 square feet; currently BSL - 2 but being converted to BSL - 3 • Status : Operational; acquisition completed on October 1 st , 2021 Advanced Development Center (ADC) – North Dartmouth, MA • Function : Development and clinical scale manufacturing of live - virus vaccines to support Phase 1 and Phase 2 trials • Description : ~45,000 square feet, under construction, planned BSL - 2 • Status : Expected to be partially operational in first half 2022 Commercial Manufacturing Center (CMC) – Hamilton, MT • Function : Phase 3 and Commercial scale manufacturing of live - virus vaccines • Description : ~44 acre green field site, planned BSL - 2 • Status : Planning for site enabling work in 2022 Architectural Rendering

66 INFECTIOUS DISEASE PORTFOLIO © 2022 Tonix Pharmaceuticals Holding Corp. American Pandemic Preparedness Plan (AP3) • “Platforms” – Foundation of Pandemic Response ‒ Key element of AP3 from White House Office of Science and Technology Policy or OSTP 1,2 ▪ 100 days to human trials ▪ Technologies that do not require sterile injection • TNX - 801/ - 1840/ - 1850 (live virus RPV) platform addresses OSTP requirements 1,2 ‒ Our goal is to be able to test new live virus vaccines against novel pathogens within the 100 days of obtaining sequence ▪ RDC is equipped to make new vaccines ▪ ADC will be equipped to make clinical trial material ▪ CMC is planned to make commercial scale material 1 Sept 3, 2021 (https:// www.whitehouse.gov/wp - content/uploads/2021/09/American - Pandemic - Preparedness - Transforming - Our - Capabilities - Final - For - Web.pdf) 2 Sept 3, 2021 (https:// www.whitehouse.gov/briefing - room/statements - releases/2021/09/03/fact - sheet - biden - administration - to - transform - capabilities - for - pandemic - preparedness/)

67 INFECTIOUS DISEASE PORTFOLIO © 2022 Tonix Pharmaceuticals Holding Corp. Small Molecule COVID - 19 Therapeutics The only COVID - 19 antiviral that is FDA approved is Remdesivir/Veklury® ‒ Gilead – Intravenous ( i.v. ) medicine ‒ FDA approved for patients who are at least 12 years old and require hospitalization ‒ May shorten the time to recover from acute COVID - 19 ‒ World Health Organization has recommended against its use 1 ‒ Resistance reported 2 Antivirals available under Emergency Use Authorization (EUA) ‒ Pfizer – PAXLOVID Œ (PF - 07321332; ritonavir) - oral protease C3L inhibitor - Emergency Use Authorization (EUA) ‒ Merck/Ridgeback – Lagevrio® (molnupiravir,) – oral polymerase inhibitor - EUA 3 Concerns about antiviral efficacy ‒ Veklury resistance reported 2 ‒ Lagevrio efficacy was not repeated in second cohort of Phase 3 trial 4 1 World Health Organization (2021). Therapeutics and COVID - 19: living guideline, 6 July 2021 (Report). ( http://apps.who.int/iris/handle/10665/342368) 2 https://yaledailynews.com/blog/2021/12/02/yale - scientists - identify - remdesivir - resistance - in - immunocompromised - covid - 19 - patient/ 3 www.merck.com/news/merck - announces - supply - agreement - with - u - s - government - for - molnupiravir - an - investigational - oral - antiviral - candidate - for - treatment - of - mild - to - moderate - covid - 19 4 www.merck.com/news/merck - announces - supply - agreement - with - u - s - government - for - molnupiravir - an - investigational - oral - antiviral - candidate - for - treatment - of - mild - to - moderate - covid - 19
INFECTIOUS DISEASE PORTFOLIO Patents Filed TNX - 3500*: COVID - 19 Antiviral Treatment Sangivamycin © 2022 Tonix Pharmaceuticals Holding Corp. PROFILE New variants heighten need for therapeutics NIH Treatment Guidelines for COVID - 19 are mixed on use of remdesivir Potential monotherapy antiviral 1,2 • 65 times more potent than remdesivir in inhibiting SARS - CoV - 2 as demonstrated in cell culture infectivity studies (dose to achieve IC 90 ) Potential combination therapy with remdesivir 1,2 • TNX - 3500 antiviral effect is additive when combined with remdesivir and reduces the amount of each drug necessary for an IC 90 • Combination therapies for other viruses have reduced the emergence of drug resistant viral strains DEVELOPMENT PROGRAM Market Entry: COVID - 19 Antiviral Additional Indications: MERS, Ebola, Lassa, Oncology Status: Preclinical Next Steps: 2Q 2022 Initiate Animal Studies MERS = Middle East Respiratory Syndrome; NIH = National Institutes of Health; PK = pharmacokinetics. *TNX - 3500 is in the pre - IND stage of development and has not been approved for any indication. 68 1 Bennett RP et al. Viruses . 2020;13(1):52. doi: 10.3390/v13010052 2 Bennett, RP et al. JCI Insight . 2021 in press preview (10.1172/jci.insight.153165)

69 INFECTIOUS DISEASE PORTFOLIO © 2022 Tonix Pharmaceuticals Holding Corp. Monoclonal Antibody COVID - 19 Therapeutics Monoclonal antibodies (mAbs) (EUA) – 3 with US Emergency Use Authorization 1 ‒ Vir/GSK – XEVURDY® (sotrovimab) 1 – ONLY mAb that was active against omicron, but now withdrawn from distribution in 8 states because of insufficient activity against BA.2 2 ‒ Lilly - bebtelovimab – EUA for treatment of mild or moderate COVID 3 ‒ AstraZeneca – Evusheld (Tixagevimab/cilgavimab) – EUA for long term prophylaxis New mAbs under development 4 ‒ AstraZeneca – AZD7442 – EUA request submitted 5 ‒ Brii Biosciences – BRII - 196 and BRII - 198 6 ‒ Adagio Therapeutics – ADG20 7 ‒ Many other companies 8 Concerns about efficacy of mAbs against new variants ‒ Regeneron/Genentech - REGEN - COV® Casirivimab/imdevimab ▪ EUA revised Jan ‘22 to susceptible variants – unlikely to be effective against omicron ‒ Eli Lilly/AbCellera – Bamlanivimab/etesevimab ▪ EUA revised Jan ‘22 to susceptible variants – unlikely to be effective against omicron ‒ Vir/GSK – XEVURDY® (sotrovimab) 1 – – unlikely to be effective against BA.2 2 ‒ Delta and Omicron variants have many changes in the spike protein, which is the target of current mAbs 1 Indicated for individuals with mild - to - moderate COVID - 19 who are at high risk for progression to severe disease; 11 Dec 7, 2021 Glaxo Says Its Covid - 19 Antibody Drug Works Against Omicron – WSJ 2 Brennan, Z. Endpoints , March 28, 2022 0US halts use of GSK/Vir monoclonal in 8 states as FDA says it can't defeat new Omicron subvariant. endpts.com/us - halts - use - of - gsk - vir - monoclonal - in - 8 - states - as - fda - says - it - cant - defeat - new - omicron - subvariant/ 3 https://investor.lilly.com/news - releases/news - release - details/lillys - bebtelovimab - receives - emergency - use - authorization 4 Dolgin, E. Nature Biotechnology volume 39, pages783 – 785 (2021) https://doi.org/10.1038/s41587 - 021 - 00980 - x 5 https:// www.cnbc.com/2021/11/18/astrazeneca - antibody - drug - 83percent - effective - at - preventing - covid - trial.html 6 https:://endpts.com/brii - bio - gets - all - hands - on - deck - for - covid - 19 - antibody - hunt - leveraging - chinese - partners - work - with - recovered - p atients/ 7 https://endpts.com/qa - tillman - gerngross - explains - why - his - covid - mab - will - have - an - edge - over - an - already - crowded - field/ 8 e.g., Centivax, Corat Therapeutics, IDBiologics, Leyden Labs, Memo Therapeutics and SpikImm

70 INFECTIOUS DISEASE PORTFOLIO TNX - 3600*: COVID - 1 9 Therapeutics Fully Human Monoclonal Antibody Platform PROFILE Collaboration with Columbia University Human monoclonal antibodies (mAbs) generated from COVID - 19 convalescent patients Potential monotherapies • Plan to seek indication similar to current EUA therapeutic mAbs for treating individuals with mild - to - moderate COVID - 19 who are at high risk for progression to severe disease Potential combination therapy with other antibodies • Combination therapies for other anti - CoV - 2 monoclonal antibodies are believed to have reduced the emergence of drug resistant viral strains DEVELOPMENT PROGRAM Market Entry: COVID - 19 Therapeutic Additional Indications: Symptomatic COVID in patients with risk factors for poor outcome Status: Preclinical Next Steps: Study inhibition of SARS CoV - 2 variants in tissue culture; 2Q 2022 Initiate Animal Studies *TNX - 3600 is in the pre - IND stage of development and has not been approved for any indication. © 2022 Tonix Pharmaceuticals Holding Corp. 1 Waltz, E. Nature. “Does the World Need an Omicron Vaccine?” 28 Jan 2022 https:// www.nature.com/articles/d41586 - 022 - 00199 - z Given the unpredictable trajectory of the SARS - CoV - 2 virus and new variants 1 , we seek to contribute to a broad set of monoclonal antibodies from a variety of patients, that can be scaled up quickly and potentially combined with other monoclonal antibodies.
71 INFECTIOUS DISEASE PORTFOLIO Patents Filed TNX - 3700*: COVID - 19 Vaccine Zinc Nanoparticle (ZNP) Formulation for mRNA Vaccines © 2022 Tonix Pharmaceuticals Holding Corp. PROFILE Collaboration with Kansas State University ZNP technology is a potential replacement for the Lipid Nanoparticle (LNP) technology of current mRNA vaccines Potential improved stability • Plan to seek initial indications as booster, similar to the current EUA and FDA approved mRNA vaccines • Improved stability would facilitate shipping and storage Addresses limitations in current mRNA vaccines which require ultra - cold storage and shipping • Stability issues limit use in less developed countries DEVELOPMENT PROGRAM Market Entry: Booster for COVID - 19 Vaccines Additional Indications: COVID - 19 vaccine for naïve individuals Status: Preclinical Next Steps: Research at K - State on CoV - 2 spike based vaccine in tissue culture and animals; 2Q 2022 Initiate Animal Studies *TNX - 3700 is in the pre - IND stage of development and has not been approved for any indication.

FUTURE OUTLOOK © 2022 Tonix Pharmaceuticals Holding Corp.
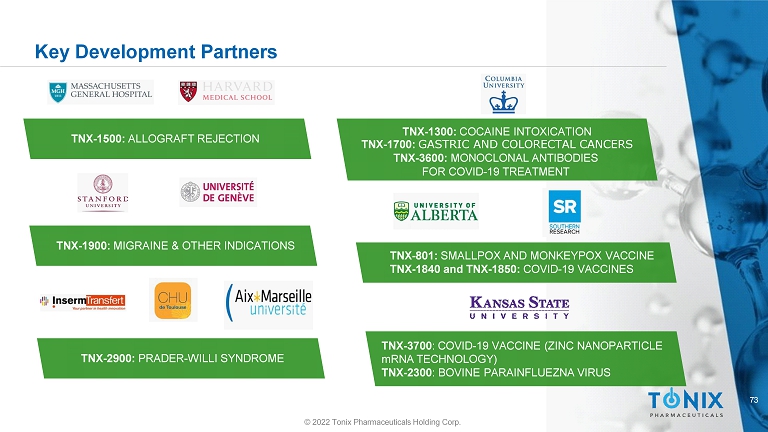
73 TNX - 1300: COCAINE INTOXICATION TNX - 1700: GASTRIC AND COLORECTAL CANCERS TNX - 3600: MONOCLONAL ANTIBODIES FOR COVID - 19 TREATMENT Key Development Partners TNX - 1500: ALLOGRAFT REJECTION TNX - 1900: MIGRAINE & OTHER INDICATIONS TNX - 801: SMALLPOX AND MONKEYPOX VACCINE TNX - 1840 and TNX - 1850: COVID - 19 VACCINES TNX - 2900: PRADER - WILLI SYNDROME TNX - 3700 : COVID - 19 VACCINE (ZINC NANOPARTICLE mRNA TECHNOLOGY) TNX - 2300 : BOVINE PARAINFLUEZNA VIRUS © 2022 Tonix Pharmaceuticals Holding Corp.

74 Expected Clinical Trial Initiations □ 2 nd Quarter 2022 Phase 2 OL safety study start of TNX - 1300 in ED setting for cocaine intoxication □ 2 nd Quarter 2022 Phase 2 study start of TNX - 102 SL for the treatment of PTSD in Kenya □ 2 nd Quarter 2022 Phase 2 study start of TNX - 102 SL for the treatment of Long COVID © 2022 Tonix Pharmaceuticals Holding Corp. □ 2 nd Half 2022 □ 2 nd Half 2022 □ 1 st Quarter 2023 Phase 2 study start of TNX - 1900 for the treatment of migraine Phase 1 study start of TNX - 1500 for prevention of allograft rejection Phase 2 study start of TNX - 601 CR for the treatment of major depressive disorder Milestones: Recently Completed and Upcoming* * We cannot predict whether the global COVID - 19 pandemic will impact the timing of these milestones. Non - human primate positive efficacy data from TNX - 1800 in COVID - 19 models reported Topline data from Phase 3 F306/RALLY study of TNX - 102 SL for the management of fibromyalgia □ x 1 st Quarter 2021 □ x 1 st Quarter 2022 □ x 2 nd Quarter 2022 Phase 3 F307/RESILIENT study start of TNX - 102 SL for the management of fibromyalgia Expected Data □ 1 st Quarter 2023 Interim analysis results of Phase 3 F307/RESILIENT study of TNX - 102 SL in fibromyalgia

75 Management Team Seth Lederman, MD Co - Founder, CEO & Chairman Gregory Sullivan, MD Chief Medical Officer Bradley Saenger, CPA Chief Financial Officer Jessica Morris Chief Operating Officer © 2022 Tonix Pharmaceuticals Holding Corp.

THANK YOU © 2022 Tonix Pharmaceuticals Holding Corp.