
Tonix Pharmaceuticals Holding Corp. 8-K
Exhibit 99.02

© 2023 Tonix Pharmaceuticals Holding Corp. Tonix Pharmaceuticals TNX - 102 SL (sublingual cyclobenzaprine) Fibromyalgia Phase 3 RESILIENT Study ( TNX - CY - F307 ) Topline December 20, 2023 Version P0512 December 18 , 2023 (Doc 1357 )

2 © 2023 Tonix Pharmaceuticals Holding Corp. Cautionary Note on Forward - Looking Statements Certain statements in this presentation regarding strategic plans, expectations and objectives for future operations or results are “forward - looking statements” as defined by the Private Securities Litigation Reform Act of 1995. These statements may be identified by the use of forward - looking words such as “anticipate,” “believe,” “forecast,” “estimate” and “intend,” among others. These forward - looking statements are based on Tonix’s current expectations and actual results could differ materially. There are a number of factors that could cause actual events to differ materially from those indicated by such forward - looking statements. These factors include, but are not limited to, the risks related to failure to obtain FDA clearances or approvals and noncompliance with FDA regulations; risks related to the failure to successfully market any of our products; risks related to the timing and progress of clinical development of our product candidates; our need for additional financing; uncertainties of patent protection and litigation; uncertainties of government or third party payor reimbursement; limited research and development efforts and dependence upon third parties; and substantial competition. As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products. The forward - looking statements in this presentation are made as of the date of this presentation, even if subsequently made available by Tonix on its website or otherwise. Tonix does not undertake an obligation to update or revise any forward - looking statement, except as required by law. Investors should read the risk factors set forth in the Annual Report on Form 10 - K for the year ended December 31, 2022, as filed with the Securities and Exchange Commission (the “SEC”) on March 13, 2023, and periodic reports and current reports filed with the SEC on or after the date thereof. All of Tonix's forward - looking statements are expressly qualified by all such risk factors and other cautionary statements.
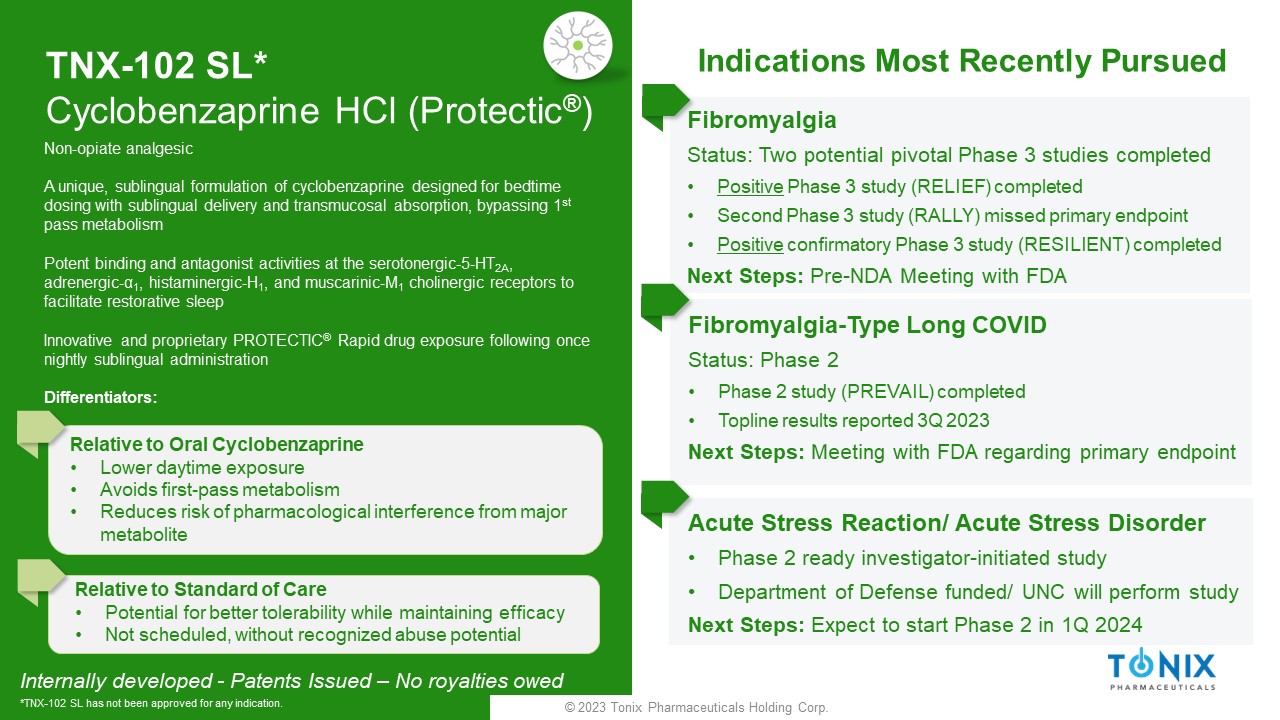
© 2023 Tonix Pharmaceuticals Holding Corp. TNX - 102 SL* Cyclobenzaprine HCl ( Protectic ® ) Fibromyalgia Status: Two potential pivotal Phase 3 studies completed • P ositive Phase 3 study (RELIEF) completed • Second Phase 3 study (RALLY) missed primary endpoint • Positive confirmatory Phase 3 study (RESILIENT) completed Next Steps: Pre - NDA Meeting with FDA Fibromyalgia - Type Long COVID Status: Phase 2 • Phase 2 study (PREVAIL) completed • Topline results reported 3Q 2023 Next Steps: Meeting with FDA regarding primary endpoint Internally developed - Patents Issued – No royalties owed *TNX - 102 SL has not been approved for any indication. Non - opiate analgesic A unique, sublingual formulation of cyclobenzaprine designed for bedtime dosing with sublingual delivery and transmucosal absorption, bypassing 1 st pass metabolism Potent binding and antagonist activities at the serotonergic - 5 - HT 2A , adrenergic - α 1 , histaminergic - H 1 , and muscarinic - M 1 cholinergic receptors to facilitate restorative sleep Innovative and proprietary PROTECTIC ® Rapid drug exposure following once nightly sublingual administration Differentiators: Relative to Oral Cyclobenzaprine • Lower daytime exposure • Avoids first - pass metabolism • Reduces risk of pharmacological interference from major metabolite Relative to Standard of Care • Potential for better tolerability while maintaining efficacy • Not scheduled, without recognized abuse potential Indications Most Recently Pursued Acute Stress Reaction/ Acute Stress Disorder • Phase 2 ready investigator - initiated study • Department of Defense funded/ UNC will perform study Next Steps: Expect to start Phase 2 in 1Q 2024

4 © 2023 Tonix Pharmaceuticals Holding Corp. TNX - 102 SL: Phase 3 RESILIENT Study Design General s tudy c haracteristics: • Randomized, double - blind, multicenter, placebo - controlle d study in fibromyalgia • 33 U.S. sites enrolled 457 participants with fibromyalgia as defined by 2016 Revisions to the 2010/2011 FM Diagnostic C riteria ^ Primary Endpoint: • Change from baseline to Week 14 (TNX - 102 SL vs. placebo) in weekly averages of daily diary average pain severity score • Primary Endpoint, p - value = 0.00005 Placebo once - daily at bedtime TNX - 102 SL once - daily at bedtime 5.6 mg (2 x 2.8 mg tablets) * * Two - week run - in at 2.8 mg dose at bedtime followed by 12 weeks at 5.6 mg dose ClinicalTrials.gov Identifier: NCT05273749 Study Title: A Phase 3 Study to Evaluate the Efficacy and Safety of TNX - 102 SL Taken Daily in Patients With Fibromyalgia (RESILIENT) Trial ID: TNY - CY - F307 (‘F307’) 14 weeks ^ Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Häuser W, Katz RL, Mease PJ, Russell AS, Russell IJ, Walitt B. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin Arthritis Rheum. 2016; 46(3):319 - 329.
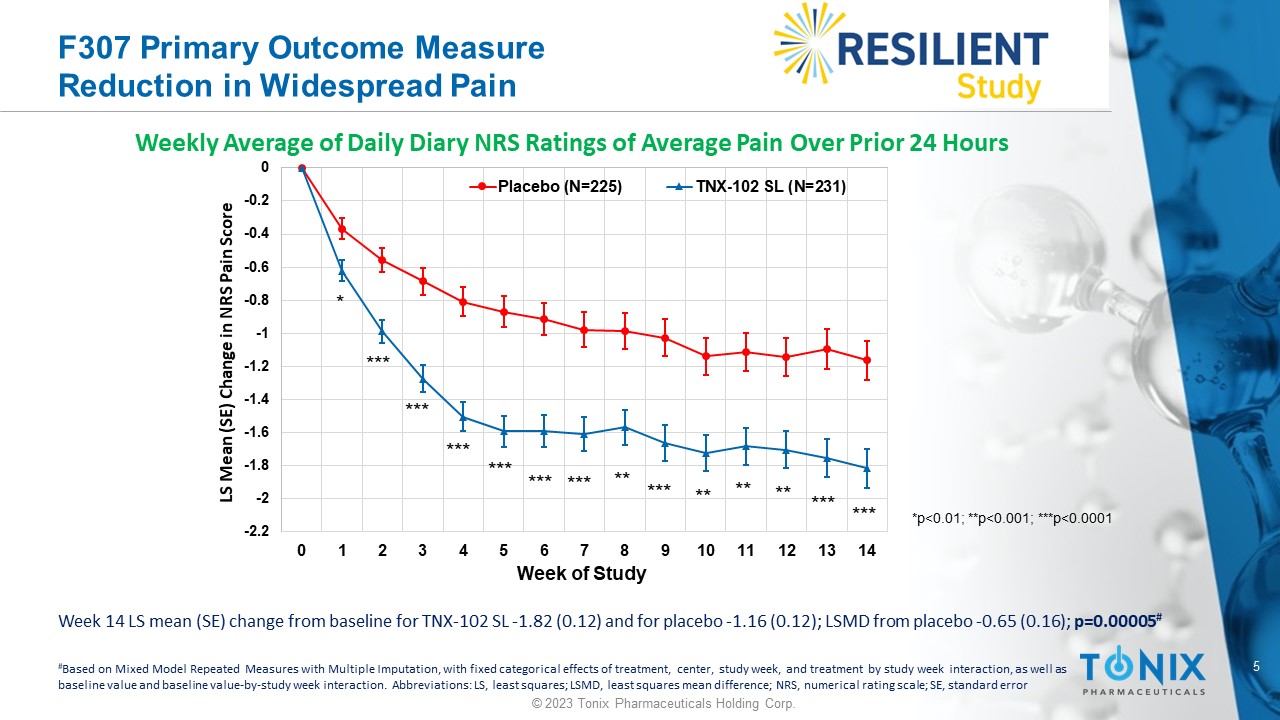
5 © 2023 Tonix Pharmaceuticals Holding Corp. F307 Primary Outcome Measure Reduction in Widespread Pain Weekly Average of Daily Diary NRS Ratings of Average Pain Over Prior 24 Hours -2.2 -2 -1.8 -1.6 -1.4 -1.2 -1 -0.8 -0.6 -0.4 -0.2 0 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 LS Mean (SE) Change in NRS Pain Score Week of Study Placebo (N=225) TNX-102 SL (N=231) * *** *** ** ** ** *** *** *** *** *** *** *** ** *p<0.01; **p<0.001; ***p<0.0001 Week 14 LS mean (SE) change from baseline for TNX - 102 SL - 1.82 (0.12) and for placebo - 1.16 (0.12); LSMD from placebo - 0.65 (0.1 6); p=0.00005 # # Based on Mixed Model Repeated Measures with Multiple Imputation, with fixed categorical effects of treatment, center, study w eek , and treatment by study week interaction, as well as baseline value and baseline value - by - study week interaction. Abbreviations: LS, least squares; LSMD, least squares mean differe nce; NRS, numerical rating scale; SE, standard error
6 © 2023 Tonix Pharmaceuticals Holding Corp. Pre - Specified Primary Endpoint Summary • TNX - 102 SL demonstrated statistically significant improvement in mean weekly pain scores over placebo at Week 14 • p - value of 0.00005 is highly statistically significant Additional Findings • Effect size 0.38 • All pre - specified sensitivity analyses of the primary endpoint show statistical significance ( p ≤ 0.001) • Rapid onset of action: nominal p - values <0.01 at each weekly time point, including Week 1
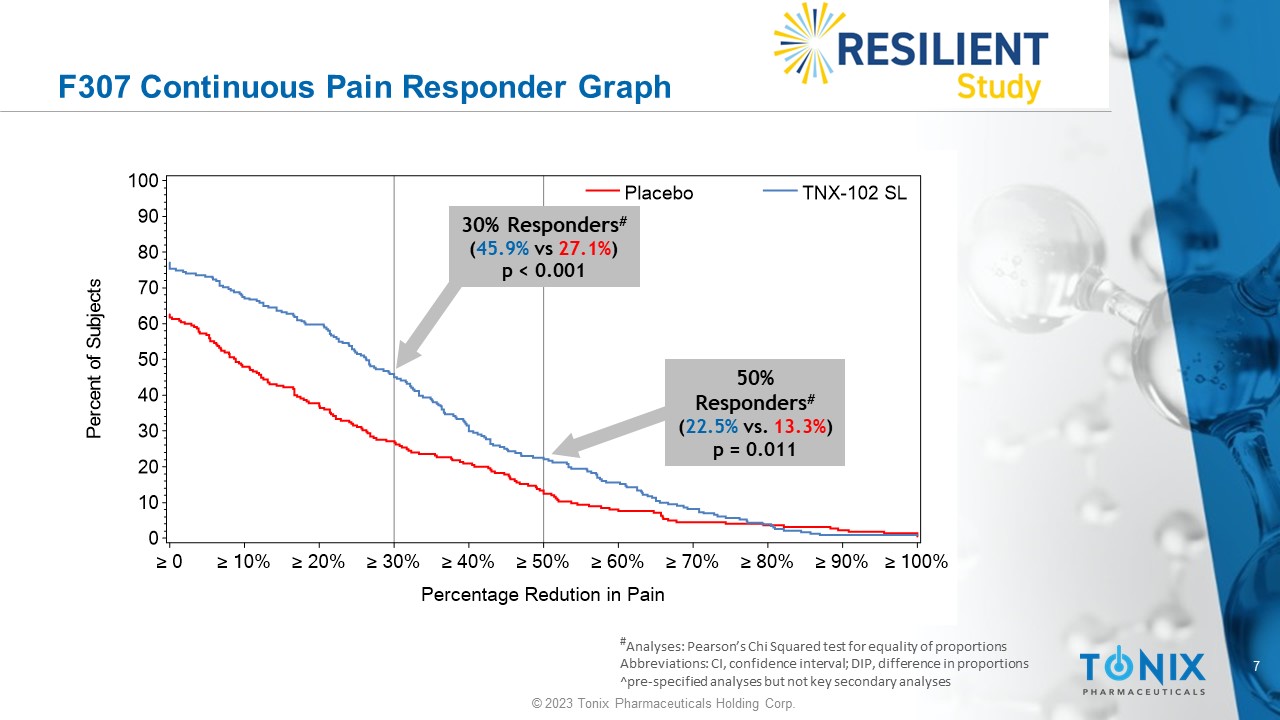
7 © 2023 Tonix Pharmaceuticals Holding Corp. F307 Continuous Pain Responder Graph # Analyses: Pearson’s Chi Squared test for equality of proportions Abbreviations: CI, confidence interval; DIP, difference in proportions ^pre - specified analyses but not key secondary analyses P e r c e n t o f S u b j e c t s 0 10 20 30 40 50 60 70 80 90 100 Percentage Redution in Pain ≥ 0 ≥ 10% ≥ 20% ≥ 30% ≥ 40% ≥ 50% ≥ 60% ≥ 70% ≥ 80% ≥ 90% ≥ 100% Placebo TNX-102 SL 30% Responders # ( 45.9% vs 27.1% ) p < 0.001 50% Responders # ( 22.5% vs. 13.3% ) p = 0.011
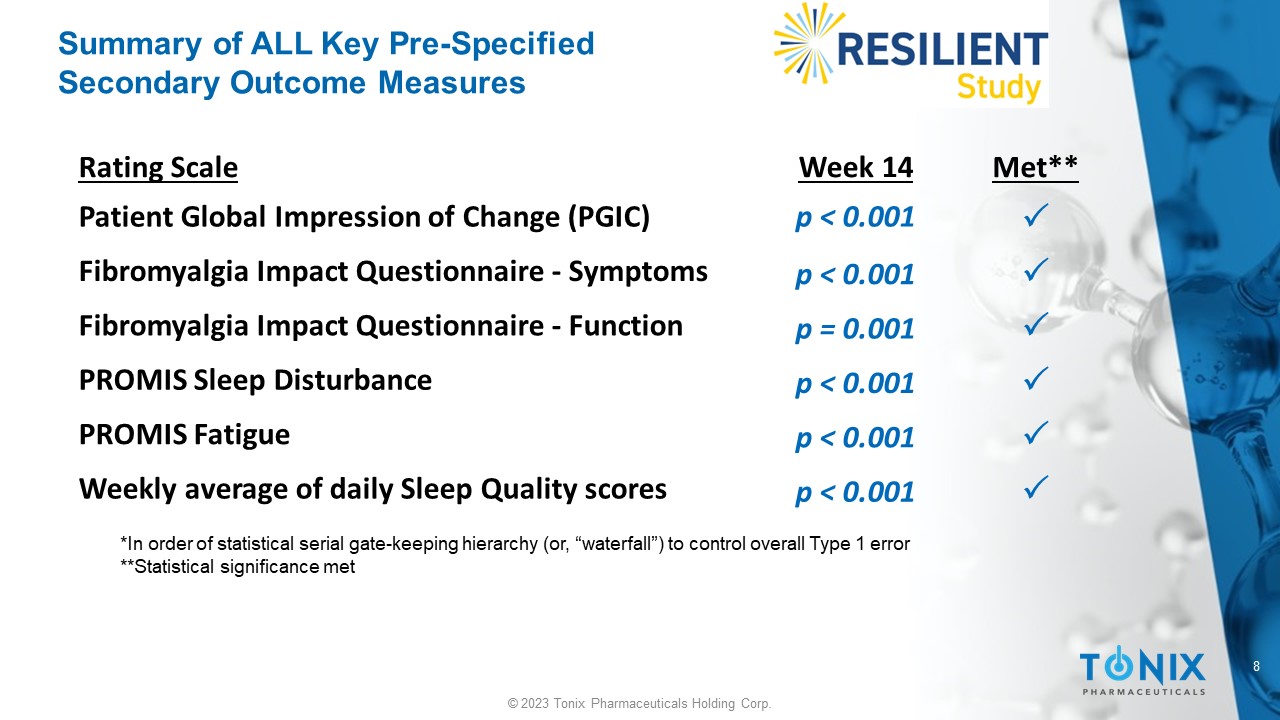
8 © 2023 Tonix Pharmaceuticals Holding Corp. Summary of ALL Key Pre - Specified Secondary Outcome Measures *In order of statistical serial gate - keeping hierarchy (or, “waterfall”) to control overall Type 1 error **Statistical significance met Met** Week 14 Rating Scale p < 0.001 Patient Global Impression of Change (PGIC) p < 0.001 Fibromyalgia Impact Questionnaire - Symptoms p = 0.001 Fibromyalgia Impact Questionnaire - Function p < 0.001 PROMIS Sleep Disturbance p < 0.001 PROMIS Fatigue p < 0.001 Weekly average of daily Sleep Quality scores
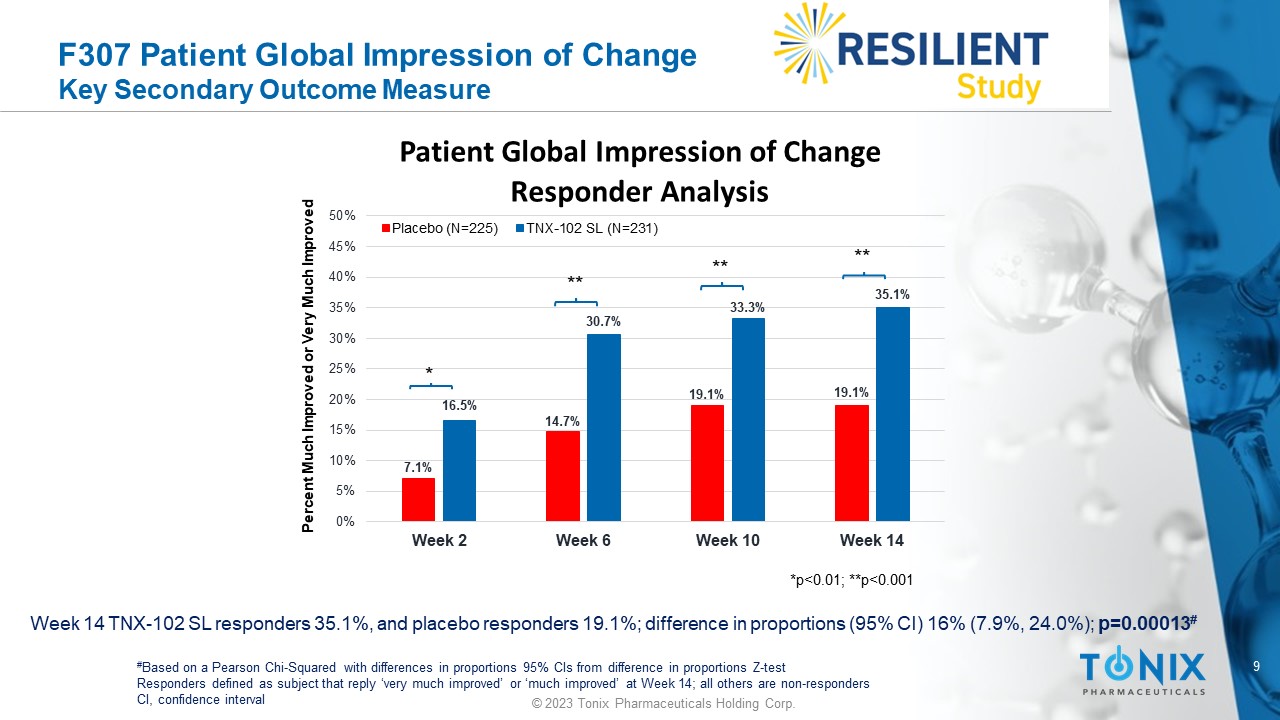
9 © 2023 Tonix Pharmaceuticals Holding Corp. F307 Patient Global Impression of Change Key Secondary Outcome Measure Week 14 TNX - 102 SL responders 35.1%, and placebo responders 19.1%; difference in proportions (95% CI) 16% (7.9%, 24.0%); p=0.00013 # # Based on a Pearson Chi - Squared with differences in proportions 95% CIs from difference in proportions Z - test Responders defined as subject that reply ‘very much improved’ or ‘much improved’ at Week 14; all others are non - responders CI, confidence interval 7.1% 14.7% 19.1% 19.1% 16.5% 30.7% 33.3% 35.1% 0% 5% 10% 15% 20% 25% 30% 35% 40% 45% 50% Week 2 Week 6 Week 10 Week 14 Patient Global Impression of Change Responder Analysis Placebo (N=225) TNX-102 SL (N=231) Percent Much Improved or Very Much Improved *p<0.01; **p<0.001 * ** ** **
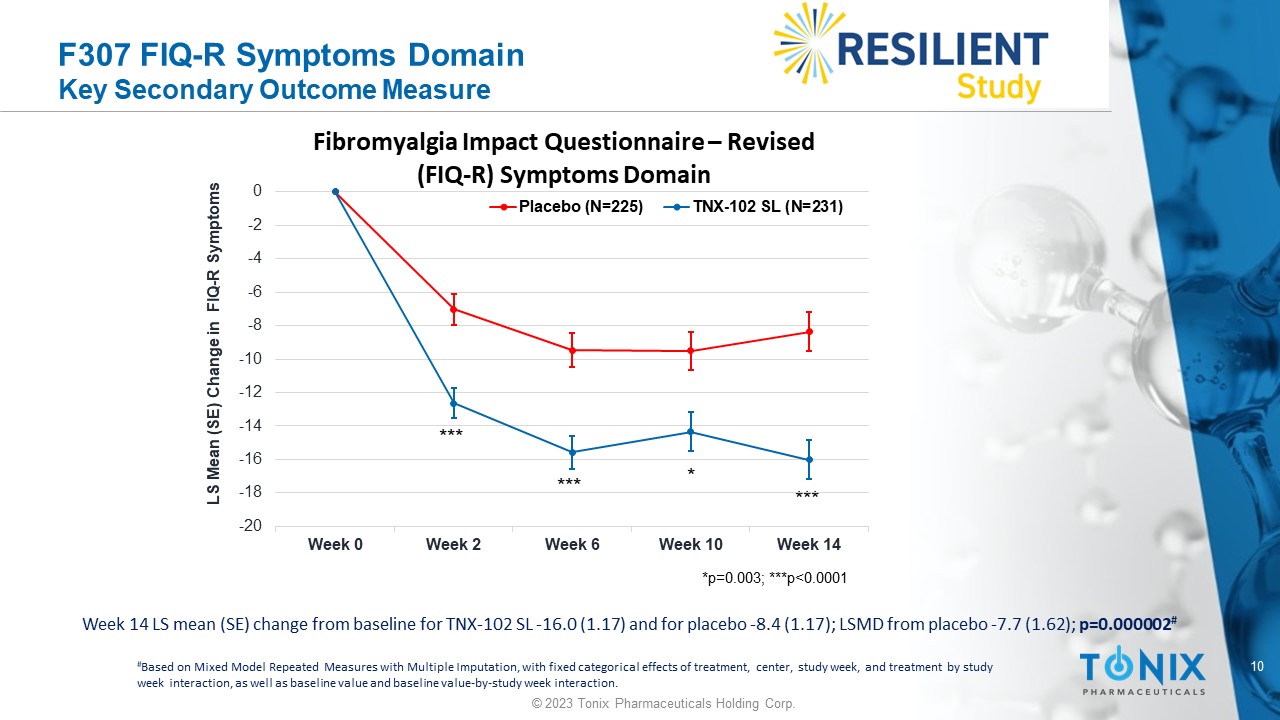
10 © 2023 Tonix Pharmaceuticals Holding Corp. F307 FIQ - R Symptoms Domain Key Secondary Outcome Measure * *** *** *** Week 14 LS mean (SE) change from baseline for TNX - 102 SL - 16.0 (1.17) and for placebo - 8.4 (1.17); LSMD from placebo - 7.7 (1.62) ; p=0.000002 # # Based on Mixed Model Repeated Measures with Multiple Imputation, with fixed categorical effects of treatment, center, study w eek , and treatment by study week interaction, as well as baseline value and baseline value - by - study week interaction. -20 -18 -16 -14 -12 -10 -8 -6 -4 -2 0 Week 0 Week 2 Week 6 Week 10 Week 14 LS Mean (SE) Change in FIQ - R Symptoms Fibromyalgia Impact Questionnaire – Revised (FIQ - R) Symptoms Domain Placebo (N=225) TNX-102 SL (N=231) *p=0.003; ***p<0.0001
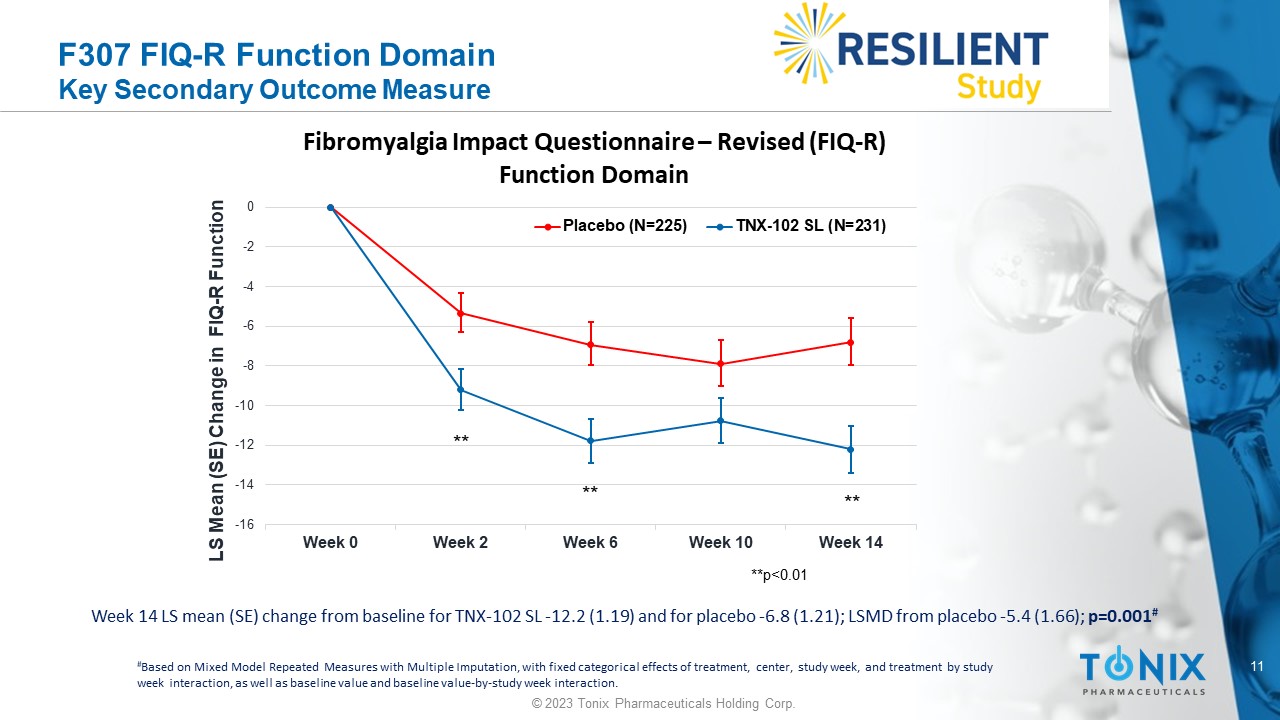
11 © 2023 Tonix Pharmaceuticals Holding Corp. F307 FIQ - R Function Domain Key Secondary Outcome Measure Week 14 LS mean (SE) change from baseline for TNX - 102 SL - 12.2 (1.19) and for placebo - 6.8 (1.21); LSMD from placebo - 5.4 (1.66) ; p=0.001 # # Based on Mixed Model Repeated Measures with Multiple Imputation, with fixed categorical effects of treatment, center, study w eek , and treatment by study week interaction, as well as baseline value and baseline value - by - study week interaction. -16 -14 -12 -10 -8 -6 -4 -2 0 Week 0 Week 2 Week 6 Week 10 Week 14 LS Mean (SE) Change in FIQ - R Function Fibromyalgia Impact Questionnaire – Revised (FIQ - R) Function Domain Placebo (N=225) TNX-102 SL (N=231) **p<0.01 ** ** **
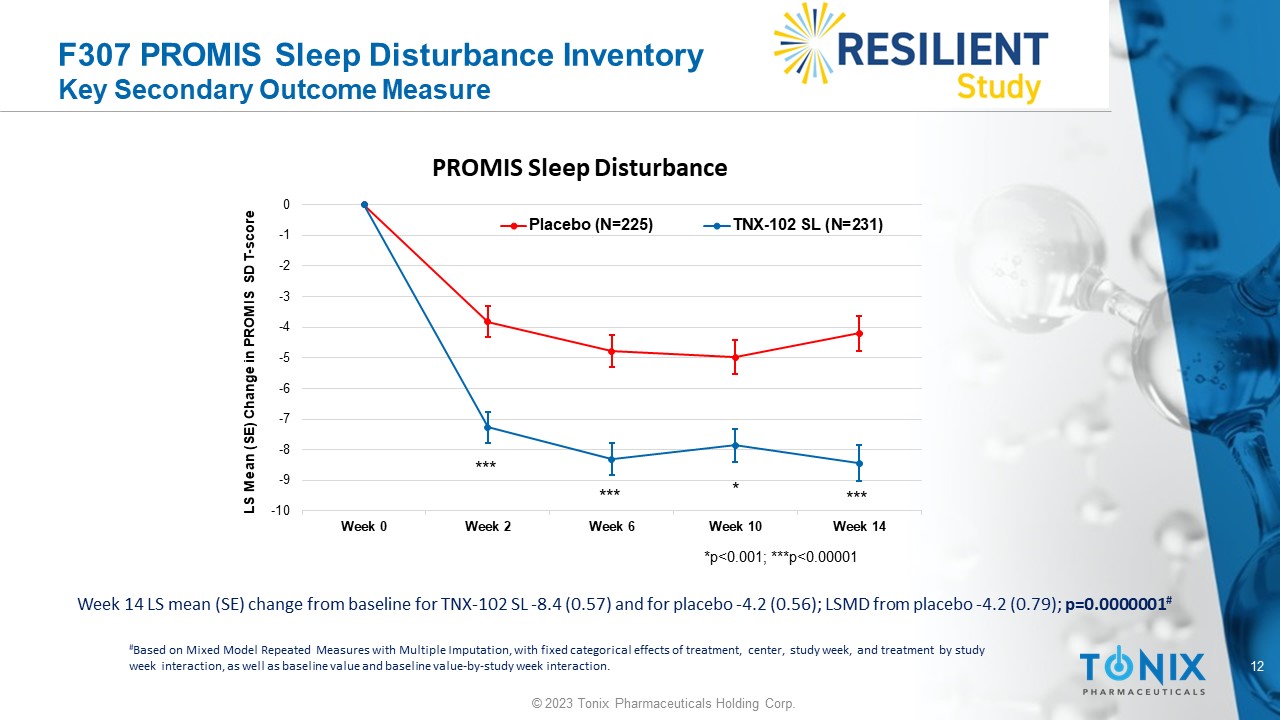
12 © 2023 Tonix Pharmaceuticals Holding Corp. F307 PROMIS Sleep Disturbance Inventory Key Secondary Outcome Measure Week 14 LS mean (SE) change from baseline for TNX - 102 SL - 8.4 (0.57) and for placebo - 4.2 (0.56); LSMD from placebo - 4.2 (0.79); p=0.0000001 # # Based on Mixed Model Repeated Measures with Multiple Imputation, with fixed categorical effects of treatment, center, study w eek , and treatment by study week interaction, as well as baseline value and baseline value - by - study week interaction. -10 -9 -8 -7 -6 -5 -4 -3 -2 -1 0 Week 0 Week 2 Week 6 Week 10 Week 14 LS Mean (SE) Change in PROMIS SD T - score PROMIS Sleep Disturbance Placebo (N=225) TNX-102 SL (N=231) *p<0.001; ***p<0.00001 * *** *** ***
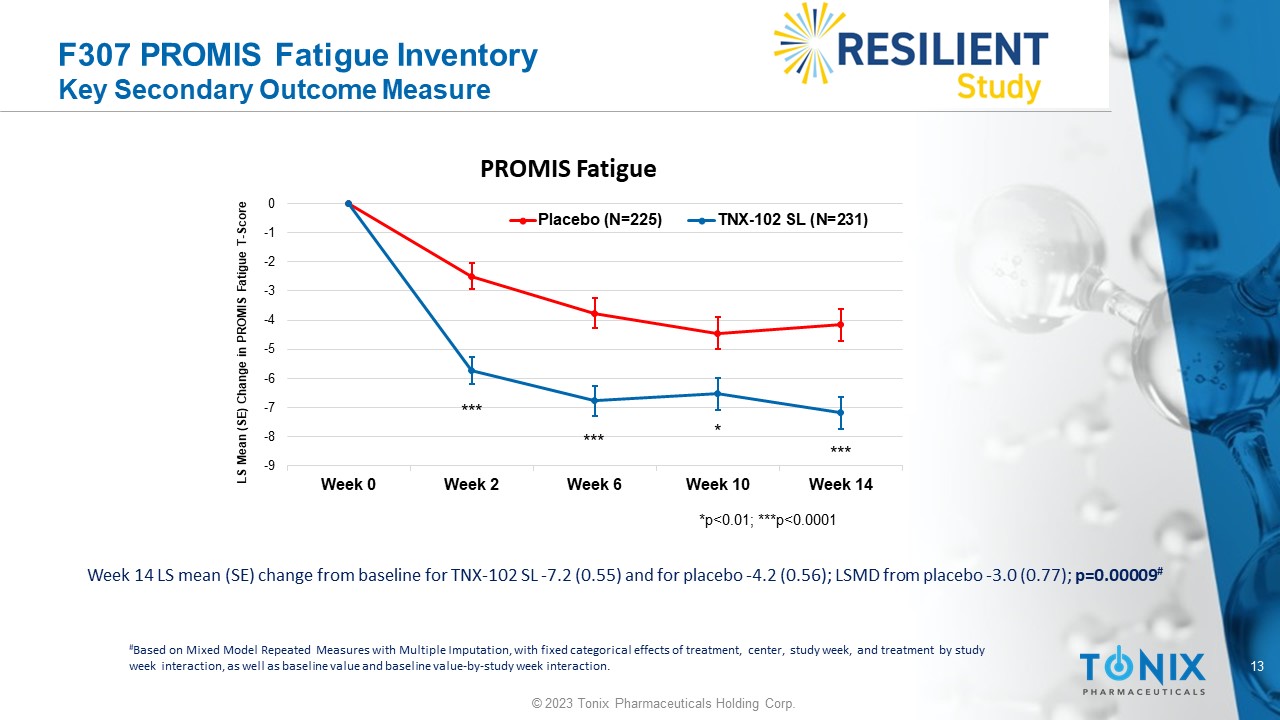
13 © 2023 Tonix Pharmaceuticals Holding Corp. F307 PROMIS Fatigue Inventory Key Secondary Outcome Measure Week 14 LS mean (SE) change from baseline for TNX - 102 SL - 7.2 (0.55) and for placebo - 4.2 (0.56); LSMD from placebo - 3.0 (0.77); p=0.00009 # # Based on Mixed Model Repeated Measures with Multiple Imputation, with fixed categorical effects of treatment, center, study w eek , and treatment by study week interaction, as well as baseline value and baseline value - by - study week interaction. -9 -8 -7 -6 -5 -4 -3 -2 -1 0 Week 0 Week 2 Week 6 Week 10 Week 14 LS Mean (SE) Change in PROMIS Fatigue T - Score PROMIS Fatigue Placebo (N=225) TNX-102 SL (N=231) *p<0.01; ***p<0.0001 * *** *** ***
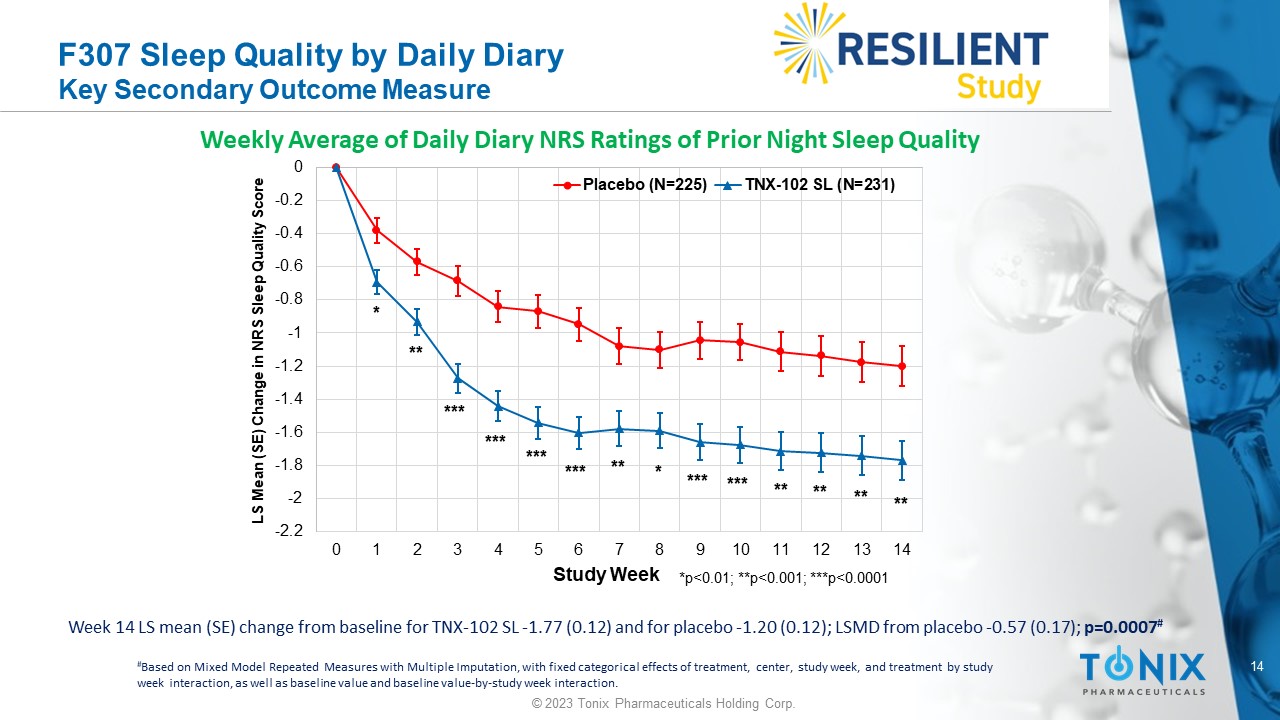
14 © 2023 Tonix Pharmaceuticals Holding Corp. F307 Sleep Quality by Daily Diary Key Secondary Outcome Measure Week 14 LS mean (SE) change from baseline for TNX - 102 SL - 1.77 (0.12) and for placebo - 1.20 (0.12); LSMD from placebo - 0.57 (0.1 7); p=0.0007 # Weekly Average of Daily Diary NRS Ratings of Prior Night Sleep Quality -2.2 -2 -1.8 -1.6 -1.4 -1.2 -1 -0.8 -0.6 -0.4 -0.2 0 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 LS Mean (SE) Change in NRS Sleep Quality Score Study Week Placebo (N=225) TNX-102 SL (N=231) *p<0.01; **p<0.001; ***p<0.0001 *** * ** ** * ** *** ** ** ** *** *** *** *** # Based on Mixed Model Repeated Measures with Multiple Imputation, with fixed categorical effects of treatment, center, study w eek , and treatment by study week interaction, as well as baseline value and baseline value - by - study week interaction.
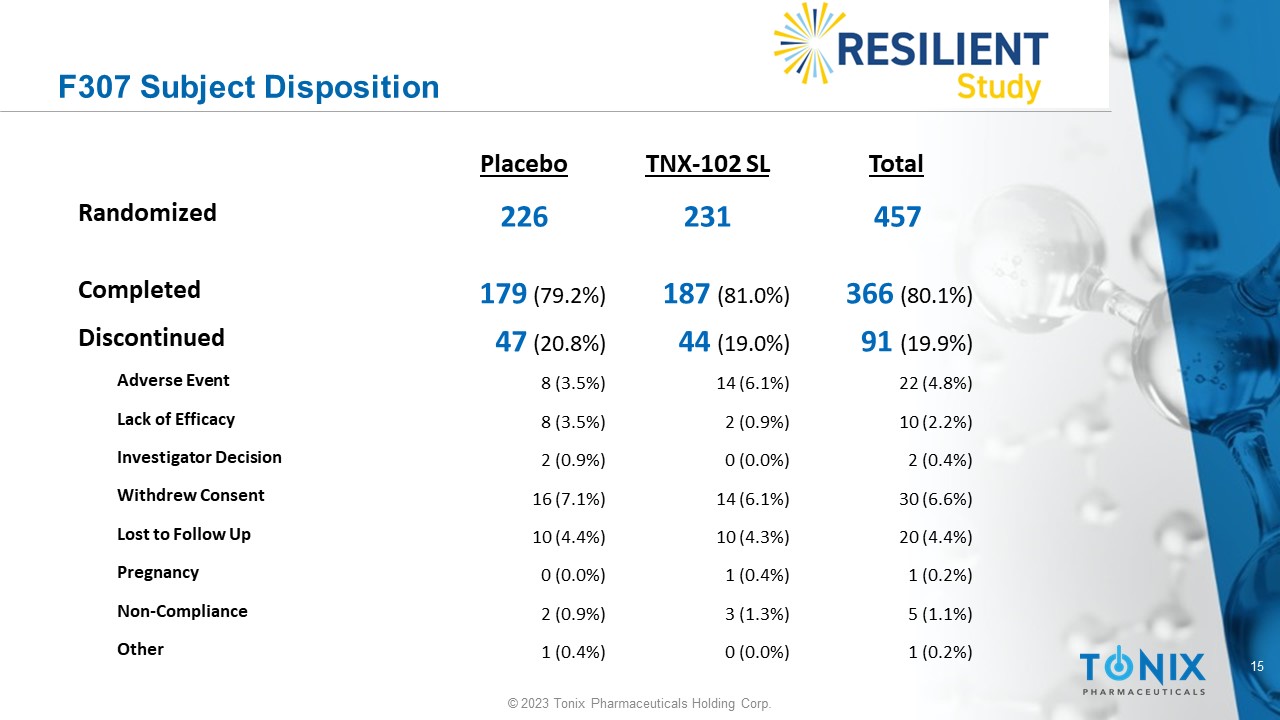
15 © 2023 Tonix Pharmaceuticals Holding Corp. F307 Subject Disposition Total TNX - 102 SL Placebo 457 231 226 Randomized 366 (80.1%) 187 (81.0%) 179 (79.2%) Completed 91 (19.9%) 44 (19.0%) 47 (20.8%) Discontinued 22 (4.8%) 14 (6.1%) 8 (3.5%) Adverse Event 10 (2.2%) 2 (0.9%) 8 (3.5%) Lack of Efficacy 2 (0.4%) 0 (0.0%) 2 (0.9%) Investigator Decision 30 (6.6%) 14 (6.1%) 16 (7.1%) Withdrew Consent 20 (4.4%) 10 (4.3%) 10 (4.4%) Lost to Follow Up 1 (0.2%) 1 (0.4%) 0 (0.0%) Pregnancy 5 (1.1%) 3 (1.3%) 2 (0.9%) Non - Compliance 1 (0.2%) 0 (0.0%) 1 (0.4%) Other

16 © 2023 Tonix Pharmaceuticals Holding Corp. F307 Safety Summary Among participants randomized to TNX - 102 SL and to placebo, 81.0% and 79.6%, respectively, completed the study TNX - 102 SL was generally well tolerated with an adverse event (AE) profile comparable to prior fibromyalgia studies • No new safety signals were observed • AE - related study discontinuations occurred in 6.1% and 3.6% of patients in the TNX - 102 SL and placebo groups, respectively • Events rated as mild or moderate made up 97.2% of AEs on placebo and 99.1% on TNX - 102 SL • As observed in prior studies with TNX - 102 SL, oral administration site AEs were higher in TNX - 102 SL than placebo, 42.9% and 10. 2%, respectively ‒ Most common oral AEs were oral hypoaesthesia, product taste abnormal, oral paraesthesia, and tongue discomfort (see table on nex t slide) ‒ Nearly all of these common oral AEs were temporally related to dosing and lasted <60 minutes • Serious Adverse Events (SAEs) ‒ Three placebo participants experienced an SAE: ▪ 1. Pneumonia, 2. Muscular weakness, and 3. Hypertension/Angina/Coronary Artery Disease ‒ Two TNX - 102 SL participants experienced an SAE ▪ 1. Renal carcinoma deemed not related to study drug ▪ 2. Acute pancreatitis with onset 14 days after completion of treatment phase, deemed ‘possibly related’* to study drug ⁃ Outcome: ‘Recovered/Resolved’ ⁃ *Note: participant was non - compliant with end of treatment study visits, and the last dose before onset of SAE was not known at the time that relationship with study drug was assessed by Investigator and Sponsor
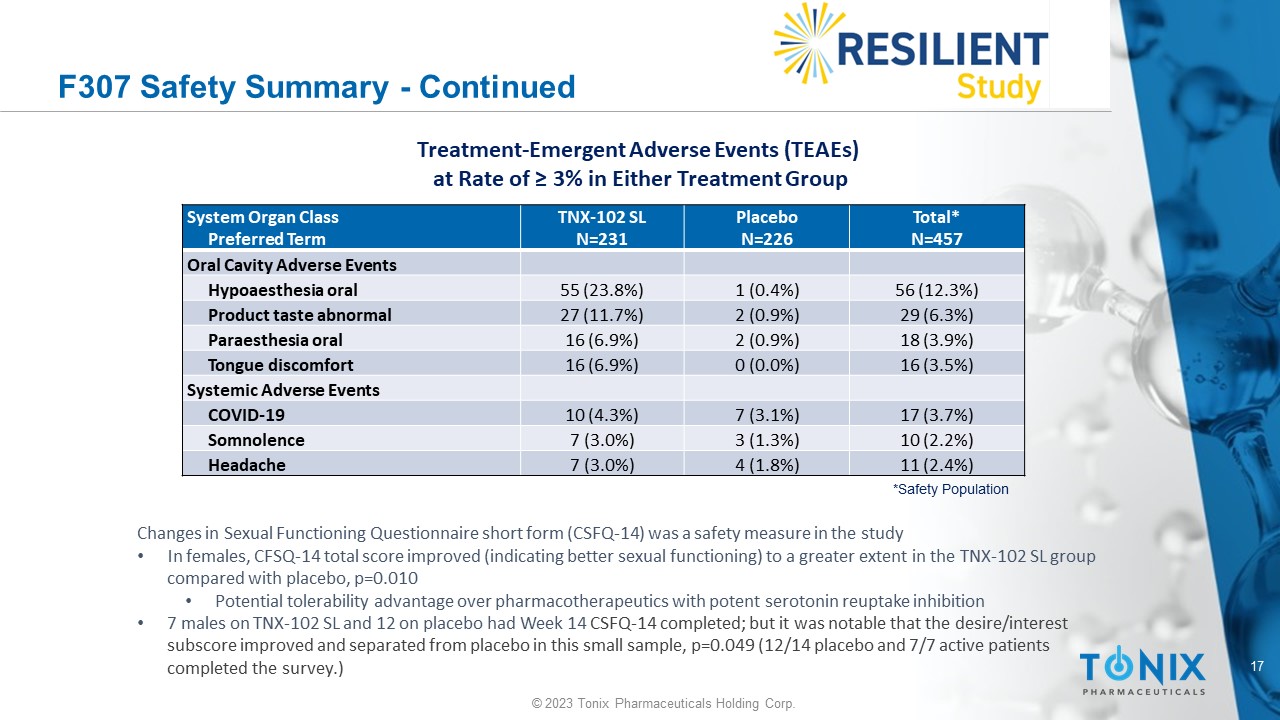
17 © 2023 Tonix Pharmaceuticals Holding Corp. F307 Safety Summary - Continued Total* N=457 Placebo N=226 TNX - 102 SL N=231 System Organ Class Preferred Term Oral Cavity Adverse Events 56 (12.3%) 1 (0.4%) 55 (23.8%) Hypoaesthesia oral 29 (6.3%) 2 (0.9%) 27 (11.7%) Product taste abnormal 18 (3.9%) 2 (0.9%) 16 (6.9%) Paraesthesia oral 16 (3.5%) 0 (0.0%) 16 (6.9%) Tongue discomfort Systemic Adverse Events 17 (3.7%) 7 (3.1%) 10 (4.3%) COVID - 19 10 (2.2%) 3 (1.3%) 7 (3.0%) Somnolence 11 (2.4%) 4 (1.8%) 7 (3.0%) Headache *Safety Population Treatment - Emergent Adverse Events (TEAEs) at Rate of ≥ 3% in Either Treatment Group Changes in Sexual Functioning Questionnaire short form (CSFQ - 14) was a safety measure in the study • In females, CFSQ - 14 total score improved (indicating better sexual functioning) to a greater extent in the TNX - 102 SL group compared with placebo, p=0.010 • Potential tolerability advantage over pharmacotherapeutics with potent serotonin reuptake inhibition • 7 males on TNX - 102 SL and 12 on placebo had Week 14 CSFQ - 14 completed; but it was notable that the desire/interest subscore improved and separated from placebo in this small sample, p=0.049 (12/14 placebo and 7/7 active patients completed the survey.)
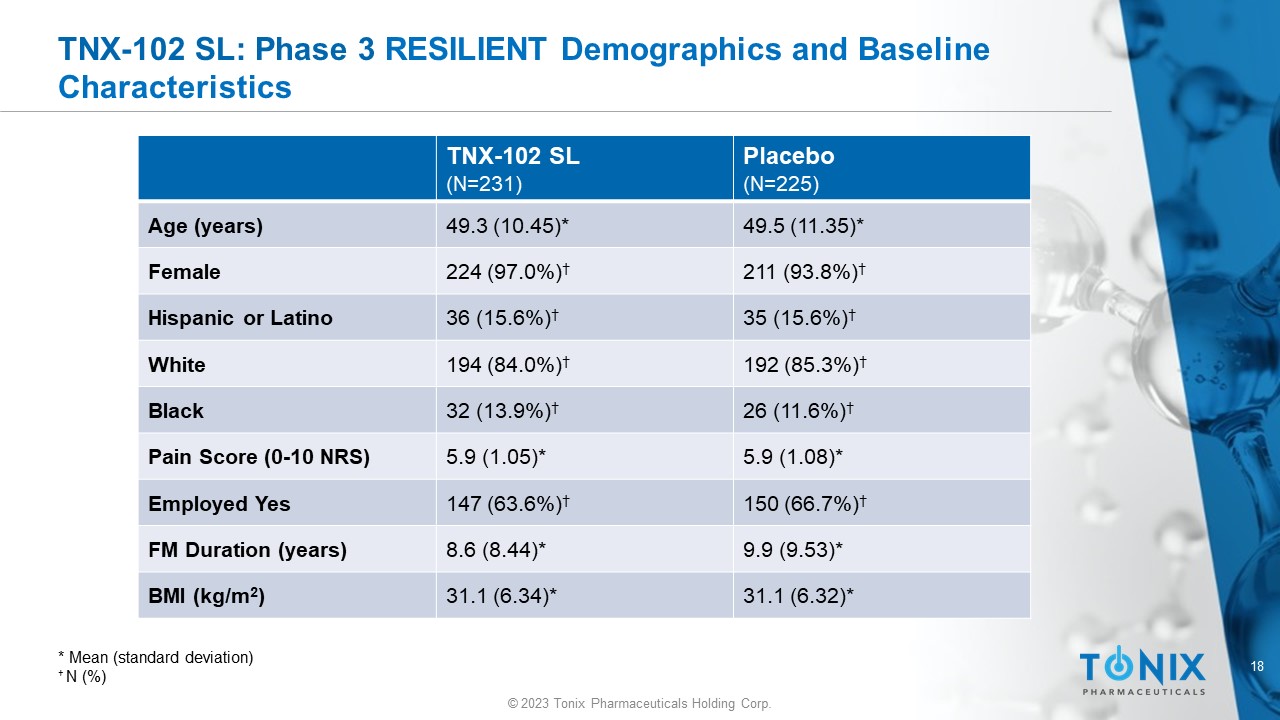
18 © 2023 Tonix Pharmaceuticals Holding Corp. TNX - 102 SL: Phase 3 RESILIENT Demographics and Baseline Characteristics Placebo (N=225) TNX - 102 SL (N=231) 49.5 (11.35)* 49.3 (10.45)* Age (years) 211 (93.8%) † 224 (97.0%) † Female 35 (15.6%) † 36 (15.6%) † Hispanic or Latino 192 (85.3%) † 194 (84.0%) † White 26 (11.6%) † 32 (13.9%) † Black 5.9 (1.08)* 5.9 (1.05)* Pain Score (0 - 10 NRS) 150 (66.7%) † 147 (63.6%) † Employed Yes 9.9 (9.53)* 8.6 (8.44)* FM Duration (years) 31.1 (6.32)* 31.1 (6.34)* BMI (kg/m 2 ) * Mean ( s tandard deviation) † N (%)
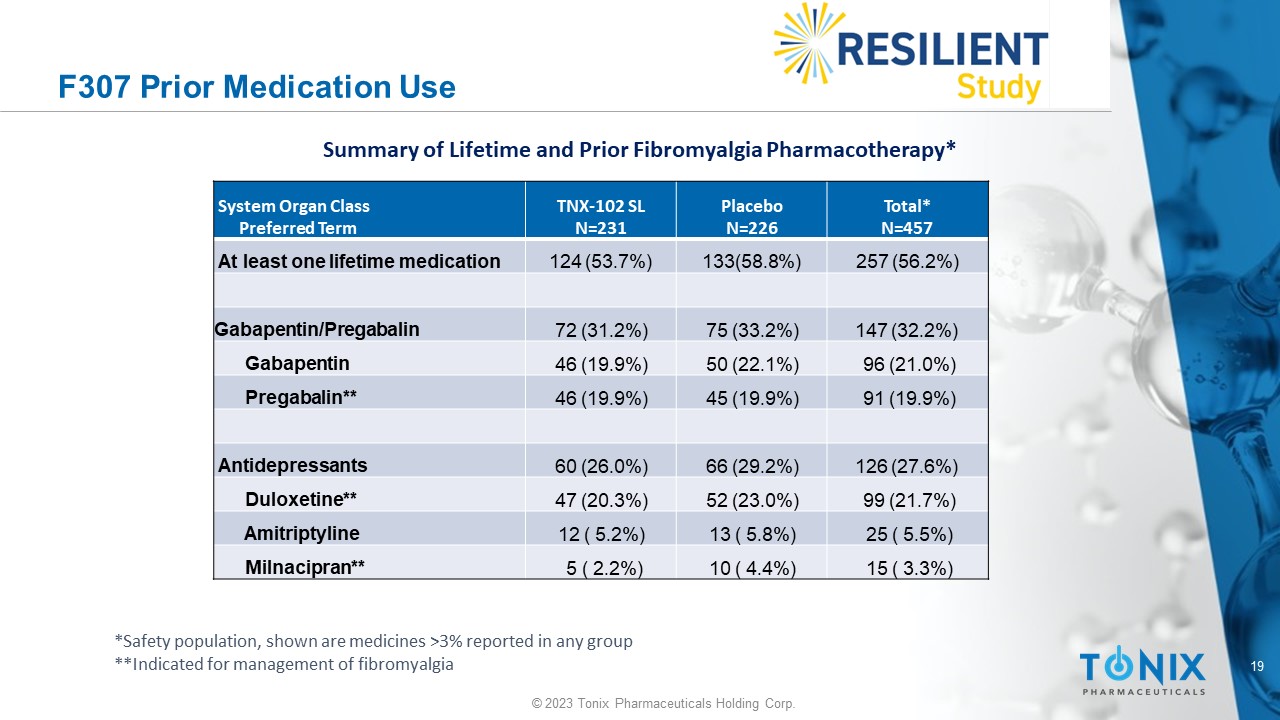
19 © 2023 Tonix Pharmaceuticals Holding Corp. F307 Prior Medication Use Total* N=457 Placebo N=226 TNX - 102 SL N=231 System Organ Class Preferred Term 257 (56.2%) 133(58.8%) 124 (53.7%) At least one lifetime medication 147 (32.2%) 75 (33.2%) 72 (31.2%) Gabapentin/Pregabalin 96 (21.0%) 50 (22.1%) 46 (19.9%) Gabapentin 91 (19.9%) 45 (19.9%) 46 (19.9%) Pregabalin** 126 (27.6%) 66 (29.2%) 60 (26.0%) Antidepressants 99 (21.7%) 52 (23.0%) 47 (20.3%) Duloxetine** 25 ( 5.5%) 13 ( 5.8%) 12 ( 5.2%) Amitriptyline 15 ( 3.3%) 10 ( 4.4%) 5 ( 2.2%) Milnacipran** Summary of Lifetime and Prior Fibromyalgia Pharmacotherapy* *Safety population, shown are medicines >3% reported in any group **Indicated for management of fibromyalgia
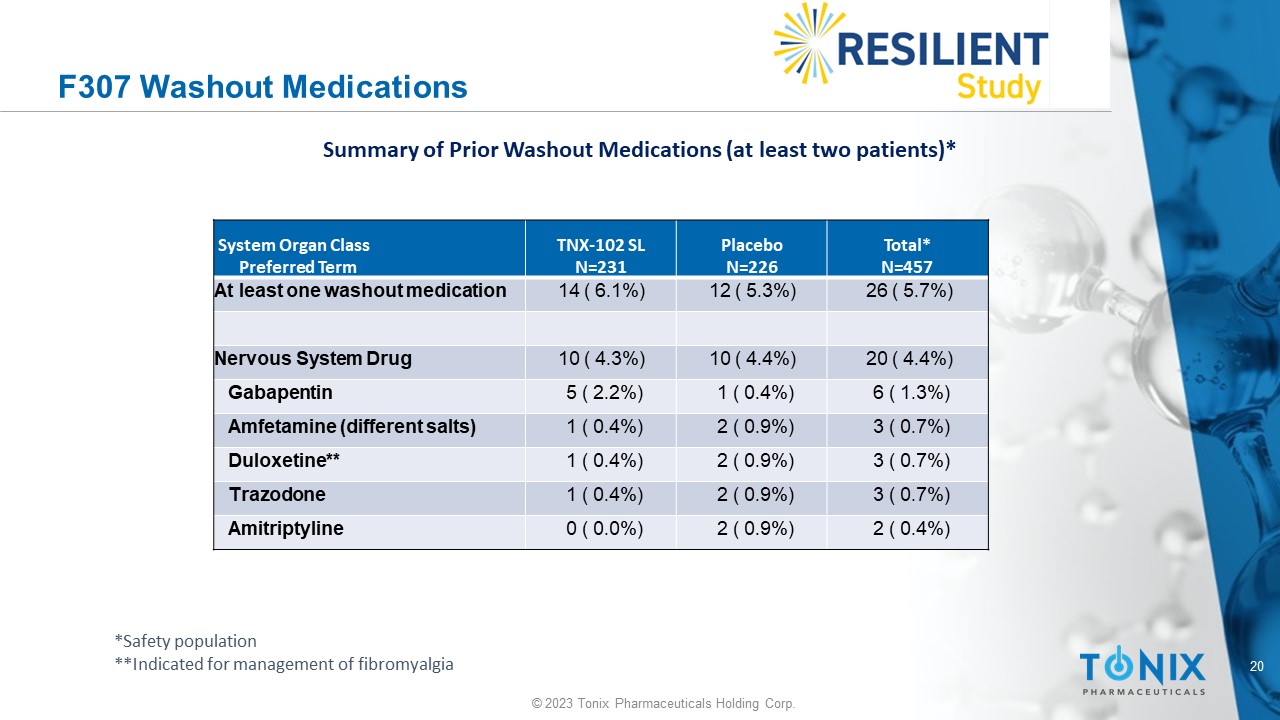
20 © 2023 Tonix Pharmaceuticals Holding Corp. F307 Washout Medications Total* N=457 Placebo N=226 TNX - 102 SL N=231 System Organ Class Preferred Term 26 ( 5.7%) 12 ( 5.3%) 14 ( 6.1%) At least one washout medication 20 ( 4.4%) 10 ( 4.4%) 10 ( 4.3%) Nervous System Drug 6 ( 1.3%) 1 ( 0.4%) 5 ( 2.2%) Gabapentin 3 ( 0.7%) 2 ( 0.9%) 1 ( 0.4%) Amfetamine (different salts) 3 ( 0.7%) 2 ( 0.9%) 1 ( 0.4%) Duloxetine** 3 ( 0.7%) 2 ( 0.9%) 1 ( 0.4%) Trazodone 2 ( 0.4%) 2 ( 0.9%) 0 ( 0.0%) Amitriptyline Summary of Prior Washout Medications (at least two patients)* *Safety population **Indicated for management of fibromyalgia

21 © 2023 Tonix Pharmaceuticals Holding Corp. F307 Characteristics of Study Population Pain Scores • Patients are asked to record “their average pain” for each day ‒ ‘Average’ pain for the day will almost always be lower than ‘worst’ pain for a patient’s day • Baseline pain for randomization a) A mean pain intensity score ≥4 and ≤9 on the 11 - point (0 - 10) NRS scale for the 7 days immediately preceding Visit 2, and b) No more than 2 individual days with a score <4 on the 7 days immediately preceding Visit 2, and c) No score of 10 on any of the 7 days immediately preceding Visit 2, and d) Pain scores recorded on at least 5 out of the 7 days immediately preceding Visit 2 • Mean Pain score for Baseline (BL) for the RESILIENT study was 5.9 ‒ Using the same method, BL for F304 (RELIEF) was 6.1 and BL for F306 (RALLY) was 6.0 • Breakthrough pain ‒ No explicit rescue algorithm ‒ 10 participants took an opiate during the study (6 on TNX - 102 SL and 4 on placebo)

22 © 2023 Tonix Pharmaceuticals Holding Corp. PROFILE DEVELOPMENT PROGRAM Patents Issued TNX - 102 SL*: Fibromyalgia Cyclobenzaprine Protectic ® Sublingual Tablets Fibromyalgia (FM) is a chronic pain disorder resulting from amplified sensory and pain signaling within the CNS • Afflicts an estimated 6 - 12 million adults in the U.S., the majority of whom are women 1 • Symptoms include chronic widespread pain, nonrestorative sleep, fatigue, and cognitive dysfunction • Patients struggle with daily activities, have impaired quality of life, and frequently are disabled • Physicians and patients report common dissatisfaction with currently marketed products Market Entry: Fibromyalgia Additional Indications: Long COVID, Acute Stress Disorder (ASD), PTSD, Agitation in Alzheimer’s, Alcohol Use Disorder Status: One Positive Phase 3 study RELIEF completed 2 Second Phase 3 study RALLY missed primary endpoint Confirmatory Phase 3 study RESILIENT positive, p - value = 0.00005 Next Steps: Pre - NDA meeting with FDA *TNX - 102 SL has not been approved for any indication. 1 American Chronic Pain Association (www.theacpa.org, 2019) 2 Lederman et al., (2023) Arthritis Care & Research "Efficacy and Safety of TNX - 102 SL (Sublingual Cyclobenzaprine) for the Treatment of Fibromyalgia: Results From the RELIEF Trial ", doi : 10.1002/acr.25142. Epub ahead of print. PMID: 37165930. When the check engine light malfunctions, the light is on even though the car is not malfunctioning

© 2023 Tonix Pharmaceuticals Holding Corp. THANK YOU Confidential

24 © 2023 Tonix Pharmaceuticals Holding Corp. Overview of TNX - 102 SL Scientific Rationale for Protectic ® Formulation • Engenders unique pharmacokinetic and pharmacodynamic properties that emphasize sleep properties of cyclobenzaprine while minimizing undesirable properties • Potential therapeutic value in a constellation of disorders where sleep disturbances are: • Co - morbid • Involved in the onset, progression and severity of the disease Protectic ® proprietary formulation of cyclobenzaprine that supports sublingual administration
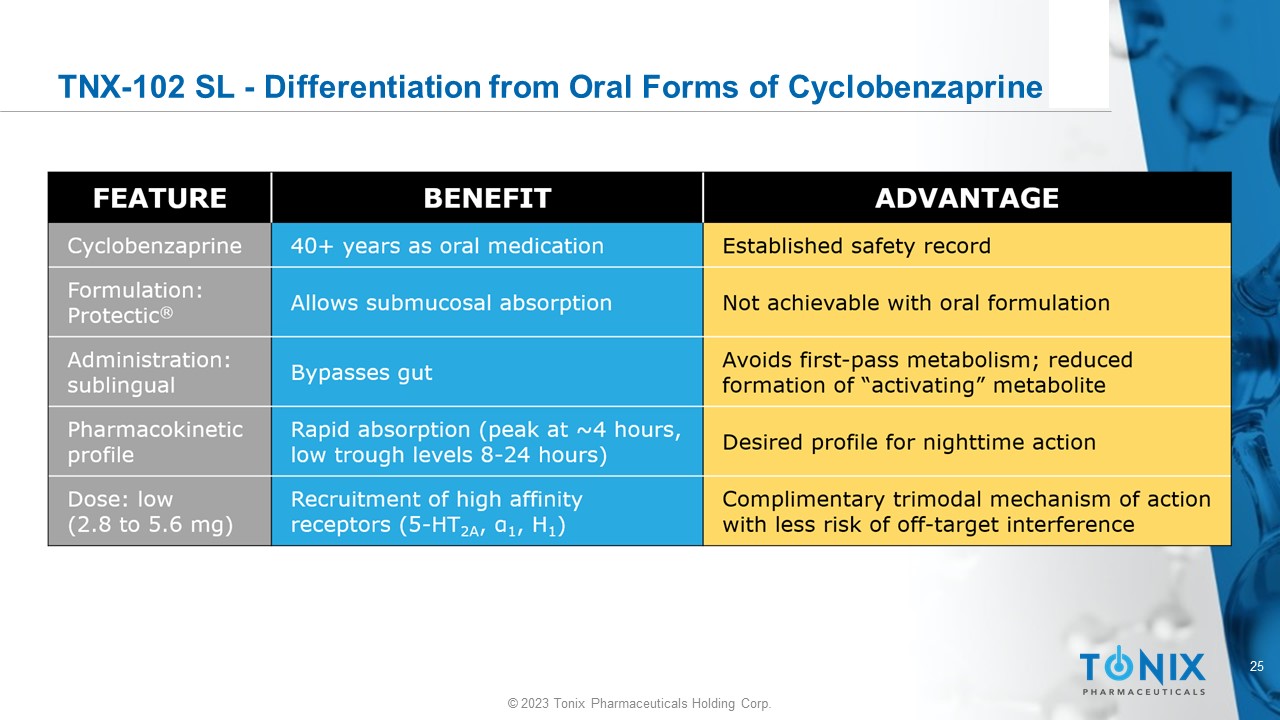
25 © 2023 Tonix Pharmaceuticals Holding Corp. TNX - 102 SL - Differentiation from Oral Forms of Cyclobenzaprine

26 © 2023 Tonix Pharmaceuticals Holding Corp. TNX - 102 SL – Experience in Muscle Spasm • Flexeril ® approval in 1977 received by Merck for the treatment of muscle spasm ‒ 10 mg T.I.D. for acute use (2 - 3 weeks) ‒ 1999 OTC AdCom Briefing Package noted original NDA included “ 8 long term safety studies in which patients with various neurologic disorders received cyclobenzaprine up to 80 mg per day for 1 month up to 3 years .” • 6 published studies in fibromyalgia prior to Tonix program ‒ N=246, placebo controlled, 4 - 24 week treatment periods ‒ Generally well tolerated, although equivocal efficacy across trials ‒ Relatively high doses of the immediate - release (IR) oral formulation may have clouded clear efficacy signal with typical adverse effects of IR such as high rates of drowsiness and dry mouth • Over 45 years of post - marketing safety data ‒ In recent years, ~20 million prescriptions and ~ 1 billion tablets dispensed per year ‒ Chronic cyclobenzaprine use is common ( ~12% of users) ‒ Post - marketing surveillance program following 7,607 patients (including 297 treated with 10 mg for > 30 days) found incidence of most common AEs much lower than in controlled studies • Extensive Post - Marketing Experience Including Long - Term Utilization
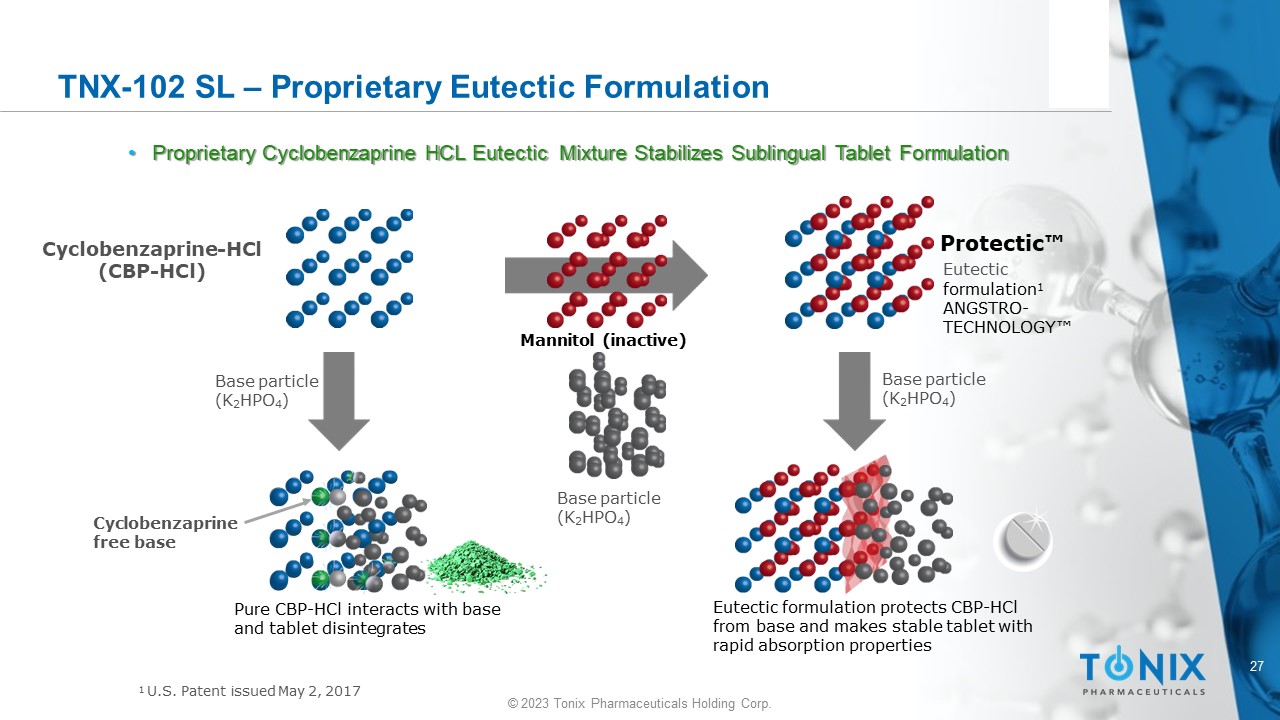
27 © 2023 Tonix Pharmaceuticals Holding Corp. TNX - 102 SL – Proprietary Eutectic Formulation • Proprietary Cyclobenzaprine HCL Eutectic Mixture Stabilizes Sublingual Tablet Formulation Base particle (K 2 HPO 4 ) Base particle (K 2 HPO 4 ) Base particle (K 2 HPO 4 ) C y cl o be n z a p r i n e - HCl (CBP - HCl) Eutectic formulation protects CBP - HCl from base and makes stable tablet with rapid absorption properties Pure CBP - HCl interacts with base and tablet disintegrates Cy c l ob en zapr ine free base Protectic Œ Eutectic formulation 1 ANGSTRO - T E C H NO L O GY Œ Mannitol (inactive) 1 U.S. Patent issued May 2, 2017
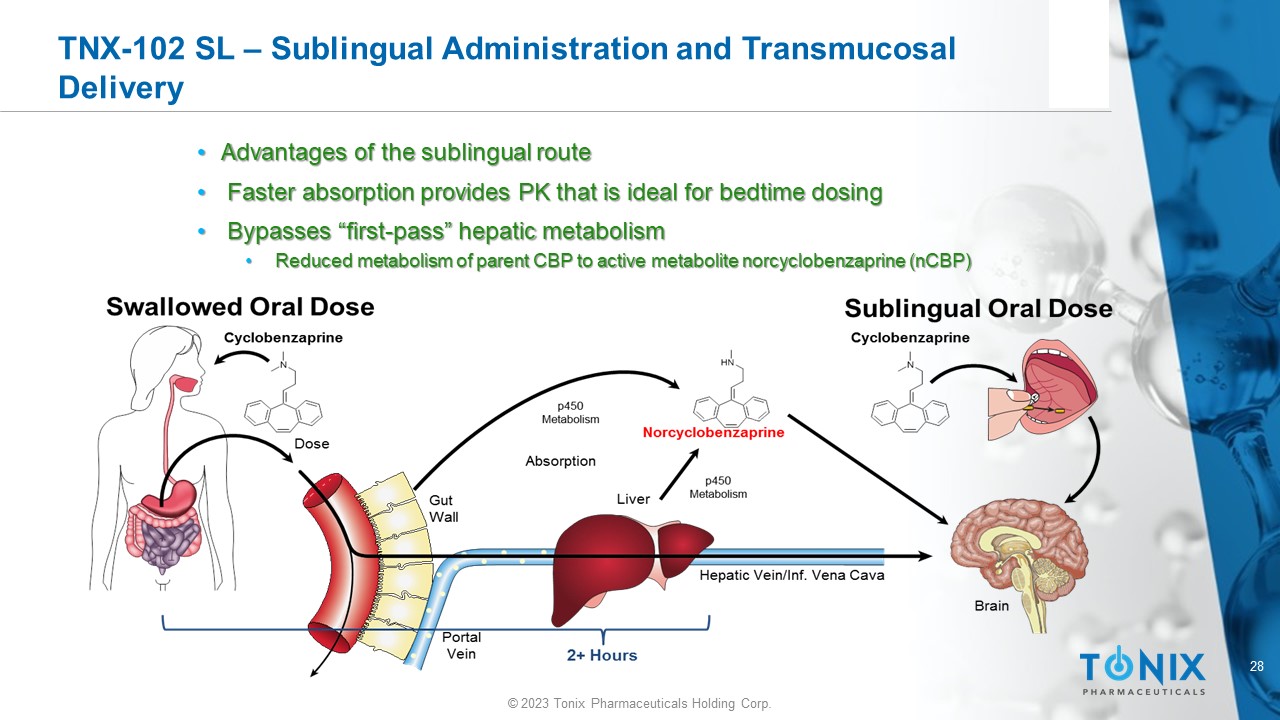
28 © 2023 Tonix Pharmaceuticals Holding Corp. TNX - 102 SL – Sublingual Administration and Transmucosal Delivery • Advantages of the sublingual route • Faster absorption provides PK that is ideal for bedtime dosing • Bypasses “first - pass” hepatic metabolism • Reduced metabolism of parent CBP to active metabolite norcyclobenzaprine (nCBP)

29 © 2023 Tonix Pharmaceuticals Holding Corp. TNX - 102 SL 5.6 mg: Fibromyalgia Three Phase 3 Trials Completed Phase 3 Study, RESILIENT, compared TNX - 102 SL 5.6 mg and placebo • First patient enrolled in April 2022, completed p - value = 0.00005 • Parallel design, double - blind, randomized placebo - controlled study, all US sites • Primary endpoint is pain at Week 14 analyzed by MMRM with MI • All continuous key secondaries by MMRM with MI • Same key secondary efficacy endpoints as F304 RELIEF and F306 RALLY studies Phase 3 Study, RALLY, comparison of TNX - 102 SL 5.6 mg and placebo • As expected from interim analysis results published in July 2021, RALLY Study missed primary endpoint • Unexpected ~80% increase in adverse event - related discontinuations in both drug and placebo arms • Multiple imputation approach on 'Missing Data' attenuated statistical significance of efficacy endpoints’ • TNX - 102 SL was generally well tolerated with overall adverse event profile comparable to prior studies; no new safety signals observed

30 © 2023 Tonix Pharmaceuticals Holding Corp. TNX - 102 SL: Fibromyalgia Program Update Phase 3 Study, RELIEF, compared TNX - 102 SL 5.6 mg and placebo • First patient enrolled in December 2019, completed p - value = 0.010 • Parallel design, double - blind, randomized placebo - controlled study, all US sites • Primary endpoint is pain at Week 14 analyzed by MMRM with MI
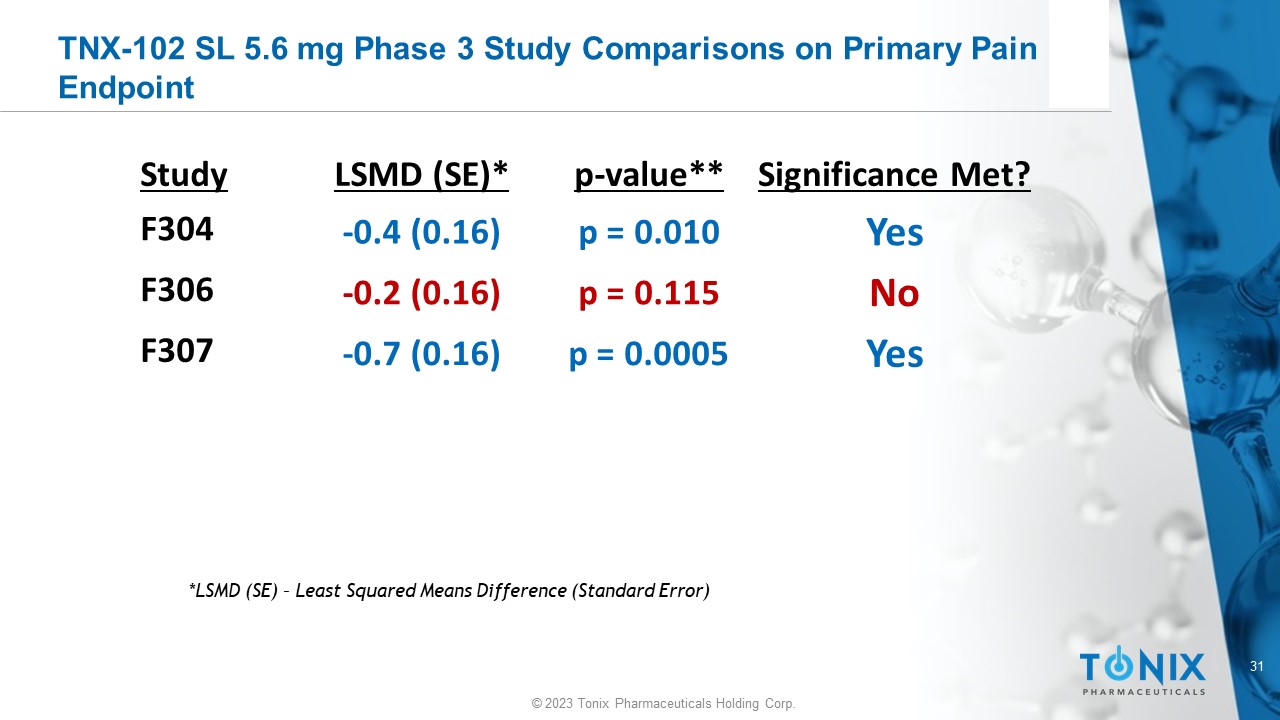
31 © 2023 Tonix Pharmaceuticals Holding Corp. TNX - 102 SL 5.6 mg Phase 3 Study Comparisons on Primary Pain Endpoint Significance Met? p - value** LSMD (SE)* Study Yes p = 0.010 - 0.4 (0.16) F304 No p = 0.115 - 0.2 (0.16) F306 Yes p = 0.0005 - 0.7 (0.16) F307 *LSMD (SE) – Least Squared Means Difference (Standard Error)
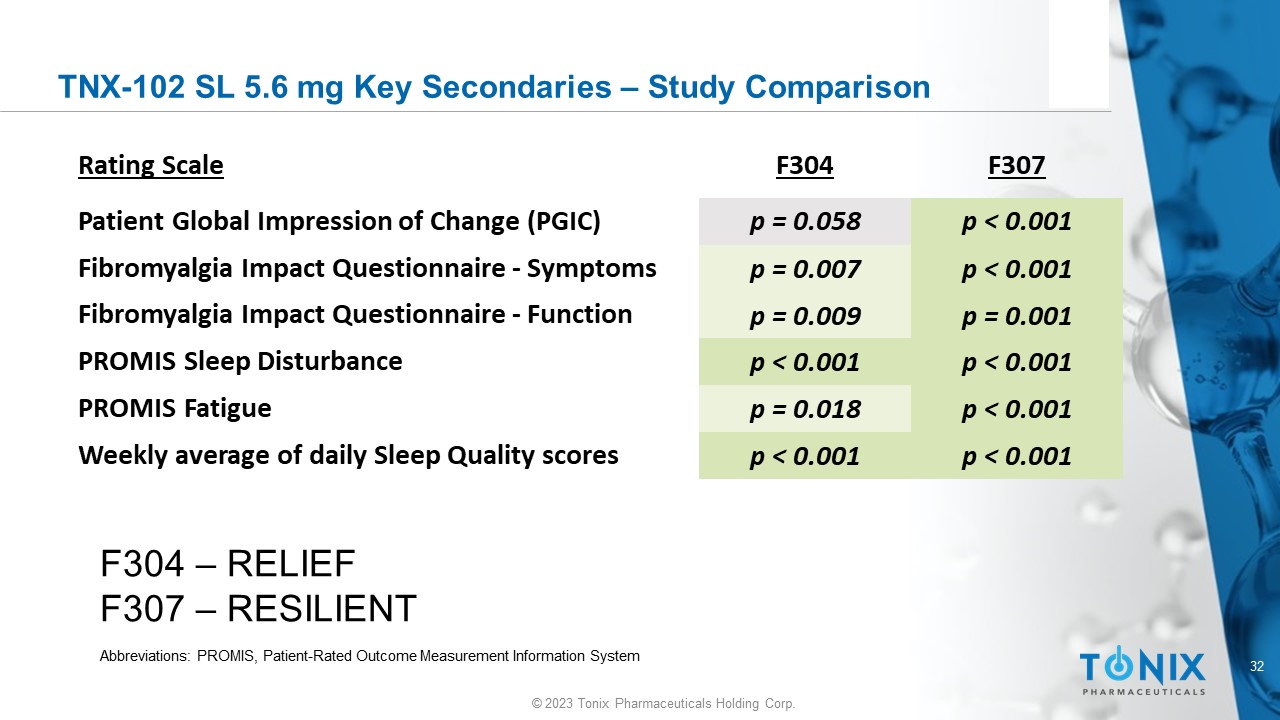
32 © 2023 Tonix Pharmaceuticals Holding Corp. TNX - 102 SL 5.6 mg Key Secondaries – Study Comparison F307 F304 Rating Scale p < 0.001 p = 0.058 Patient Global Impression of Change (PGIC) p < 0.001 p = 0.007 Fibromyalgia Impact Questionnaire - Symptoms p = 0.001 p = 0.009 Fibromyalgia Impact Questionnaire - Function p < 0.001 p < 0.001 PROMIS Sleep Disturbance p < 0.001 p = 0.018 PROMIS Fatigue p < 0.001 p < 0.001 Weekly average of daily Sleep Quality scores F304 – RELIEF F307 – RESILIENT Abbreviations: PROMIS, Patient - Rated Outcome Measurement Information System
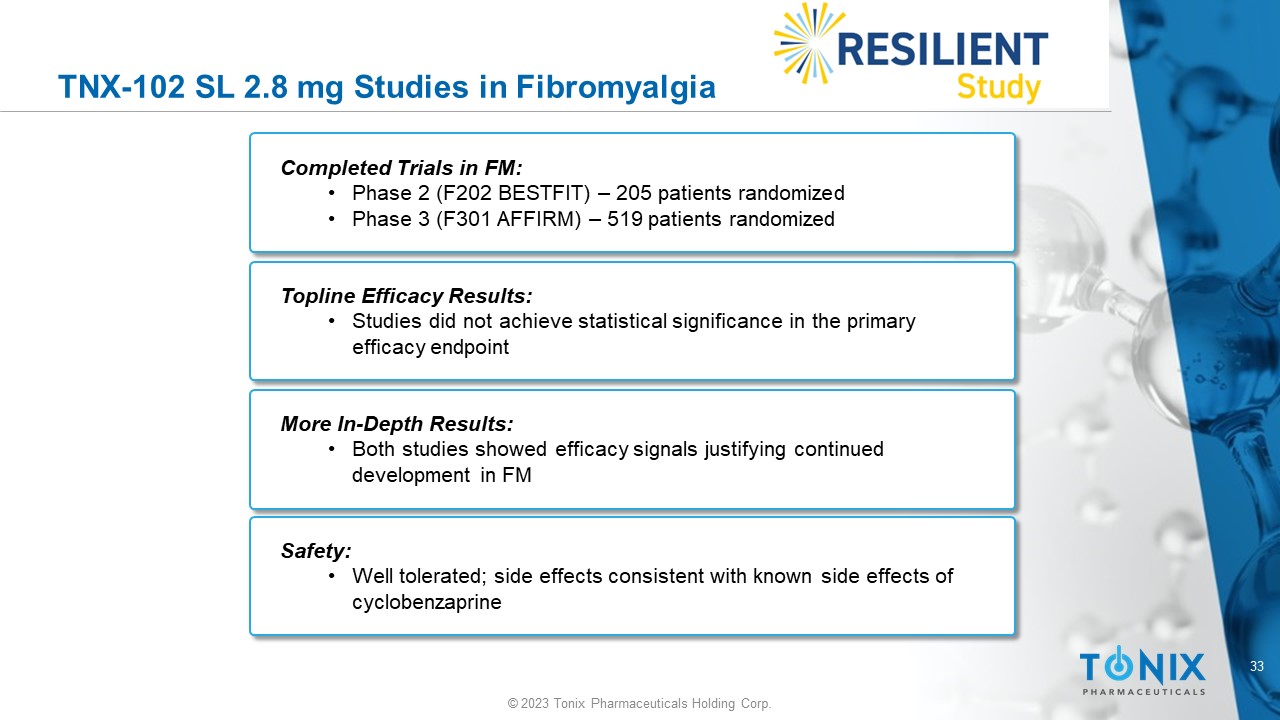
33 © 2023 Tonix Pharmaceuticals Holding Corp. TNX - 102 SL 2.8 mg Studies in Fibromyalgia Completed Trials in FM: • Phase 2 (F202 BESTFIT) – 205 patients randomized • Phase 3 (F301 AFFIRM) – 519 patients randomized Topline Efficacy Results: • Studies did not achieve statistical significance in the primary efficacy endpoint More In - Depth Results: • Both studies showed efficacy signals justifying continued development in FM Safety: • Well tolerated; side effects consistent with known side effects of cyclobenzaprine
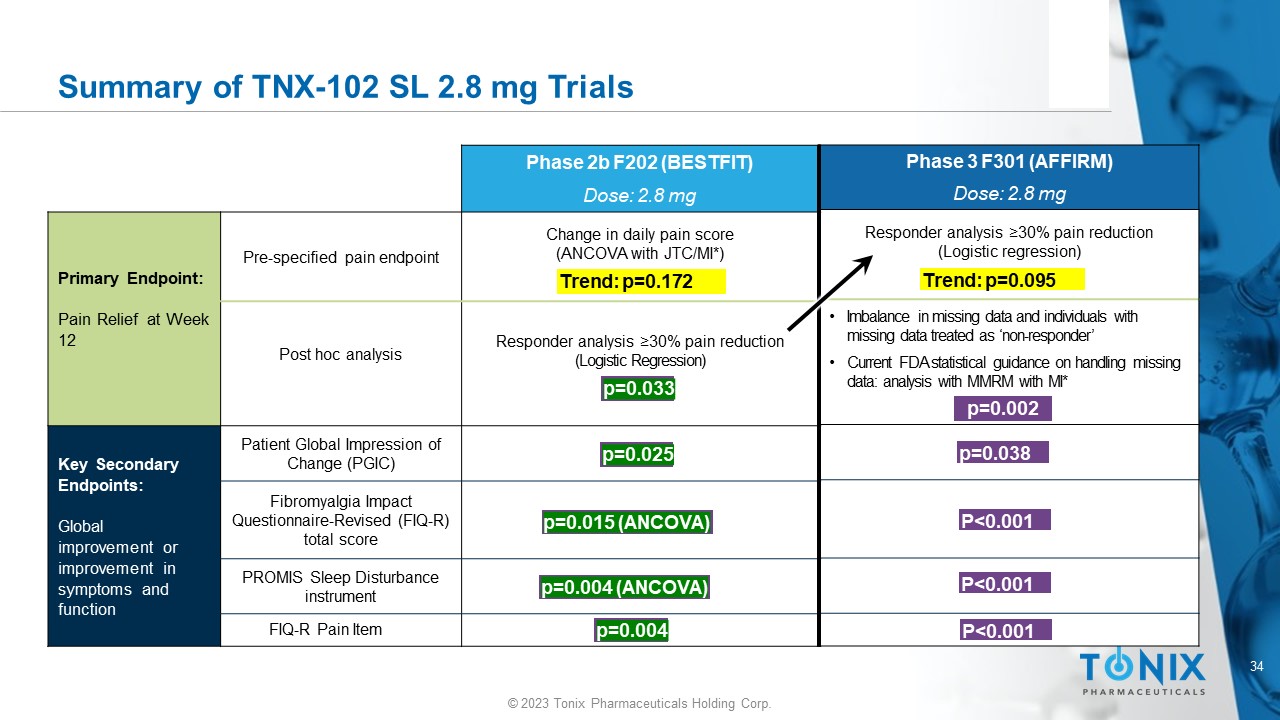
34 © 2023 Tonix Pharmaceuticals Holding Corp. Summary of TNX - 102 SL 2.8 mg Trials p=0.005 p=0.038 P<0.001 P<0.001 P<0.001 Phase 3 F301 (AFFIRM) Dose: 2.8 mg Responder analysis ≥ 30% pain reduction (Logistic regression) • Imbalance in missing data and individuals with missing data treated as ‘non - responder’ • Current FDA statistical guidance on handling missing data: analysis with MMRM with MI* p=0.002 p=0.038 P<0.001 P<0.001 P<0.001 Trend: p=0.095 Phase 2b F202 (BESTFIT) Dose: 2.8 mg Change in daily pain score (ANCOVA with JTC/MI*) Pre - specified pain endpoint Primary Endpoint: Pain Relief at Week 12 Responder analysis ≥30% pain reduction (Logistic Regression) Post hoc analysis Patient Global Impression of Change (PGIC) Key Secondary Endpoints: Global improvement or improvement in symptoms and function Fibromyalgia Impact Questionnaire - Revised (FIQ - R) total score PROMIS Sleep Disturbance instrument FIQ - R Pain Item Trend: p=0.172 p=0.033 p=0.025 p=0.015 (ANCOVA) p=0.004 (ANCOVA) p=0.004
35 © 2023 Tonix Pharmaceuticals Holding Corp. TNX - 102 SL Dose Response Response Dosage Dose - Response Inconsistent response No clear response Consistent response Consistent but trade - offs* (AEs) Basic Pharmacology • Dose can make the difference in the strength of the response
36 © 2023 Tonix Pharmaceuticals Holding Corp. Chronic Overlapping Pain Conditions (COPC) Believed to Result from Shared Brain Processes • COPC is a set of disorders that coaggregate ; these disorders can include but are not limited to 1,2 : • Temporomandibular disorder • Fibromyalgia • Irritable bowel syndrome • Vulvodynia • CFS/ME 3 • Interstitial cystitis/painful bladder syndrome • Endometriosis • Chronic tension - type headache • Migraine headache • Chronic lower back pain 1 Maixner W, et al. J Pain . 2016;17(9 Suppl):T93 - T107. 2 Veasley C, et al. http://www.chronicpainresearch. org/public/CPRA_WhitePaper_2015 - FINAL - Digital.pdf. Published May 2015. Accesse d July 26, 2021. 3 CFS/ME – chronic fatigue syndrome/ myalgic encephalomyelitis • Similar central mechanisms play significant roles in all pain conditions, even those with known peripheral contributions 1,2
37 © 2023 Tonix Pharmaceuticals Holding Corp. Role of Infections in Triggering Fibromyalgia or Chronic fatigue (CFS) - Like Illnesses Infection initiates an autoreactive process, which affects several functions, including brain and energy metabolism 2 - 7 • Infections can trigger any of these conditions in approximately 10% of exposed individuals • The initial location of the infection determines the subsequent pain syndrome • Any type of infectious diarrhea will trigger irritable bowel syndrome (IBS) in 10% to 20% of those exposed 1 Department of Health and Human Services, Office of the Assistant Secretary for Health. 2022. National Research Action Plan on Lo ng COVID, 200 Independence Ave SW, Washington, DC 20201. 2 Blomberg J, et al. Front Immunol. 2018;9:229. Published 2018 Feb 15. 3 Warren JW, et al. Urology. 2008;71(6):1085 - 1090. 4 Buskila D, et al. Autoimmun Rev. 2008;8(1):41 - 43. 5 Hickie I, et al. BMJ. 2006;333(7568):575. 6 Parry SD, et al. Am J Gastroenterol. 2003;98(9):1970 - 1975. 7 Halvorson HA, et al. Am J Gastroenterol. 2006;101(8):1894 - 1942. • Symptoms of Long COVID, like multi - site pain, fatigue and insomnia, are the hallmarks of chronic pain syndromes like fibromyalgia and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). • In August 2022, the HHS released the National Research Action Plan on Long COVID 1 which endorses the connection between Long COVID and chronic fatigue syndrome.
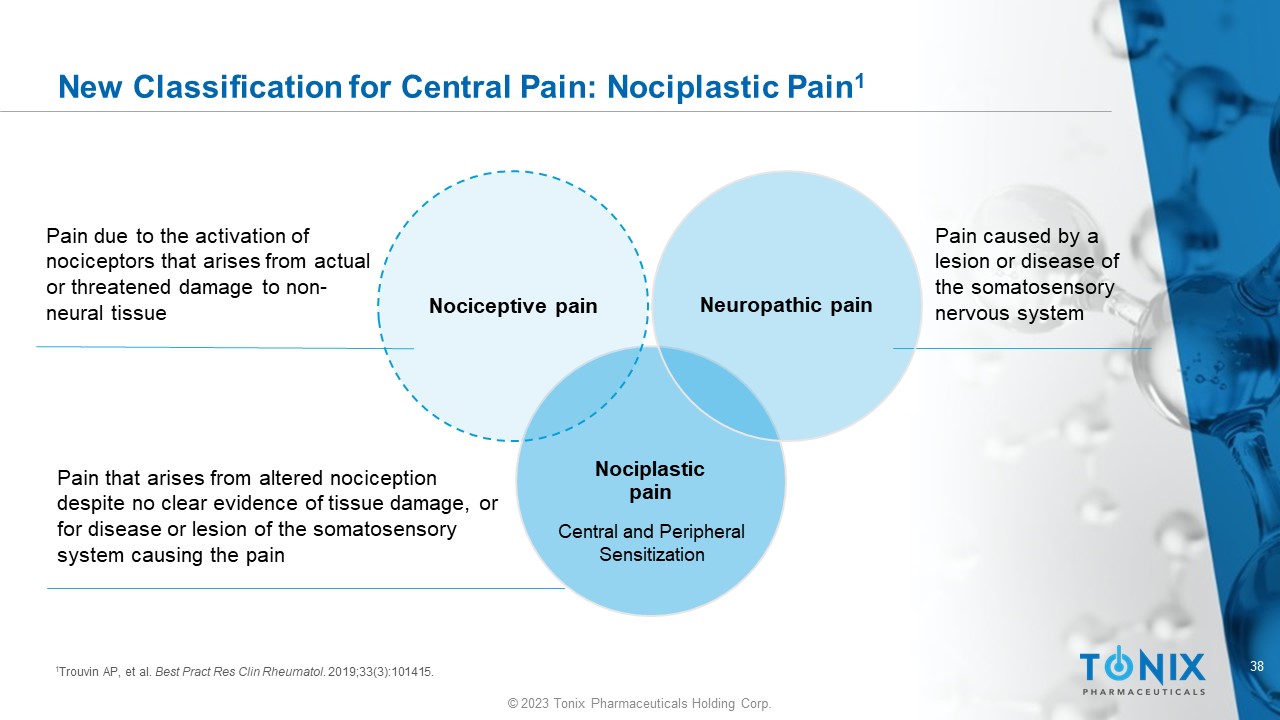
38 © 2023 Tonix Pharmaceuticals Holding Corp. New Classification for Central Pain: Nociplastic Pain 1 Nociplastic pain Nociceptive pain Neuropathic pain Pain due to the activation of nociceptors that arises from actual or threatened damage to non - neural tissue Pain that arises from altered nociception despite no clear evidence of tissue damage, or for disease or lesion of the somatosensory system causing the pain Pain caused by a lesion or disease of the somatosensory nervous system 1 Trouvin AP, et al. Best Pract Res Clin Rheumatol . 2019;33(3):101415. Central and Peripheral Sensitization
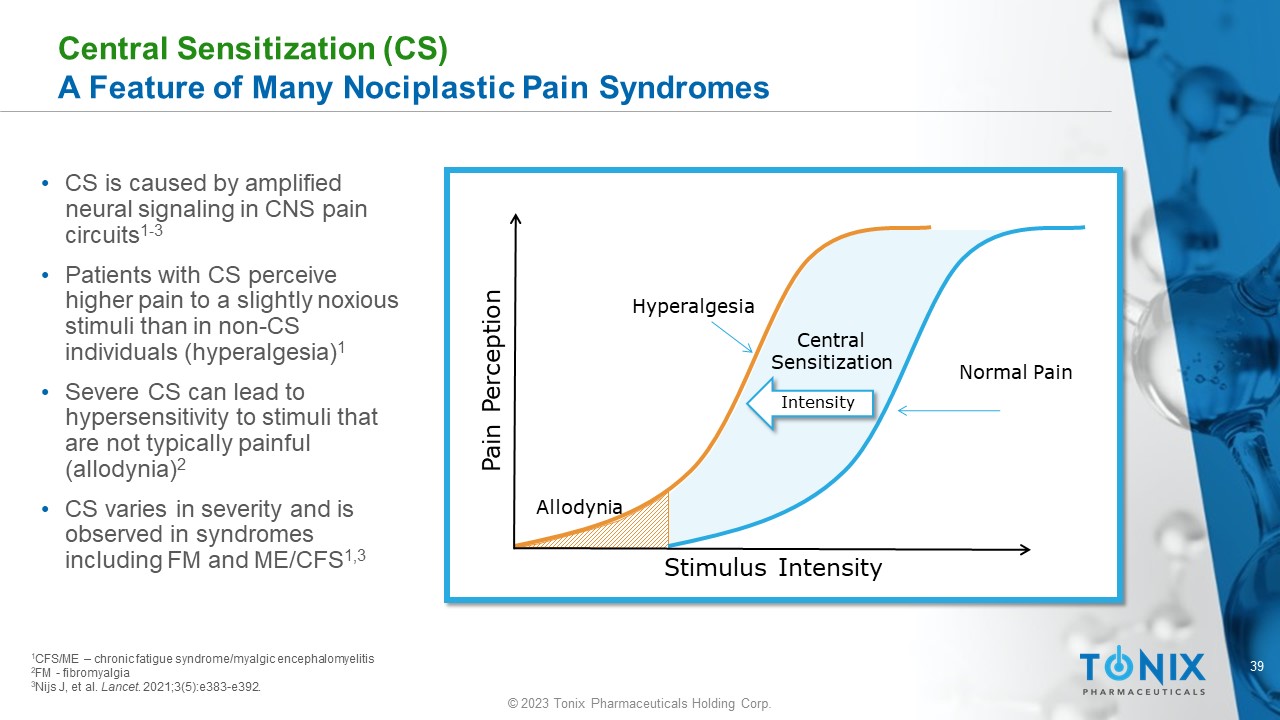
39 © 2023 Tonix Pharmaceuticals Holding Corp. Central Sensitization (CS) A Feature of Many Nociplastic Pain Syndromes • CS is caused by amplified neural signaling in CNS pain circuits 1 - 3 • Patients with CS perceive higher pain to a slightly noxious stimuli than in non - CS individuals (hyperalgesia) 1 • Severe CS can lead to hypersensitivity to stimuli that are not typically painful (allodynia) 2 • CS varies in severity and is observed in syndromes including FM and ME/CFS 1,3 Stimulus Intensity Pain Perception Normal Pain Hyperalgesia Allodynia Central Sensitization Intensity 1 CFS/ME – chronic fatigue syndrome/ myalgic encephalomyelitis 2 FM - fibromyalgia 3 Nijs J, et al. Lancet . 2021;3(5):e383 - e392.
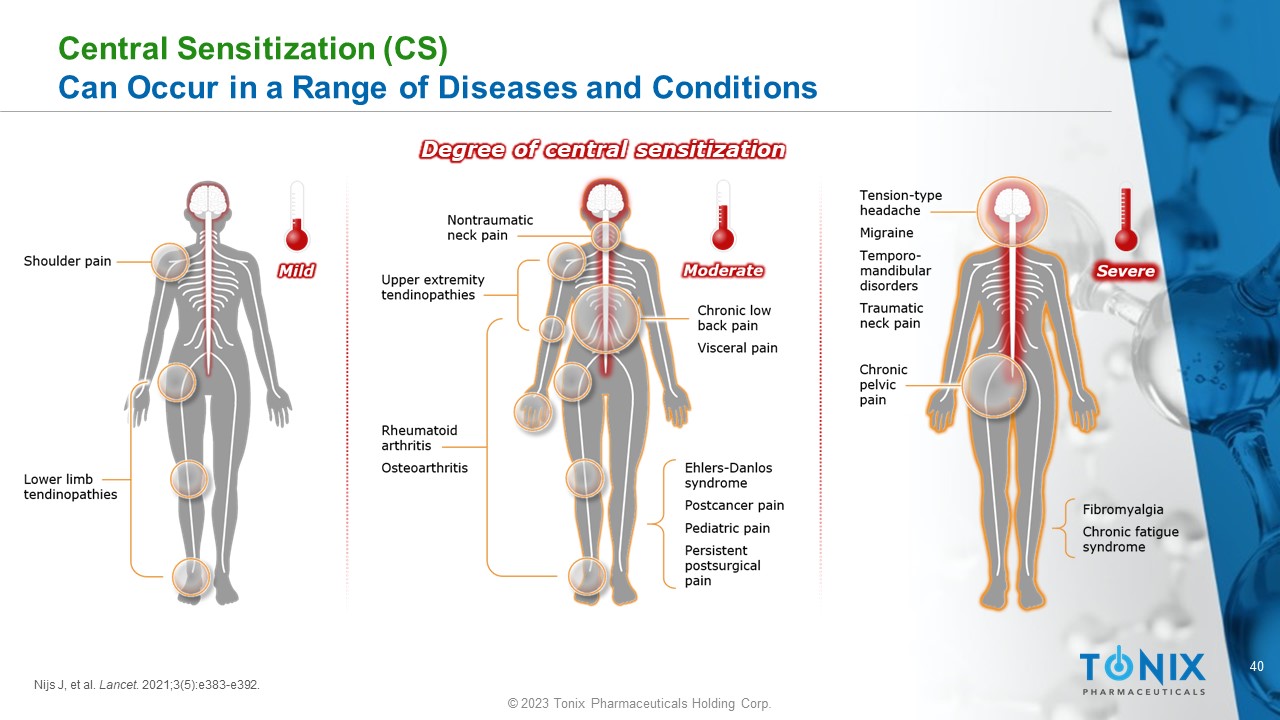
40 © 2023 Tonix Pharmaceuticals Holding Corp. Central Sensitization (CS) Can Occur in a Range of Diseases and Conditions Degree of central sensitization Nijs J, et al. Lancet . 2021;3(5):e383 - e392.

© 2023 Tonix Pharmaceuticals Holding Corp. THANK YOU