UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of report (date of earliest event reported):
(Exact name of registrant as specified in its charter)
|
(State or Other Jurisdiction of Incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
(Address of principal executive offices) (Zip Code)
Registrant’s telephone number, including area
code:
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered |
| The |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 7.01 | Regulation FD Disclosure. |
On December 20, 2023, Tonix Pharmaceuticals Holding Corp. (the “Company”) announced that the Phase 3 RESILIENT study evaluating its TNX-102 SL (cyclobenzaprine HCl sublingual tablets) product candidate for the management of fibromyalgia met its pre-specified primary endpoint in the second of two positive Phase 3 clinical trials. A copy of the press release that discusses this matter is filed as Exhibit 99.01 and hereto and incorporated herein by reference.
The Company presented certain information regarding the Company and its product candidates on December 19, 2023. A copy of the presentation, which may contain nonpublic information, is filed as Exhibit 99.02 hereto and incorporated herein by reference.
The information in this Item 7.01 of this Current Report on Form 8-K, including Exhibits 99.01 and 99.02 attached hereto, shall not be deemed “filed” for purposes of Section 18 of the United States Securities Exchange Act of 1934 (the “Exchange Act”) or otherwise subject to the liabilities of that section, nor shall they be deemed incorporated by reference in any filing under the United States Securities Act of 1933 or the Exchange Act, except as shall be expressly set forth by specific reference in such a filing.
| Item 8.01 | Other Events. |
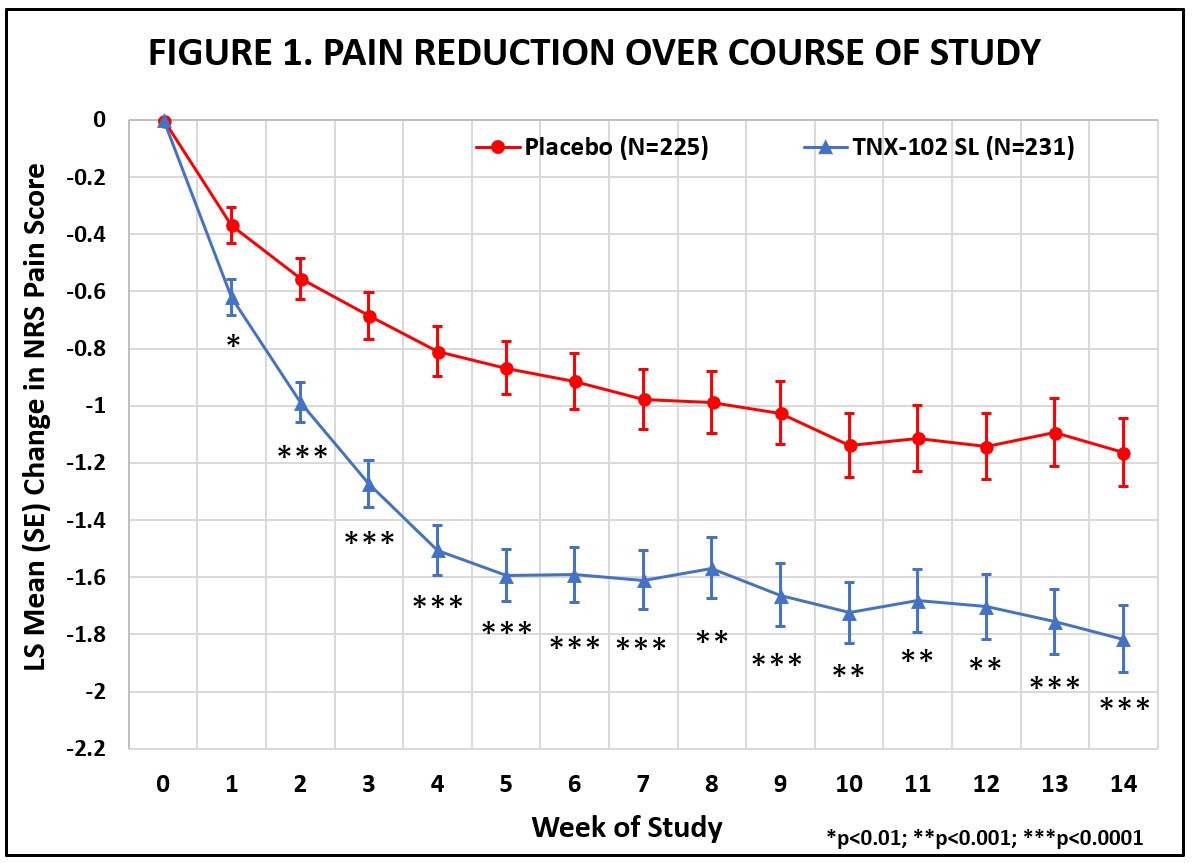
On December 20, 2023, the Company announced that the Phase 3 RESILIENT study evaluating TNX-102 SL met its pre-specified primary endpoint in the second of two positive Phase 3 clinical trials, significantly reducing daily pain compared to placebo (p=0.00005) in participants with fibromyalgia. Statistically significant and clinically meaningful results were also seen in all key secondary endpoints related to improving sleep quality, reducing fatigue, and improving overall fibromyalgia symptoms and function. Additionally, as it relates to improving daily pain, treatment with TNX-102 SL showed a clinically meaningful analgesic effect size of 0.38, with rapid onset of action, separating from placebo for each week of the study. TNX-102 SL was well tolerated with an adverse event profile comparable to prior studies, and no new safety signals were observed. The Company plans to submit a new drug application (“NDA”) to the U.S. Food and Drug Administration (“FDA”) in the second half of 2024 for TNX-102 SL for the management of fibromyalgia. The Company believes it is on track to supply TNX-102 in the U.S. upon FDA approval. The Company’s cash spend will be prioritized for the advancement this program toward FDA approval.
Table 1. Results of Primary and Secondary Endpoints for the Phase 3 RESILIENT Study of TNX-102 SL
| Outcome Measure at Week 14 | Intent-to-Treat Analysis1 | P-value | ||||||
| Primary Endpoint | ||||||||
| Daily Pain Diary, NRS | Mean Change from Baseline2 | 0.00005* | ||||||
| Key Secondary Endpoints | ||||||||
| Non-specific | ||||||||
| Patient Global Impression of Change | Proportion "Much" or "Very Much Improved"3 | <0.001* | ||||||
| Fibromyalgia Syndrome-Related | ||||||||
| FIQ-R Symptom Domain | Mean Change from Baseline | <0.001* | ||||||
| FIQ-R Function Domain | Mean Change from Baseline | 0.001* | ||||||
| PROMIS Sleep Disturbance | Mean Change from Baseline | <0.001* | ||||||
| PROMIS Fatigue | Mean Change from Baseline | <0.001* | ||||||
| Daily Sleep Quality Diary, NRS | Mean Change from Baseline | <0.001* | ||||||
| Abbreviations: FIQ-R = Fibromyalgia Impact Questionnaire - Revised; NRS = Numeric Rating Scale; PROMIS = Patient-Reported Outcomes Measurement Information System | ||||||||
| * statistically significant; to control for overall type 1 error, a pre-specified, serial gatekeeping procedure was utilized. | ||||||||
| 1Analysis by mixed model repeated measures with multiple imputation unless otherwise indicated. | ||||||||
|
2Primary endpoint analysis for FDA approvals of Cymbalta® and Lyrica® in fibromyalgia. 3Pearson’s chi-squared test, with missing data considered non-responders
| ||||||||
Summary of Topline Results of the RESILIENT Study
The RESILIENT study achieved statistical significance on the pre-specified primary efficacy endpoint: change from baseline in the weekly average of daily diary pain severity numerical rating scale (NRS) scores for TNX-102 SL 5.6 mg (LS mean [SE]: -1.8 [0.12] units) versus placebo (-1.2 [0.12] units), analyzed by mixed model repeated measures with multiple imputation (LS mean [SE] difference: -0.7 [0.16] units, p=0.00005, Table 1). All pre-specified sensitivity analyses of the primary endpoint were also statistically significant (p<0.001).

Abbreviations: LS = least squares; NRS = numerical rating scale; SE = standard error
The statistically significant improvement in pain is further substantiated when diary pain was analyzed by another standard statistical approach, a 30 percent responder analysis, with 45.9% on active and 27.1% on placebo having a 30 percent or greater reduction in pain (Pearson Chi-Squared Test; difference in proportions [95% CI]: 18.8% [10.1%, 27.4%]; nominal p<0.001).
TNX-102 SL showed statistical significance (≤0.001) on all six pre-specified key secondary efficacy outcome measures (Table 1).
Consistent with the proposed mechanism that TNX-102 SL acts in fibromyalgia through improving sleep quality, TNX-102 SL showed statistically significant improvement of sleep by two main measures. For the daily diary sleep quality ratings, improvement in sleep quality for TNX-102 SL (-1.8 [0.12] units) was greater than that of placebo (-1.2 [0.12] units; LS mean [SE] difference from placebo: -0.6 [0.17] units; p<0.001). For the PROMIS Sleep Disturbance instrument, TNX-102 SL also demonstrated greater improvement over placebo on T-scores (LS mean [SE] difference from placebo: -4.2 [0.79] units; p<0.001). TNX-102 SL showed improvement over placebo on the PROMIS Fatigue instrument T-scores (-3.0 [0.77] units; p<0.001).
At week 14 on the Fibromyalgia Impact Questionnaire – Revised (FIQ-R), there was greater improvement with TNX-102 SL than with placebo (LS mean [SE] difference from placebo: -7.7 (1.62), p<0.001). TNX-102 SL resulted in greater improvement on FIQ-R Function (LS mean [SE] difference from placebo: -5.4 [1.66], p=0.001). TNX-102 SL also separated from placebo on the FIQ-R Impact domain (nominal p=0.001).
Safety Results of the Phase 3 RESILIENT Study
In the RESILIENT study, TNX-102 SL was well tolerated, with no new safety signals observed. Among participants randomized to the TNX-102 SL and placebo arms, 81.0% and 79.2%, respectively, completed the 14-week dosing period. Administration site reactions were the most commonly reported adverse events and were higher in the TNX-102 SL treatment group. Hypoaesthesia oral and paraesthesia oral, product taste abnormal, and tongue discomfort were local effects nearly always temporally related to dose administration and transiently expressed (<60 minutes) in most occurrences. The treatment-emergent adverse events that occurred at a rate of 3.0% or greater in either arm were these four oral adverse events, along with COVID-19, somnolence, and headache. Adverse events resulted in premature study discontinuation in 6.1% of those who received TNX-102 SL compared with 3.5% of placebo recipients. There were a total of seven serious adverse events in five patients, five of which were experienced by three patients in the placebo arm, and two of which were in the TNX-102 SL arm. Of the two in the TNX-102 SL arm, one was renal cancer, deemed unrelated to study drug, and the other was acute pancreatitis with onset 14 days after dosing was completed and reported as possibly related to study drug.
Table 2. Treatment-emergent adverse events at a rate of 3% or greater in either treatment arm
| TNX-102 SL (N=231) | Placebo (N=226) | Total (N=457)* | ||||||||||
| Administration Site Reactions | N | % | N | % | N | % | ||||||
| Hypoaethesia oral | 55 | 23.8% | 1 | 0.4% | 56 | 12.3% | ||||||
| Product taste abnormal | 27 | 11.7% | 2 | 0.9% | 29 | 6.3% | ||||||
| Paraesthesia oral | 16 | 6.9% | 2 | 0.9% | 18 | 3.9% | ||||||
| Tongue discomfort | 16 | 6.9% | 0 | 0.0% | 16 | 3.5% | ||||||
| Systemic | ||||||||||||
| Adverse Events | N | % | N | % | N | % | ||||||
| COVID-19 | 10 | 4.3% | 7 | 3.1% | 17 | 3.7% | ||||||
| Somnolence | 7 | 3.0% | 3 | 1.3% | 10 | 2.2% | ||||||
| Headache | 7 | 3.0% | 4 | 1.8% | 11 | 2.4% | ||||||
*Safety Population
In females, the total score on the Changes in Sexual Functioning Questionnaire short form (CSFQ-14) at Week 14 improved (indicating better sexual functioning) in the TNX-102 SL group compared with placebo (nominal p=0.010 by analysis of covariance). This potentially indicates an important tolerability advantage over pharmacotherapeutics which potently inhibit reuptake of serotonin. The low percentage of males in the safety population (<5%) did not allow meaningful analysis of the CSFQ-14 data.
Separately, the Company completed the analysis of the Phase 2 PREVENTION study of its TNX-1900 (intranasal potentiated oxytocin) product candidate for the prevention of migraine headaches in chronic migraineurs. The trial did not meet the primary endpoint as measured by a reduction from 28-day run-in baseline in the mean number of migraine headache days during the last 28 days of the treatment phase. PREVENTION was a small proof-of-concept study, and with 88 patients enrolled across three arms (TNX-1900 30 IU QD, TNX-1900 30 IU BID and placebo), was not powered to result in a statistically significant outcome. For the primary outcome of monthly migraine days during the last 28 days of treatment, the placebo group saw a least squares mean (LSmean) change from baseline of -8.2 (1.4) [mean (standard error)]; the 30 IU group reported a LSmean change of -7.8 (1.2) and the 60 IU group had -5.8 (1.3). The comparison between placebo and TNX-1900 30 IU favored placebo with a difference of 0.4 (1.5) migraine days and an effect size of 0.07; the comparison between placebo and TNX-1900 60 IU favored placebo with a difference of 2.4 (1.5) migraine days and an effect size of 0.42. In the trial, TNX-1900 was generally well-tolerated with no treatment-emergent serious or severe adverse events.
| Item 9.01 | Financial Statements and Exhibits. |
| (d) |
Exhibit No. |
Description. | ||
|
104 |
Press Release, dated December 20, 2023 Investor Presentation by the Company Cover Page Interactive Data File (embedded within the Inline XBRL document) |
SIGNATURE
Pursuant to the requirement of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| TONIX PHARMACEUTICALS HOLDING CORP. | |||
| Date: December 20, 2023 | By: | /s/ Bradley Saenger | |
| Bradley Saenger | |||
| Chief Financial Officer | |||